Abstract
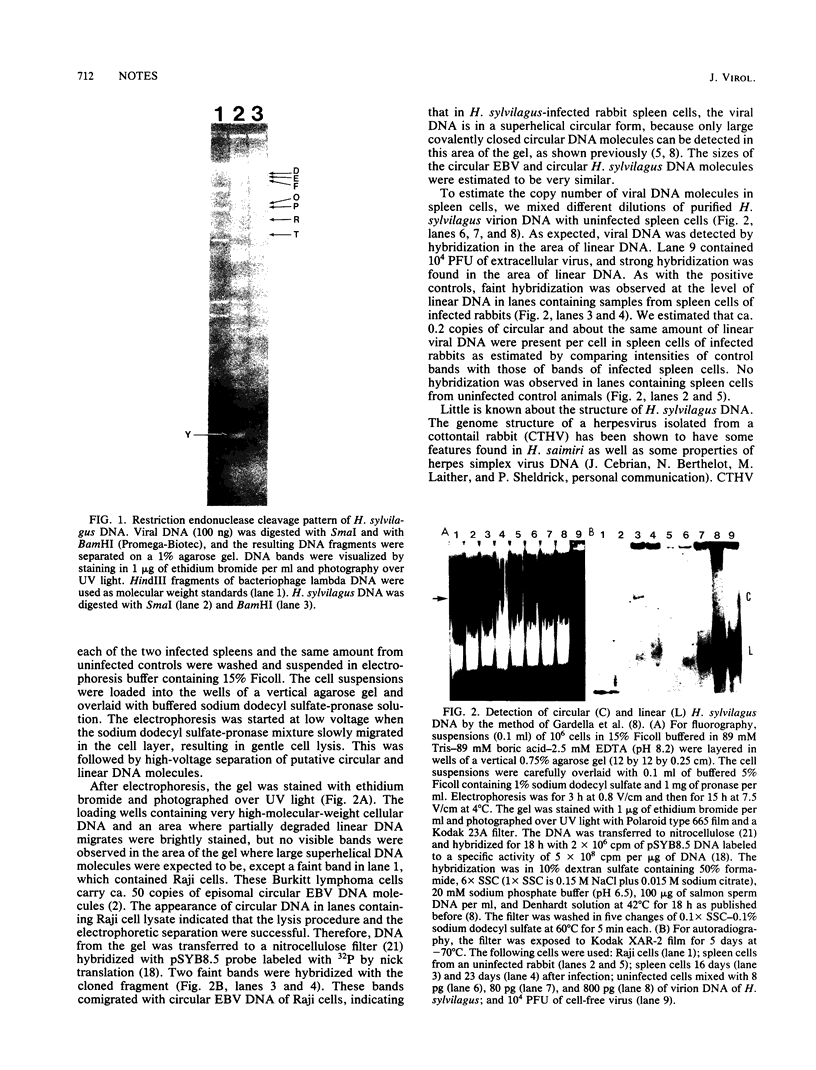
Cottontail rabbits (Sylvilagus floridanus) were infected with Herpesvirus sylvilagus, and spleen cells were analyzed for the presence of virus-specific, covalently closed circular, and linear DNA molecules by a simple electrophoretic technique, followed by transfer to nitrocellulose filters and hybridization with cloned viral DNA (Gardella et al., J. Virol. 50:248-254, 1984). Approximately 0.2 copies per cell of circular DNA and 0.2 copies per cell of linear DNA were detected by hybridization with a cloned viral DNA fragment. The size of the viral DNA was estimated at ca. 158 kilobase pairs. Restriction endonuclease patterns suggested structural similarities to cottontail herpesvirus DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs R., Rouhandeh H. Herpesvirus sylvilagus I. Polypeptides of virions and nucleocapsids. J Virol. 1982 Mar;41(3):1063–1072. doi: 10.1128/jvi.41.3.1063-1072.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby B. M., Shaw J. E., Elion G. B., Pagano J. S. Effect of acyclovir [9-(2-hydroxyethoxymethyl)guanine] on Epstein-Barr virus DNA replication. J Virol. 1980 May;34(2):560–568. doi: 10.1128/jvi.34.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Wolf H. Purification and properties of Herpesvirus saimiri DNA. Virology. 1974 Mar;58(1):55–64. doi: 10.1016/0042-6822(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Gardella T., Medveczky P., Sairenji T., Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984 Apr;50(1):248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodheart C. R., Plummer G. The densities of herpesviral DNAs. Prog Med Virol. 1975;19:324–352. [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze H. C. Induction of lymphoid hyperplasia and lymphoma-like disease in rabbits by Herpesvirus sylvilagus. Int J Cancer. 1971 Nov 15;8(3):514–522. doi: 10.1002/ijc.2910080320. [DOI] [PubMed] [Google Scholar]
- Hinze H. C. New member of the herpesvirus group isolated from wild cottontail rabbits. Infect Immun. 1971 Feb;3(2):350–354. doi: 10.1128/iai.3.2.350-354.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Werner F. J., Bauer I., Fleckenstein B. Structure of nonintegrated, circular Herpesvirus saimiri and Herpesvirus ateles genomes in tumor cell lines and in vitro-transformed cells. J Virol. 1982 Oct;44(1):295–310. doi: 10.1128/jvi.44.1.295-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez L. V., Hunt R. D., King N. W., Barahona H. H., Daniel M. D., Fraser C. E., Garcia F. G. Herpesvirus ateles, a new lymphoma virus of monkeys. Nat New Biol. 1972 Feb 9;235(58):182–184. doi: 10.1038/newbio235182b0. [DOI] [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., García F. G., Fraser C. E. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab Anim Care. 1969 Jun;19(3):378–386. [PubMed] [Google Scholar]
- Powell P. C., Payne L. N., Frazier J. A., Rennie M. T lymphoblastoid cell lines from Marek's disease lymphomas. Nature. 1974 Sep 6;251(5470):79–80. doi: 10.1038/251079a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rziha H. J., Bauer B. Circular forms of viral DNA in Marek's disease virus-transformed lymphoblastoid cells. Arch Virol. 1982;72(3):211–216. doi: 10.1007/BF01348966. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Werner F. J., Bornkamm G. W., Fleckenstein B. Episomal viral DNA in a Herpesvirus saimiri-transformed lymphoid cell line. J Virol. 1977 Jun;22(3):794–803. doi: 10.1128/jvi.22.3.794-803.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima Y., Tanaka A., Nonoyama M. Inhibition of productive replication of Epstein-Barr virus DNA by phosphonoacetic acid. Virology. 1976 May;71(1):352–354. doi: 10.1016/0042-6822(76)90119-7. [DOI] [PubMed] [Google Scholar]