Abstract
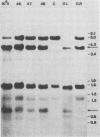
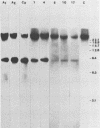
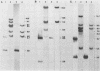
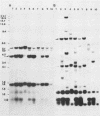
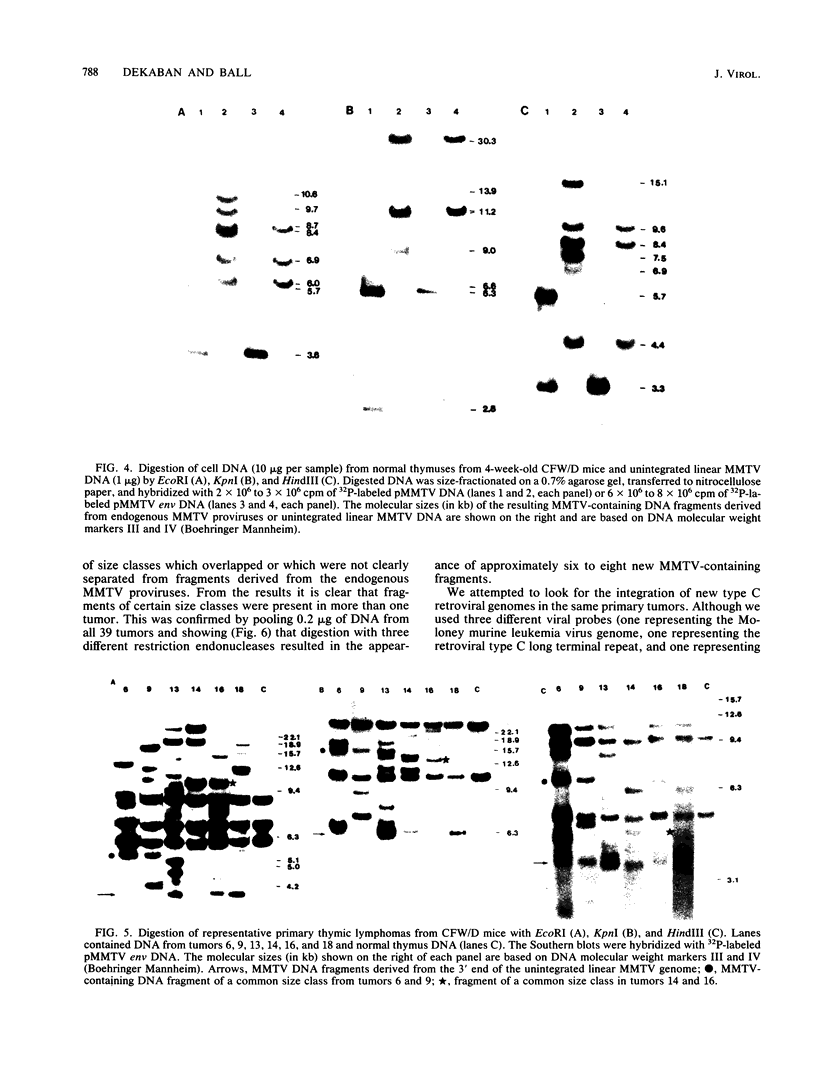
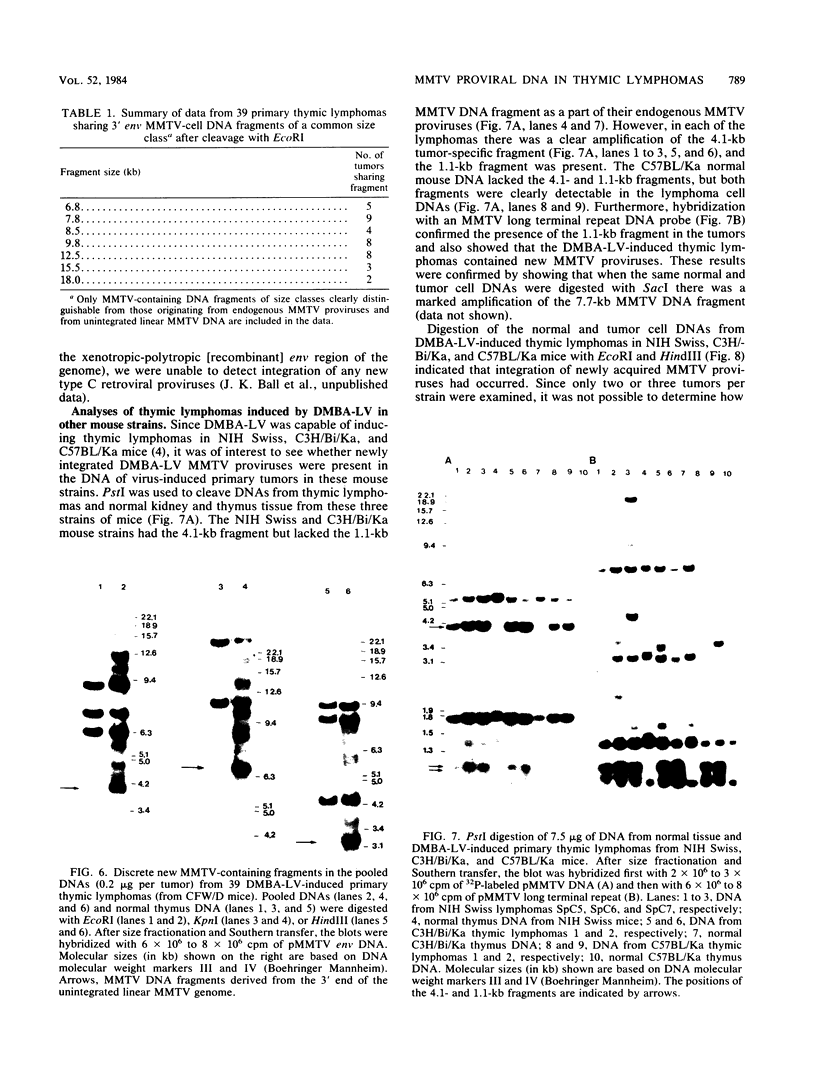
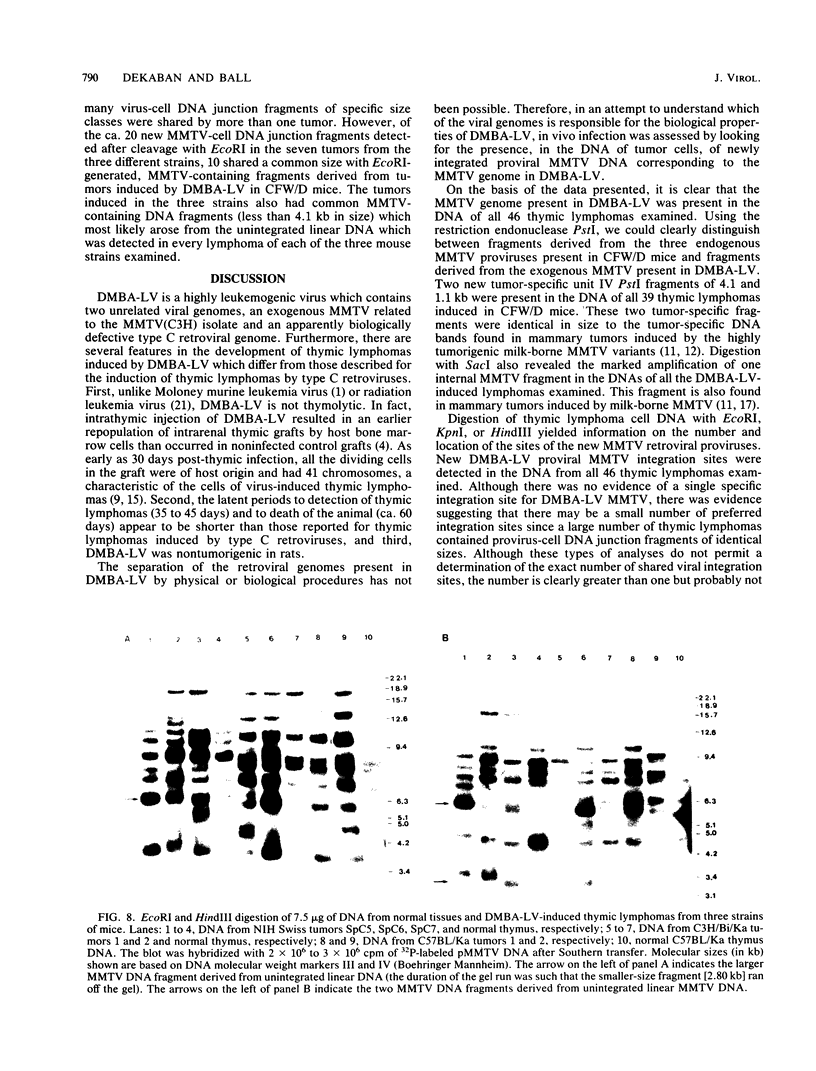
In previous studies we described the isolation and characterization of a highly leukemogenic virus, DMBA-LV, isolated from a transplanted, chemical carcinogen-induced thymic lymphoma. The virus is composed of a mixture of two unrelated retroviral genomes, one highly related to type B milk-borne mouse mammary tumor virus isolates and the other partially related to type C viral genomes. In the present study, primary thymic lymphomas induced by DMBA-LV in CFW/D, NIH Swiss, C3H/Bi/Ka, and C57BL/Ka mice were assessed for the presence of newly integrated type B retroviral DNA. All 46 primary thymic lymphomas examined contained one to four newly acquired murine mammary tumor virus proviruses. Based on the sizes of provirus-cell DNA junction fragments, the integration of newly acquired murine mammary tumor virus proviruses did not appear to be random.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball J. K., Dawson D. A. Biological effects of the neonatal injection of 7,12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1969 Apr;42(4):579–591. [PubMed] [Google Scholar]
- Ball J. K., Dekaban G. A., McCarter J. A., Loosmore S. M. Molecular biological characterization of a highly leukaemogenic virus isolated from the mouse. III. Identity with mouse mammary tumour virus. J Gen Virol. 1983 Oct;64(Pt 10):2177–2190. doi: 10.1099/0022-1317-64-10-2177. [DOI] [PubMed] [Google Scholar]
- Ball J. K. Leukemogenesis by an endogenous virus isolated from the CFW mouse. II. Early effects of virus on thymus gland and bone marrow cell populations. J Natl Cancer Inst. 1979 Jun;62(6):1517–1522. [PubMed] [Google Scholar]
- Ball J. K., McCarter J. A. Biological characterization of a leukemogenic virus isolated from the CFW mouse. Cancer Res. 1979 Aug;39(8):3080–3088. [PubMed] [Google Scholar]
- Bentvelzen P., Brinkhof J. Organ distribution of exogenous murine mammary tumour virus as determined by bioassay. Eur J Cancer. 1977 Mar 29;13(3):241–245. doi: 10.1016/0014-2964(77)90211-0. [DOI] [PubMed] [Google Scholar]
- Canaani E., Aaronson S. A. Restriction enzyme analysis of mouse cellular type C viral DNA: emergence of new viral sequences in spontaneous AKR/J lymphomas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1677–1681. doi: 10.1073/pnas.76.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan F. P., Ball J. K., Sergovich F. R. Trisomy no. 15 in murine thymomas induced by chemical carcinogens, X-irradiation, and an endogenous murine leukemia virus. J Natl Cancer Inst. 1979 Mar;62(3):605–610. doi: 10.1093/jnci/62.3.605. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Proviruses of mouse mammary tumor virus in normal and neoplastic tissues from GR and C3Hf mouse strains. J Virol. 1980 Aug;35(2):298–305. doi: 10.1128/jvi.35.2.298-305.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE HARVEN E., FRIEND C. Electron microscopy of Swiss mouse leukemia virus. Natl Cancer Inst Monogr. 1960 Sep;4:291–311. [PubMed] [Google Scholar]
- Dalton A. J., Potter M. Electron microscopic study of the mammary tumor agent in plasma cell tumors. J Natl Cancer Inst. 1968 Jun;40(6):1375–1385. [PubMed] [Google Scholar]
- Dofuku R., Biedler J. L., Spengler B. A., Old L. J. Trisomy of chromosome 15 in spontaneous leukemia of AKR mice. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1515–1517. doi: 10.1073/pnas.72.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J., Risser R. Amplification and novel locations of endogenous mouse mammary tumor virus genomes in mouse T-cell lymphomas. J Virol. 1984 Jan;49(1):92–101. doi: 10.1128/jvi.49.1.92-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning T. G., Puma J. P., Cardiff R. D. Selective amplification of mouse mammary tumor virus in mammary tumors of GR mice. J Virol. 1980 Oct;36(1):109–114. doi: 10.1128/jvi.36.1.109-114.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay F. W., Clarke J. K., Dermott E. Direct cell to cell transfer of Bittner virus. J Gen Virol. 1970 Apr;7(1):75–79. doi: 10.1099/0022-1317-7-1-75. [DOI] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran-Ghera N. The mechanism of radiation action in leukemogenesis. 3. Thymolytic effect induced by the leukemogenic agent. Isr J Med Sci. 1968 Nov-Dec;4(6):1169–1180. [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Jaenisch R. Conformation of free and of integrated Moloney leukemia virus proviral DNA in preleukemic and leukemic BALB/Mo mice. Virology. 1980 Feb;101(1):111–123. doi: 10.1016/0042-6822(80)90488-2. [DOI] [PubMed] [Google Scholar]
- Karande K. A., Joshi B. J., Talageri V. R., Dumaswala R. U., Ranadive K. J. Intracytoplasmic type A particles from mammary tumours and leukaemias of strain ICRC mice. Br J Cancer. 1979 Feb;39(2):132–142. doi: 10.1038/bjc.1979.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Fung Y. K., Majors J. E., Bishop J. M., Varmus H. E. Synthesis of plus strands of retroviral DNA in cells infected with avian sarcoma virus and mouse mammary tumor virus. J Virol. 1981 Jan;37(1):127–138. doi: 10.1128/jvi.37.1.127-138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E., Hilkens J., Hilgers J., Groner B., Hynes N. E. Acquisition of proviral DNA of mouse mammary tumor virus in thymic leukemia cells from GR mice. J Virol. 1982 Sep;43(3):819–829. doi: 10.1128/jvi.43.3.819-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983 Jun;33(2):369–377. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- Pourreau-Schneider N., Stephens R. J., Gardner W. U. Viral inclusions and other cytoplasmic components in a Leydig cell murine tumor: an electron microscopic study. Int J Cancer. 1968 Jan 15;3(1):155–162. doi: 10.1002/ijc.2910030119. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. B., Long C. A., Sheffield J. B., Tamura A., Tanaka H. Murine mammary tumor virus deficient in the major glycoprotein: biochemical and biological studies on virions produced by a lymphoma cell line. Virology. 1980 Jul 30;104(2):279–293. doi: 10.1016/0042-6822(80)90333-5. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk W. J., Emmelot P., Hilgers J., Kamlag D., Nusse R., Feltkamp C. A. Quantitation of virus-induced (MLr) and normal (Thy.1.2) cell surface antigens in isolated plasma membranes and the extracellular ascites fluid of mouse leukemia cells. Cancer Res. 1975 Oct;35(10):2743–2751. [PubMed] [Google Scholar]
- van Blitterswijk W. J., Emmelot P., Hilkmann H. A., Hilgers J., Feltkamp C. A. Rigid plasma-membrane-derived vesicles, enriched in tumour-associated surface antigens (MLr), occurring in the ascites fluid of a murine leukaemia (GRSL). Int J Cancer. 1979 Jan 15;23(1):62–70. doi: 10.1002/ijc.2910230112. [DOI] [PubMed] [Google Scholar]