Abstract
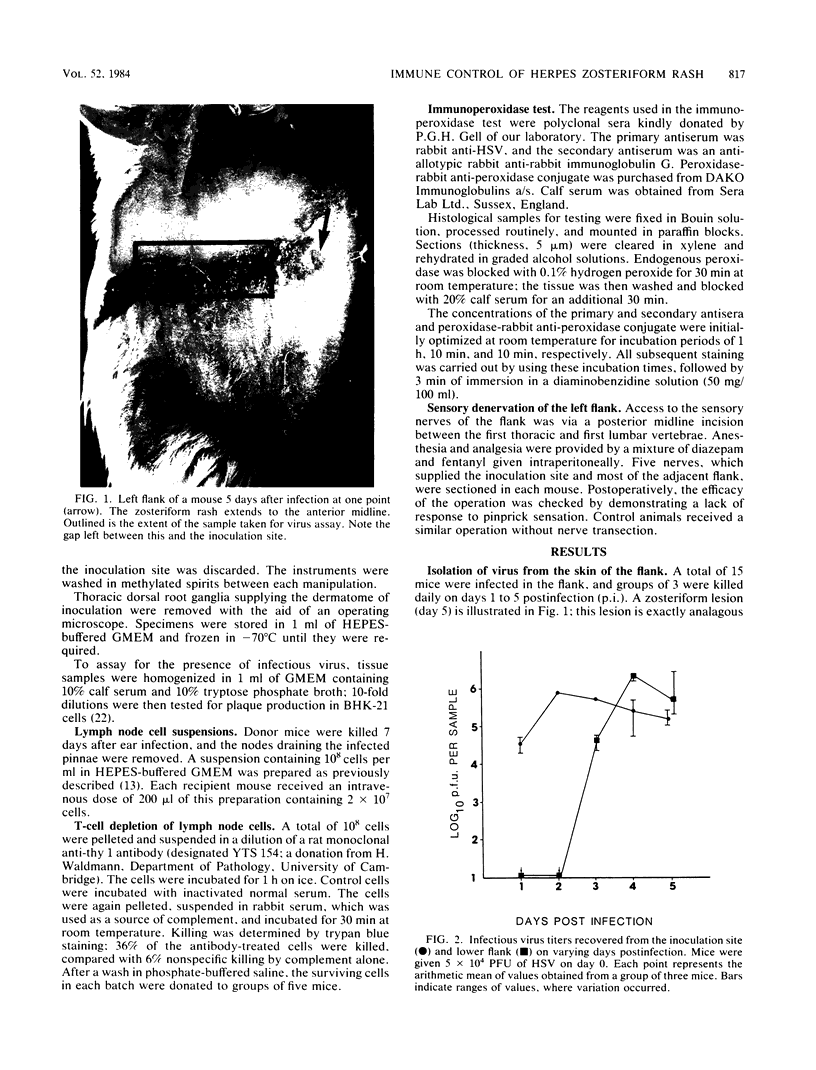
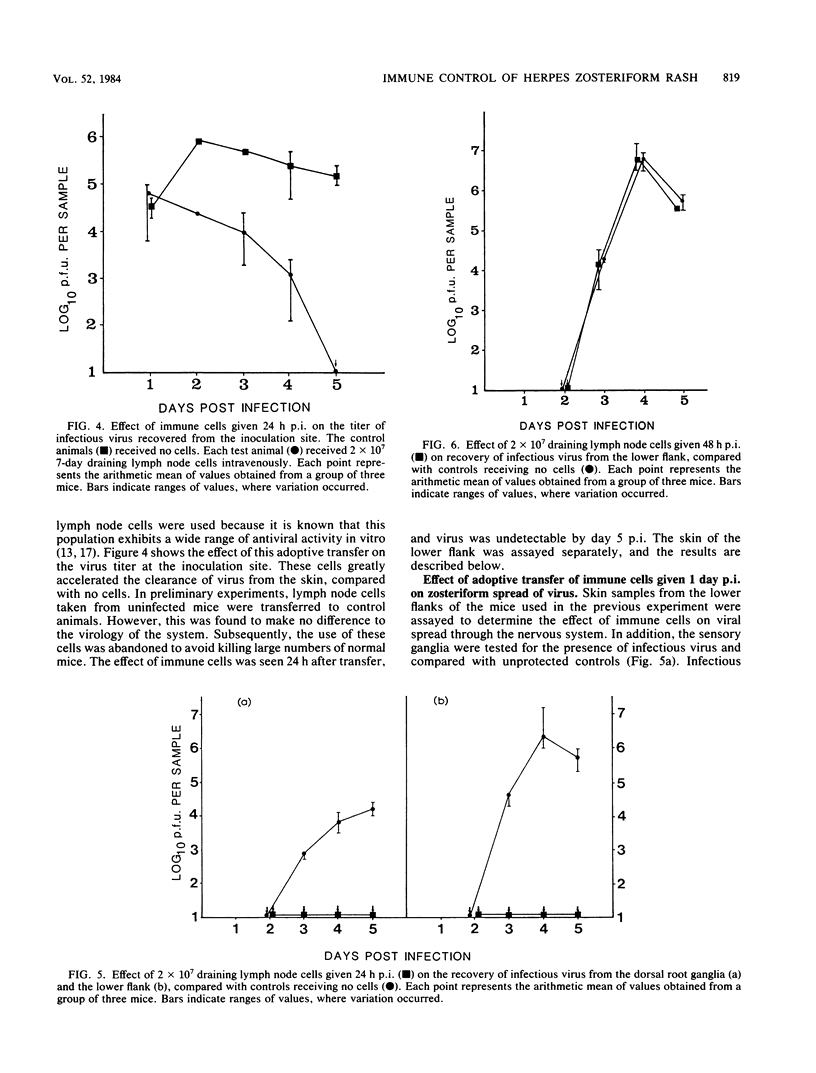
During the development of a zosteriform rash, which occurs after flank inoculation of BALB/c mice with herpes simplex virus, clinically normal skin becomes infected via nerve endings. This is analogous to the final step in the development of a recrudescent lesion, which may occur after reactivation of latent virus. Therefore, the zosteriform reaction has potential as a model with which to study the modification of such a recrudescent infection by immune processes. Using an adoptive transfer system, we confirmed that immune lymph node cells are potent in accelerating the clearance of virus from the primary site of replication (the inoculation site). This effect was T cell dependent. However, if injection of the same cell population was delayed until ganglionic infection was established, the appearance of the zosteriform rash was not prevented, and the virus titer recovered from the lower flank was not reduced. Immunoperoxidase studies showed that virus is at first highly localized to the epidermis after it emerges from nerves. As determined by conventional histology, little cellular infiltration was seen until clinical lesions were apparent. These observations indicate that recrudescent lesions appear in the presence of cell populations normally associated with rapid virus clearance; cellular immune mechanisms may be rendered ineffective owing to the lack of recruitment to the site of recrudescence until tissue breakdown instigates an inflammatory response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. 3. Regression infectious foci. J Exp Med. 1971 May 1;133(5):1090–1104. doi: 10.1084/jem.133.5.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard S. H., Cheatham W. J., Moses H. L. Electron microscopy of zosteriform herpes simplex infection in the mouse. Lab Invest. 1972 Apr;26(4):391–402. [PubMed] [Google Scholar]
- Ennis F. A. Host defense mechanisms against Herpes simplex virus. I. Control of infection in vitro by senstized spleen cells and antibody. Infect Immun. 1973 Jun;7(6):898–904. doi: 10.1128/iai.7.6.898-904.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Bell S. E., Elion G. B., Nash A. A., Wildy P. Effect of acycloguanosine treatment of acute and latent herpes simplex infections in mice. Antimicrob Agents Chemother. 1979 Apr;15(4):554–561. doi: 10.1128/aac.15.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Blyth W. A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975 Sep;28(3):341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- Kapoor A. K., Nash A. A., Wildy P. Pathogenesis of herpes simplex virus in B cell-suppressed mice: the relative roles of cell-mediated and humoral immunity. J Gen Virol. 1982 Jul;61(Pt 50):127–131. doi: 10.1099/0022-1317-61-1-127. [DOI] [PubMed] [Google Scholar]
- Kapoor A. K., Nash A. A., Wildy P., Phelan J., McLean C. S., Field H. J. Pathogenesis of herpes simplex virus in congenitally athymic mice: the relative roles of cell-mediated and humoral immunity. J Gen Virol. 1982 Jun;60(Pt 2):225–233. doi: 10.1099/0022-1317-60-2-225. [DOI] [PubMed] [Google Scholar]
- Kino Y., Hayashi Y., Hayashida I., Mori R. Dissemination of herpes simplex virus in nude mice after intracutaneous inoculation and effect of antibody on the course of infection. J Gen Virol. 1982 Dec;63(2):475–479. doi: 10.1099/0022-1317-63-2-475. [DOI] [PubMed] [Google Scholar]
- Mori R., Tasaki T., Kimura G., Takeya K. Depression of acquired resistance against herpes simplex virus infection in neonatally thymectomized mice. Arch Gesamte Virusforsch. 1967;21(3):459–462. doi: 10.1007/BF01241745. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Hayashida I., Higa K., Wada T., Mori R. Role of Lyt-1 positive immune T cells in recovery from herpes simplex virus infection in mice. Microbiol Immunol. 1982;26(4):359–362. doi: 10.1111/j.1348-0421.1982.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Oda H., Mori R., Taniguchi T. Mechanism of acquired resistance to herpes simplex virus infection as studied in nude mice. J Gen Virol. 1979 Sep;44(3):715–723. doi: 10.1099/0022-1317-44-3-715. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Field H. J., Quartey-Papafio R. Cell-mediated immunity in herpes simplex virus-infected mice: induction, characterization and antiviral effects of delayed type hypersensitivity. J Gen Virol. 1980 Jun;48(Pt 2):351–357. doi: 10.1099/0022-1317-48-2-351. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Gell P. G. Membrane phenotype of murine effector and suppressor T cells involved in delayed hypersensitivity and protective immunity to herpes simplex virus. Cell Immunol. 1983 Feb 1;75(2):348–355. doi: 10.1016/0008-8749(83)90332-5. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Gell P. G., Wildy P. Tolerance and immunity in mice infected with herpes simplex virus: simultaneous induction of protective immunity and tolerance to delayed-type hypersensitivity. Immunology. 1981 May;43(1):153–159. [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Phelan J., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: H-2 mapping of the delayed-type hypersensitivity response and the antiviral T cell response. J Immunol. 1981 Apr;126(4):1260–1262. [PubMed] [Google Scholar]
- Nash A. A., Quartey-Papafio R., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: functional analysis of lymph node cells during periods of acute and latent infection, with reference to cytotoxic and memory cells. J Gen Virol. 1980 Aug;49(2):309–317. doi: 10.1099/0022-1317-49-2-309. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Role for cell-mediated immunity in the resistance of mice to subcutaneous herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):166–172. doi: 10.1128/iai.12.1.166-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizenmaier K., Starzinski-Powitz A., Röllinghoff M., Falks D., Wagner H. T-cell-mediated cytotoxicity against herpes simplex virus-infected target cells. Nature. 1977 Feb 17;265(5595):630–632. doi: 10.1038/265630a0. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C. A sensitive and precise plaque assay for herpes virus. Nature. 1962 Sep 8;195:1028–1029. doi: 10.1038/1951028a0. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Mechanism of immunologic resistance to herpes simplex virus 1 (HSV-1) infection. J Immunol. 1976 Jan;116(1):35–40. [PubMed] [Google Scholar]
- SYDISKIS R. J., SCHULTZ I. HERPES SIMPLEX SKIN INFECTION IN MICE. J Infect Dis. 1965 Jun;115:237–246. doi: 10.1093/infdis/115.3.237. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Brandis H. Specifically immune mouse T-cells can destroy H-2 compatible murine target cells infected with herpes simplex virus types 1 or 2. Z Immunitatsforsch Immunobiol. 1977 Jul;153(2):162–173. [PubMed] [Google Scholar]
- Spruance S. L., Overall J. C., Jr, Kern E. R., Krueger G. G., Pliam V., Miller W. The natural history of recurrent herpes simplex labialis: implications for antiviral therapy. N Engl J Med. 1977 Jul 14;297(2):69–75. doi: 10.1056/NEJM197707142970201. [DOI] [PubMed] [Google Scholar]




