Abstract
Although obstructive sleep apnea and cardiovascular disease have common risk factors, epidemiologic studies show that sleep apnea increases risks for cardiovascular disease independently of individuals' demographic characteristics (i.e., age, sex, and race) or risk markers (i.e., smoking, alcohol, obesity, diabetes, dyslipidemia, atrial fibrillation, and hypertension). Individuals with severe sleep apnea are at increased risk for coronary artery disease, congestive heart failure, and stroke. The underlying mechanisms explaining associations between obstructive sleep apnea and cardiovascular disease are not entirely delineated. Several intermediary mechanisms might be involved including sustained sympathetic activation, intrathoracic pressure changes, and oxidative stress. Other abnormalities such as disorders in coagulation factors, endothelial damage, platelet activation, and increased inflammatory mediators might also play a role in the pathogenesis of cardiovascular disease. Linkage between obstructive sleep apnea and cardiovascular disease is corroborated by evidence that treatment of sleep apnea with continuous positive airway pressure reduces systolic blood pressure, improves left ventricular systolic function, and diminishes platelet activation. Several systematic studies are necessary to explicate complex associations between sleep apnea and cardiovascular disease, which may be compounded by the involvement of diseases comprising the metabolic syndrome (i.e., central obesity, hypertension, diabetes, and dyslipidemia). Large-scale, population-based studies testing causal models linking among sleep apnea, cardiovascular morbidity, and metabolic syndrome are needed.
Citation:
Jean-Louis G; Zizi F; Clark LT; Brown CD; McFarlane SI. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med 2008;4(3):261–272.
Keywords: Sleep apnea, cardiovascular disease, metabolic syndrome
Background
Decades of research have provided evidence supporting the associations between obstructive sleep apnea with cardiovascular morbidity and mortality. In the early 1980s, investigators demonstrated that subsequent to tracheostomy, patients with sleep apnea experienced substantial decrease in systemic blood pressure and diminution of cardiac arrhythmia.1 With the presentation of many case-control studies, replicating similar findings, a concern arose that initial studies may have been confounded by factors such as age, sex, and body habitus.2–4
Adequate response to address this concern required the performance of several large-scale epidemiologic studies to assess the influence of suspected confounders on the associations between sleep apnea and cardiovascular disease. Despite adequate methodological and statistical control for known covariates, relationships between these 2 conditions have persisted.5–8 Notwithstanding the significance of these findings, presently there is a growing concern that associations between sleep apnea and cardiovascular disease might be mediated by the metabolic syndrome. It is equally plausible that sleep apnea and metabolic syndrome might have synergistic health risks, since both conditions are predictive of cardiovascular morbidity and mortality.
In this paper, we review findings supporting the associations between obstructive sleep apnea and cardiovascular disease. We also present evidence supporting the potential mediating effects of the metabolic syndrome and respective intermediate mechanisms involved in proposed causal pathways. The role of each of the component disease entities of the metabolic syndrome is delineated, and pertinent clinical management issues are discussed.
Associations between Sleep Apnea and Cardiovascular Disease
Obstructive sleep apnea is a serious, potentially life-threatening condition. It is characterized by repeated cessation of breathing while sleeping, due mostly to complete or partial pharyngeal obstruction. Objectively, it is recognized by a combination of symptoms and laboratory results. These include repetitive apneas and hypopneas, which are accompanied by hypoxia, sleep arousals, and hemodynamic changes.9–12 Moreover, activation of the sympathetic nervous system during respiratory events potentiates vasoconstriction and often triggers increases in blood pressure and heart rate.10,13 Obstructive sleep apnea is also associated with several cardiorespiratory problems (e.g., loud snoring, loud gasps, and daytime breathlessness).14,15
Obstructive sleep apnea causes significant sleep disturbances, leading to excessive daytime sleepiness and fatigue, which in turn may cause vehicular and industrial accidents.16,17 When left untreated, sleep apnea gradually induces cognitive deficits and poor performance.18 According to a chart audit of 4 million beneficiaries of the Veterans Health Administration, numerous psychiatric comorbid diagnoses accompany sleep apnea including depression (21.8%), anxiety (16.7%), posttraumatic stress disorder (11.9%), psychosis (5.1), and bipolar disorders (3.3%).19
Obstructive sleep apnea is thought to be more prevalent than asthma and adult diabetes, possibly affecting more than 18 million Americans.20,21 Public health advocates think it may be as big a public health hazard as smoking.22 The National Commission on Sleep Disorders Research estimated that sleep apnea is probably responsible for 38,000 cardiovascular deaths yearly, with an associated 42 million dollars spent on related hospitalizations.23 Obstructive sleep apnea increases the risk of heart failure by 140%, the risk of stroke by 60%, and the risk of coronary heart disease by 30%.6 Thus, sleep apnea is an important target for public health interventions aiming at reducing cardiovascular disease, the leading cause of death among adults in developed countries.24
Clinical and Epidemiologic Evidence
About 3 decades ago, a concerted effort was made to study the relationships between obstructive sleep apnea and cardiovascular disease. Early studies were largely observational, but results had been very impressive. In 2 independent laboratories, investigators performed tracheostomy to alleviate comorbid conditions among patients with a diagnosis of sleep apnea. Subsequent to tracheostomy, investigators observed complete disappearance of severe atrial flutter and postoperative decrease in systemic blood pressure.25,26 Others observed normalization of blood pressure in 2 hypertensive children with severe sleep apnea within 24 hours after surgery.27 Of note, a contemporaneous study demonstrated a 10% reduction in mortality over a 10-year period among patients with sleep apnea who underwent tracheostomy, compared with those favoring conservative treatment.1
Importance of Confounding Factors
Early studies showing associations between obstructive sleep apnea and cardiovascular disease were questioned, as some could not account for the influence of confounding factors since samples were too small. Critics pointed out that obstructive sleep apnea and cardiovascular disease have common risk factors including, age, gender, race/ethnicity, and obesity, which could confound the observed associations.28 Studies also needed to take into account that patients with obstructive sleep apnea were generally unhealthy. One analysis indicated that the 10-year risk of coronary heart disease and stroke, for instance, was approximately 30% among patients with sleep apnea.29
More recent large-scale epidemiologic studies have confirmed associations between obstructive sleep apnea and cardiovascular disease, with adequate statistical control for known confounding factors.5–8 In one of those studies, sleep apnea significantly increased the risk for stroke or death (hazard ratio = 1.97; 95% CI: 1.12– 3.48) independently of other risk factors (i.e., age, sex, race, smoking, alcohol consumption, body mass index, and the presence or absence of diabetes mellitus, dyslipidemia, atrial fibrillation, and hypertension).5 This finding is consistent with a cross-sectional study showing that patients with an apnea hypopnea index (AHI) ≥ 20 had significantly greater odds for stroke (adjusted OR = 4.33; 95% CI: 1.32–14.24) than individuals without sleep apnea (AHI < 5).8
Other researchers have studied patients with sleep apnea presenting without other known medical conditions in order to rule out the influence of confounding factors.30,31 Those studies showed that patients with sleep apnea were characterized by higher levels of sympathetic nervous system activity during wakefulness as well during sleep, relative to healthy controls.30,31 Accordingly, during apnea events, oxygen levels decreased and carbon dioxide levels increased commensurately, which activated the sympathetic nervous system. Higher level of sympathetic nervous system activity induced blood vessel constriction, with blood pressure rising to 250/150 mm Hg. Those patients also exhibited faster heart rates during wakefulness.
One of the largest epidemiologic studies conducted to date, the Sleep Heart Health Study sampling 6,424 community-dwelling individuals who underwent home polysomnography, documented increased risk of coronary artery disease, congestive heart failure, and stroke among patients with severe sleep apnea.6 Specific analysis of the Sleep Heart Health Study showed that individuals with obstructive sleep apnea had 4 times the odds for atrial fibrillation (adjusted OR = 4.02; 95% CI: 1.03–15.74).32 This is consistent with data from a prospective study of consecutive patients undergoing electrocardioversion for atrial fibrillation indicating that the adjusted odds ratio for atrial fibrillation among patients with obstructive sleep apnea was 2.19 (95% CI: 1.40–3.42).33 Data from the Sleep Heart Health Study also revealed that odds for coronary heart disease (OR = 4.02; 95% CI: 1.03–15.74) and tachycardia (OR = 3.40; 95% CI: 1.03–11.20) were also significantly greater among individuals with sleep apnea.32
Proposed Mechanisms Explaining Link between Sleep Apnea and Cardiovascular Disease
The underlying mechanisms explaining the associations between obstructive sleep apnea and cardiovascular disease are not entirely understood, although several intermediate mechanisms are proposed. They include sustained sympathetic activation,34,35 changes in intrathoracic pressure,36 and oxidative stress, and consequently vascular inflammation resulting from the nocturnal hypoxia and reoxygenation cycles.37,38
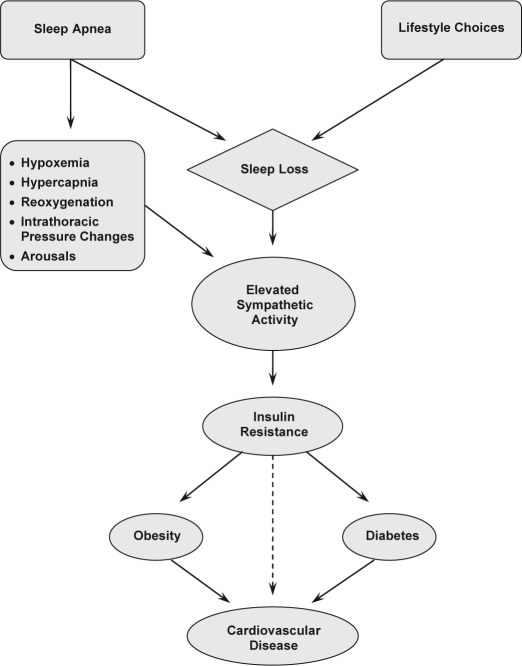
With regard to the role of sympathetic activation, investigators have posited that repetitive apneic/hypopneic events along with ensuing arterial desaturation and hypercapnia cause activation of the sympathetic nervous system (see Figure 1).34,35,38 This then results in increases in systolic blood pressure that might ultimately lead to hypertension or exacerbation of this condition. A similar mechanism might explain the link between obstructive sleep apnea and tachyarrhythmia;33 whereas bradyarrhythmia, which is more common than tachyarrhythmia,3,4 might be the resulting effect of an increase in vagal tone due to stimulation of receptor sites in the upper airway.39 Utilization of pacemakers permitting long-term monitoring of cardiac arrhythmias and ventilation parameters might offer data leading to more comprehensive explanations of those relationships.40,41
Figure 1.
Proposed pathway linking sleep loss to cardiovascular disease. Of note, obesity triggers the onset of sleep apnea in a significant number of cases.
Other abnormalities observed among patients with obstructive sleep apnea might also be involved in the pathogenesis of cardiovascular disease. These include disorders in coagulation factors, endothelial damage, platelet activation, and an increase in inflammatory mediators.38,42–45 Patients with obstructive sleep apnea have characteristically greater levels of endothelin and lower levels of nitric oxide than healthy sleepers.38,43 This elevated endothelin is believed to impair blood pressure regulation as well. Thus, patients with obstructive sleep apnea often experience greater blood vessel constriction. Notably, levels of endothelin and circulating nitric oxide invariably return to normal with continuous positive airway pressure (CPAP) treatment.43
Recently, research interests have centered on the relative contribution of oxidative stress in explaining the associations between sleep apnea and cardiovascular morbidity.38,45,46 Investigators have proposed that hypoxia, which is commonly observed in sleep apnea, promotes the formation of reactive oxygen species, which could activate the transcriptional activator hypoxia-inducible factor 1 (HIF-1), particularly during the reoxygenation period.47,48 Reactive oxygen species, regulate the activation of critical transcription factors that are redox sensitive, resulting in increased expression of genes, which encode proteins promoting adaptation to hypoxia.47 It has also been suggested that redox-sensitive transcription factors, which elicit inflammatory pathways are also activated, thereby affecting inflammatory and immune responses by promoting activation of endothelial cells, leukocytes, and platelets.38 Once activated, those cells express adhesion molecules and proinflammatory cytokines that may lead to endothelial injury and dysfunction,38 which inevitably lead to the development of cardiovascular morbidity.
Observing this chain of events, investigators surmise that atherogenesis apparently starts soon after the onset of sleep apnea.38 It is likely that substantial atherosclerotic insults are incurred by the time a diagnosis is rendered, since symptoms often become apparent around the age of 45 years.38,46 To some extent, this might explain why many patients with sleep apnea present with cardiovascular morbidity in the clinic. It is unclear whether such atherogenic damages can be reversed, but treatment can retard their progress.49 Using CPAP therapy, investigators have shown significant reductions in levels of C-reactive protein and interleukin-6,49 and atherogenic plaque regression has been observed among patients with dyslipidemia.50 Therefore, in order to prevent cardiovascular morbidity, sleep apnea diagnosis and treatment should be made as early as possible.
Evidence from CPAP Studies
The link between sleep apnea and cardiovascular disease has been further demonstrated through treatment studies of sleep apnea with nasal continuous positive airway pressure (CPAP).51–53 Those studies have shown positive results, which include reduced diurnal and nocturnal blood pressure.51,52 Among patients with heart failure and congestive heart failure, for instance, treatment of coexisting sleep apnea with CPAP reduces systolic blood pressure, improves left ventricular systolic function, and increased ejection fraction.53,54 CPAP therapy has been found to eliminate apneic episodes and associated hemodynamic changes occurring during sleep.31,55 One implication of those findings is that adverse effects of obstructive sleep apnea on cardiac functions can be lessened with CPAP administration,52 although it is not certain whether it could delay mortality among patients with central sleep apnea and heart failure.54
Sleep Apnea and Cardiovascular Disease: Role of the Metabolic Syndrome
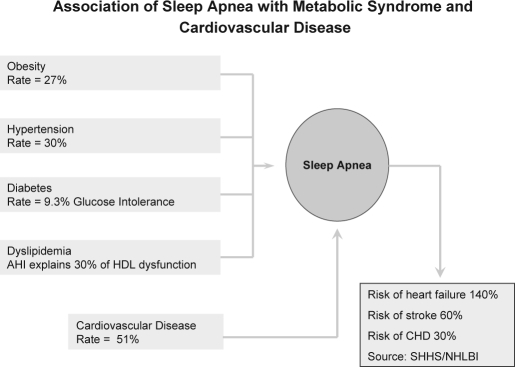
The relationships between sleep apnea and cardiovascular disease are rather complex. Several systematic studies are necessary to explicate fully the nature of those relationships. These complexities are further compounded by findings suggesting that conditions comprising the metabolic syndrome might mediate effects of sleep apnea on cardiovascular disease (see Figure 2).
Figure 2.
Prevalence of metabolic syndrome components and cardiovascular disease among patients with a diagnosis of sleep apnea; risks of cardiovascular disease for patients with sleep apnea are also indicated.
A direct link between the metabolic syndrome and sleep apnea has not been established, as previous research has considered linkage analysis only for individual components of the metabolic syndrome. Recent preliminary evidence from the Mayo Clinic suggests that the metabolic syndrome might be more prevalent among patients with obstructive sleep apnea than among individuals without OSA. Clinical data show that 60% of patients with sleep apnea had the metabolic syndrome, compared with 40% of patients without sleep apnea.56 In the following sections, we consider the role of each component of the metabolic syndrome namely obesity, hypertension, diabetes, and dyslipidemia; but first, issues pertaining to definition and prevalence of the metabolic syndrome are presented.
The Metabolic Syndrome: Definition and Prevalence
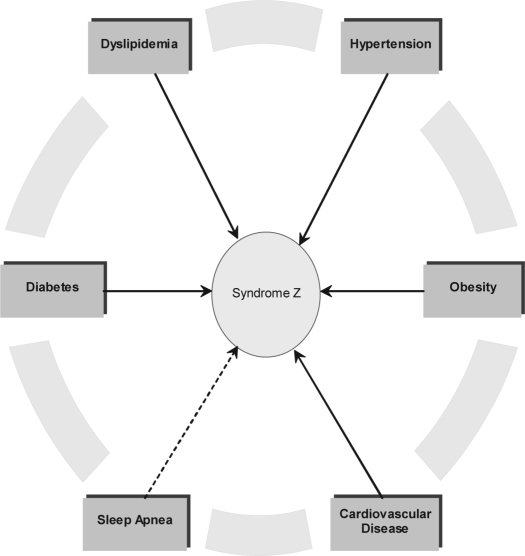
Perhaps the first observation of the metabolic syndrome was made in 1923 when a cluster of co-occurring metabolic diseases namely hyperglycemia, hypertension, obesity, and hyperuricemia was first recorded.57 This observation led to a growing area of clinical and basic research as well as one that has provided the impetus for new and innovative clinical care. In the sleep literature, the term Syndrome Z has been used to explain such interrelated diseases.58 This incorporates the typical features of the metabolic syndrome (Syndrome X) namely central obesity, hypertension, diabetes, and dyslipidemia, with the addition of sleep apnea (see Figure 3).58
Figure 3.
Interrelationships between metabolic diseases comprising the metabolic syndrome and sleep apnea. Together, these diseases are referred to as Syndrome Z in the field of sleep medicine. Available epidemiologic and clinical evidence suggests that all of these conditions interact with each other through complex, yet undifferentiated pathophysiological pathways, thereby increasing risks for cardiovascular disease.58
The metabolic syndrome is an emerging public health concern.59–62 It constitutes a collection of interrelated risk factors of metabolic origin that increase chances of developing heart disease, stroke, and diabetes. It increases the risk for coronary heart disease at any LDL-C level, and it is associated with prothrombotic and proinflammatory states, two common risk factors for coronary heart disease and diabetes mellitus.45 The metabolic syndrome is now recognized as an important contributor to the development of atherosclerosis and cardiovascular disease. Individuals presenting with characteristics of the metabolic syndrome are at increased risks of developing type 2 diabetes,62,63 and those with diabetes are at increased risks of developing cardiovascular disease.64
Individuals with metabolic syndrome have several co-occurring disorders of the body's metabolism: obesity, hypertension, dyslipidemia, and hypercholesterolemia. The causes of this condition are complex, and empirical data are in the beginning stages in shedding light on these complexities. To date, the most consistent observation has been that most patients who meet criteria for the metabolic syndrome are older and obese; they also tend to exhibit insulin resistance.65,66 However, it remains unclear whether obesity or insulin resistance is the cause of metabolic syndrome or a byproduct of a more extensive metabolic disorder. One theory postulates that insulin resistance might be the principal determinant of the metabolic syndrome.65–67 This explains in part why recent research has focused on the role of insulin resistance and glucose intolerance.
According to the third National Health and Nutrition Examination Survey (1988–1994) of 8814 adults (ages ≥ 20 years), the age-adjusted prevalence of the metabolic syndrome was 23.7%.68 Analysis of data from the National Health and Nutrition Examination Survey (1999–2002; n = 3,601) using new definition from the National Cholesterol Education Program showed that the unadjusted prevalence of the metabolic syndrome among men and women (ages ≥ 20 years) was 33.7% and 35.4%, respectively.69 Of note, the prevalence of metabolic syndrome in black women was 57% higher than in black men.68
Since the first observation of the cluster of metabolic diseases, referred to as the metabolic syndrome, many definitions have been used. As the metabolic syndrome is now recognized as a worldwide health concern, a more formal definition has been proposed by several organizations including the World Health Organization, the National Cholesterol Education Program, and the American Association of Clinical Endocrinologists. In Table 1, we compare criteria provided by the World Health Organization and the National Cholesterol Education Program. These have been set forth to allow consensus agreement in recognizing the components of the metabolic syndrome as well as in identifying high-risk individuals.60,65,70 It should be noted that other criteria have been proposed by the American Heart Association/National Heart, Lung, and Blood Institute and the European Group for Study of Insulin Resistance (see AHA/NHLBI Scientific Statement).71 In sum, they all share similar definitional criteria, although they differ somewhat regarding etiology of the metabolic syndrome and degree of importance assigned to each of its components.
Table 1.
WHO and NCEP ATP III Definition of the Metabolic Syndrome
| Characteristic | WHO | NCEP ATP III |
|---|---|---|
| Hypertension | Current antihypertensive therapy and/or BP > 140/90 | Blood pressure medication or BP >130/85 |
| Dyslipidemia | Plasma triglycerides > 1.7 mmol/L (150 mg/dL) and/or HDL < 0.9 mmol/L (35 mg/dL) in men and < 1.0 mmol/L (< 40 mg/dL) in women | Plasma triglycerides > 150 mg/dL, HDL cholesterol < 40 mg/dL in men and < 50 mg/dL in women. |
| Obesity | BMI > 30 and/or waist/hip ratio > 0.90 in men and > 0.85 in women | Waist circumference > 40 cm in men and > 50 cm in women |
| Diabetes | Type 2 diabetes or IGT | Fasting blood sugar > 110 mg/dL* |
| Other | Microalbuminuria = overnight urinary albumin excretion rate > 20 mcg/min (30 mg/g Cr) | |
| Requirements for Diagnosis | Type 2 diabetes or IGT and any 2 of the above criteria. If normal glucose tolerance is found, 3 other disorders must be present. | Any 3 of the above disorders |
Information in the table was obtained from guidelines provided by the World Health Organization (WHO) and the National Cholesterol Education Program (NCEP). BMI denoted body mass index; BP, blood pressure; HDL, high-density lipoprotein; IGT, impaired glucose tolerance.
Fasting plasma glucose was recently updated to 100 mg/dL by the American Diabetes Association.
In addition to the importance attributed to the impact of metabolic diseases in assessing cardiovascular risks, there is also considerable interest in understanding the contribution of circadian rhythms in cardiovascular events, which might indicate an interplay among sleep cycles, arousal mechanisms, and acute thrombosis.72 Recent evidence led to the belief that peripheral cells, including those of the cardiovascular system, contain a circadian clock functioning in a manner similar to the one found in the suprachiasmatic nuclei.73 Varying facets of cardiovascular physiology are subject to diurnal variation.72 Indeed, cardiovascular events including stroke, myocardial infarction, and sudden cardiac death tend to occur during specific phases of the circadian cycle.72,74 Blood pressure patterns also have a circadian component, often used to categorize individuals as dippers and nondippers.75 Preliminary evidence shows that the temporal distribution of blood pressure could be used to predict vascular events.75 Accordingly, abnormal patterns of blood pressure variation correlate with advanced target organ damage and poor cardiovascular prognosis.75–77 Important work is underway to elucidate the contribution of circadian patterns of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in blood pressure variations among sleep apnea patients.78–80AHA/NHLBI Scientific Statement
Sleep Apnea and Cardiovascular Disease: Role of Obesity
According to data from the National Center for Health Statistics, about two-thirds of American adults are either overweight (BMI > 25; 33%) or obese (BMI > 30; 31%).81 The age-adjusted prevalence of overweight/obesity in ethnic minorities, especially minority women, is higher than in whites in the United States, reaching a critical level of greater than two-thirds of the female minority population.82 Blacks are at greater risks because they are disproportionately more obese than whites (see Table 2).
Table 2.
Trends in Obesity by Ethnicity
| Ethnicity | 1991 | 1995 | 1998 | 1999 | 2000 | 2001 |
|---|---|---|---|---|---|---|
| Black | 19.3 | 22.6 | 26.9 | 27.3 | 29.3 | 31.1 |
| White | 11.3 | 14.5 | 16.6 | 17.7 | 18.5 | 19.6 |
Data originated from the Center for Disease Control; values represent percent of cases
It has long been recognized that obesity plays a pivotal role in the development of obstructive sleep apnea. It may be the most significant predictor of sleep apnea.83,84 A recent study of obese men (BMI = 30 kg/m2) without major medical illnesses indicated that 60% of them met criteria for sleep-disordered breathing and 27% had obstructive sleep apnea.85 It is estimated that 60% to 90% of patients with sleep apnea are obese (defined as BMI > 28 kg/m2), and that a BMI of 28 kg/m2 has a sensitivity of 93% and a specificity of 74% for sleep apnea.84 The risk of having moderate to severe sleep apnea over a 4-year period increases 6-fold among persons gaining 10% excess weight.86 Thus, it has been assumed that the high incidence of cardiovascular morbidity among patients with sleep apnea is explained by the presence of obesity.87,88
Most of the initial reports wrestled with the idea that associations of sleep apnea with cardiovascular morbidity were confounded by underlying obesity. Since both sleep apnea and cardiovascular morbidity are linked to obesity, some investigators have conjectured a viable hypothesis, which suggested that both conditions are caused by a defect in a common pathway potentially resulting from central (visceral) obesity. Plausibly, with the onset of sleep apnea individuals develop leptin resistance, which in turn contributes to further weight gain. Leptin is an adipocyte-derived hormone that regulates weight by controlling appetite and energy expenditure.89 We should also consider that inflammatory cytokines TNF-α and IL-6, which are associated with daytime sleepiness, might also be involved in the causal pathway, as they are elevated among obese patients with sleep apnea.80 With new empirical evidence, the debate has recently evolved, and currently the comorbid relationship between sleep apnea and cardiovascular disease is seen as a manifestation of the metabolic syndrome.
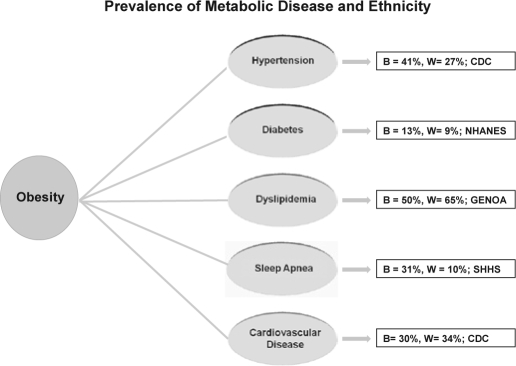
Whether, in fact, obesity is the final common pathway remains to be determined. The preponderance of evidence indicates that obesity is associated with important metabolic diseases linked to cardiovascular disease (see Figure 5), and African Americans seem particularly vulnerable. Within the context of ethnicity and sleep apnea research, disparities favoring greater rates of sleep apnea for blacks might be a function of greater odds of being overweight or obese (see Table 1). Older blacks experienced severe sleep apnea with a relative risk twice that of their white counterparts.90 This is consistent with case-control family study of sleep apnea comparing blacks and whites, ages 2 to 86 years, which has shown that 31% of blacks and 10% of whites had respiratory disturbance index greater than 10.91 Also important in that study was the observation that young blacks (≤ 25 years) may be at increased risk for sleep apnea. These findings led to research suggesting that genetic factors might underlie the susceptibility to sleep apnea among blacks.92
Figure 5.
Prevalence of metabolic diseases linked to obesity based on ethnicity: black (B) vs. white (W). Data were retrieved from the Center for Disease Control (CDC), National Health and Nutrition Examination Survey (NHANES), the Genetic Epidemiology Network of Arteriopathy (GENOA) and the Sleep Heart Health Study (SHHS).
Sleep Apnea and Cardiovascular Disease: Role of Hypertension
One of the complexities permeating relations between sleep apnea and cardiovascular disease relates to the fact that both conditions are potentially characterized by similar pathogenetic mechanisms. In effect, both sleep apnea and cardiovascular disease are linked to hypertension.93–96 Results of several multivariate analytical models have indicated that sleep apnea represents an independent risk factor for hypertension,93,97,98 and hypertension constitutes a significant predictor of cardiopulmonary deaths among patients with sleep apnea.97
Regarding the link between sleep apnea and hypertension, there are data suggesting that approximately 40% of patients with sleep apnea suffer from hypertension, whereas 30% of hypertensive patients have occult sleep apnea.94 Data from the Wisconsin Sleep Cohort Study, sampling 1060 women and men ages 30 to 60 years, showed that for individuals with a BMI of 30 kg/m2 an AHI of 15 was associated with blood pressure increases of 3.6 mm Hg for systolic (95% CI: 1.3– 6.0) and 1.8 mm Hg for diastolic (95% CI: 0.3–3.3). Further analyses indicated a dose-response relationship between sleep apnea and blood pressure, independent of confounding factors,93 which is consistent with other independent analyses.97,98
Data from the Wisconsin Sleep Cohort Study exploring relationships between sleep apnea and hypertension indicated that the severity of sleep apnea based on initial sleep studies could predict the development of new hypertension during the subsequent 4 years. Even with control for known confounders, analyses suggested the odds ratios for the presence of hypertension at follow-up were 1.42 (95% CI: 1.13–1.78) with an AHI of 0.1 to 4.9 at baseline relative to none, 2.03 (95% CI: 1.29–3.17) with an AHI of 5.0 to 14.9, and 2.89 (95% CI: 1.46–5.64) with an AHI of 15.0 or more.86,99 These data are consistent with findings of the Sleep Heart Health Study, showing that mean systolic and diastolic blood pressure and prevalence of hypertension increased significantly with increasing severity of sleep apnea.7 Reduction of sleep apnea severity through CPAP therapy results in a diminution of daytime systemic blood pressure,95 although it has not been conclusively demonstrated that CPAP treatment lowers blood pressure on a long-term basis.
Sleep Apnea and Cardiovascular Disease: Role of Diabetes
The obesity pandemic over the last decade has accompanied a rise in the prevalence of type 2 diabetes mellitus. One of the characteristic features of diabetes mellitus is the inability to regulate serum glucose levels, resulting in impaired glucose tolerance affecting 11% to 15.6% of the U.S. population.100 Both type 1 and type 2 diabetes are associated with insulin deficiency, but type 2 diabetes is further complicated by cellular resistance to insulin action.101 According to data from the Third National Health and Nutrition Examination Survey, 5.1% of U.S. adults have an existing diagnosis for diabetes; an additional 2.7% met criteria for the diagnosis, but have yet to receive one.100 Analysis from the same research group indicated that 15.6% of American adults exhibit glucose intolerance (140 mg/dL) and 6.9% showed impaired fasting glucose levels (≥ 110 mg/dL).100
On a parallel tract, there is a growing body of evidence suggesting that obstructive sleep apnea is involved in the pathogenesis of altered glucose metabolism. A number of epidemiologic and experimental studies have shown that patients with sleep apnea have increased glucose levels and increased insulin resistance,85,102–104 which might predispose afflicted individuals to developing type 2 diabetes mellitus. Cross-sectional data suggest that associations of sleep apnea with greater glucose levels and increased insulin resistance might be independent of the presence of obesity.80,85,102,105 It is known that both obese and non-obese patients with sleep apnea are insulin resistant, whereas not all apnea patients are obese. Specifically, investigators have proposed the following likely scenario. Sleep apnea causes an increase in sympathetic activity,55 and increased sympathetic activity impairs glucose homeostasis by enhancing glycogen breakdown and gluconeogenesis.106 Hence, recurrent hypoxemia along with abnormal sympathetic activity, commonly observed among patients with sleep apnea, might mediate the relationships between insulin resistance and sleep apnea (see Figure 1).
Data from the Sleep Heart Health Study indicated that the prevalence of 2-hour glucose tolerance values increased from 9.3% among patients with an AHI less than 5 to 15% among those with an AHI > 15.103 Among patients with an AHI ≥ 15, the odds of having an abnormal glucose tolerance was 1.44. Results of the study also indicated that insulin resistance was also greatest among the latter group. This study provided evidence that apnea-induced hypoxemia is associated with glucose intolerance and insulin resistance.103 Moreover, data from the Nurse's Health Study suggest that curtailed sleep duration resulting from sleep fragmentation induced by sleep apnea may also lead to the development or exacerbation of type 2 diabetes mellitus.107,108 This is corroborated by analysis of data from the Sleep Heart Health Study.109 Compared with Individuals sleeping 7 to 8 h/night, individuals sleeping ≤ 5 h or < 6 h/night had adjusted OR for diabetes of 2.51 (95% CI: 1.57–4.02) and 1.66 (95% CI: 1.15–2.39), respectively. On balance, there are data suggesting that the associations between sleep apnea and diabetes might be mediated through obesity, a common risk factor,110 although the prevalence of periodic breathing itself remained significantly higher among diabetic individuals, even after control for covariates. Thus, more definitive explanation of these relationships awaits further empirical studies.
Given the potential effects of reduced sleep on glucose metabolism, one wonders about the overall, long-term health impact of curtailed sleep time on American adults. Converging data indicate that the U.S. population has been sleeping less and less.21,111,112 Interestingly, although sleep duration is a strong predictor of morbidity and mortality, among American adults we found that the quality of one's well-being hinged on the quality, rather than on the quantity of one's sleep.113 Based on the most recent report by investigators from the University of California, San Diego who studied over 1 million men and women ages 30 to 102 years, the greatest longevity was observed among individuals sleeping 7 h/night. Those who slept ≥ 8 h experienced significantly increased mortality risks, as did participants who reported sleeping ≤ 6 h. The increased risk of mortality exceeded 15% for individuals who reportedly slept > 8.5 h or < 3.5 or 4.5 h.114,115 It would be interesting to examine whether excess mortality was associated with impaired glucose tolerance/insulin resistance or type 2 diabetes mellitus in that study. Controlled studies seem necessary to answer the question as to the correct sleep time for optimal survival and satisfying life.
If it can be demonstrated that there is an independent association of sleep apnea to impaired glucose metabolism, this might offer another mechanism to explain increased cardiovascular morbidity. Evidently, this will require better delineation of the contribution of cytokines and leptin. In support of such hypotheses, investigators have pointed out that CPAP studies produced significant improvement in insulin sensitivity and left ventricular function with a corresponding decrease in blood pressure.116 CPAP therapy can also normalize leptin levels, thereby reducing central obesity. On balance, there is evidence suggesting that CPAP treatment does not always lead to significant improvement in metabolic disorders.117 Failure to demonstrate positive CPAP effects often result from limited treatment compliance, inadequate duration of treatment, and lack of statistical power. In light of such evidence, therapeutic approaches might integrate methods to increase sleep time, both via reduction of sleep apnea severity and through lifestyle modifications. Synergistically, these would confer significant benefits in the management of cardiovascular complications resulting from uncontrolled diabetes and/or untreated sleep apnea.
Sleep Apnea and Cardiovascular Disease: Role of Dyslipidemia
Dyslipidemia is yet another condition characterizing the metabolic syndrome. It is primarily a disorder of lipoprotein metabolism, usually caused by excessively high cholesterol levels. It is a known risk factor in the development of coronary artery disease,118 and it is highly prevalent among hypertensive adults. Data from the Multi-Ethnic Study of Atherosclerosis, a multicenter study of 6814 persons ages 45 to 84 years, indicated that 29.3% met criteria for dyslipidemia.119 In that study, the prevalence of dyslipidemia was similar among ethnic groups, although a disproportionate number of blacks and Hispanics, relative to whites, did not receive treatment to control their dyslipidemia.119 These findings were inconsistent with data obtained from the GENOA study, indicating that the prevalence of dyslipidemia was significantly greater among white than among blacks. Among women, rates were 64.7% and 49.5%, respectively; whereas for men, rates were 78.4% and 56.7%, respectively.120 Patterns of treatment were nonetheless similar, favoring better treatment for whites.
Both factors involved in developing dyslipidemia (i.e., high triglyceride levels and low high-density lipoprotein) are affected by obesity, a common predictor of sleep apnea and cardiovascular morbidity. With increased adiposity, a commensurate increase in triglyceride levels is observed, whereas high-density lipoprotein levels decrease.88,121,122 Given that most of the available evidence comes from cross-sectional data, it remains a daunting task to establish directional causality. In that regard, it cannot be said that dyslipidemia causes sleep apnea or that sleep apnea causes dyslipidemia, although the two conditions tend to aggregate among patients with increased adiposity. It would certainly help to view these associations in the context of data pointing to a direct correlation between lipid profile and cortical arousals, as often observed among patients with sleep apnea. Patients with sleep apnea exhibit greater HDL dysfunction and oxidized LDL levels compared to matched control persons.123 Of note, AHI explained 30% of the variance in HDL dysfunction in sleep apnea.123
Moreover, clinical evidence indicates that patients with abnormal serum lipid/lipoprotein levels improved significantly with CPAP or bilevel PAP therapy.124 In that follow-up (6 months) treatment study, investigators observed that the mean HDL-C serum level increased significantly by 5.8%. Such therapeutic interventions are consistent with the Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III).125 Accordingly, reduction of dyslipidemia is a potential secondary target of risk-reduction therapy, which could improve management of the metabolic syndrome.
Conclusions
The aforementioned associations do not seem to be fortuitous, judging from the consistency across studies. The epidemiologic and clinical evidence summarized in this paper constitutes a growing body of literature demonstrating relationships between sleep apnea and diverse systemic abnormalities. The epidemiologic evidence is abundant, but data explaining causal pathways are lacking. It may be that associations among those metabolic disorders point to a maladaptive autonomic response of chemoreceptors, reacting to hypoxia, hypercapnia, and acidosis commonly found in sleep apnea.126 Activation of the sympathetic nervous system through hypoxia and hypercapnia triggers an inflammatory response cascading in several downstream consequences including hypertension, diabetes, and dyslipidemia,34 all of which represent significant risk factors for cardiovascular morbidity. It seems prudent, therefore, that patients with a suspicion of sleep apnea and meeting criteria for metabolic syndrome be properly evaluated and treated.
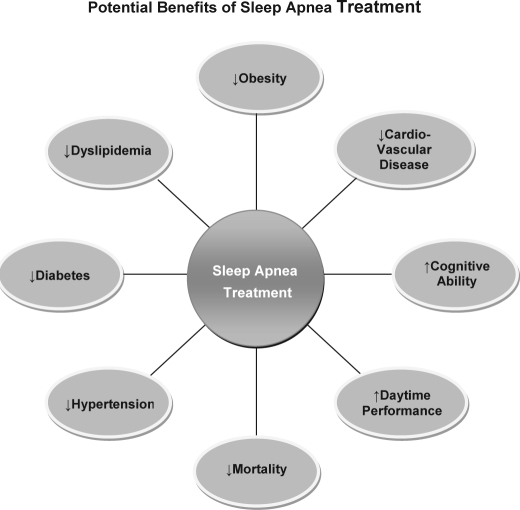
Clinical trials involving the use of CPAP or bilevel PAP therapy have delineated its positive benefits (see Figure 4). This therapeutic modality is very effective in improving left ventricular ejection fraction and quality of life, lowers blood pressure and sympathetic activity, and reduces mortality among patients with congestive heart failure.52,54,127 Among patients with coronary artery disease, CPAP treatment significantly reduces risks of cardiovascular death, acute coronary syndrome, and hospitalization for heart failure.128 Moreover, CPAP therapy has significant effects on lipid levels.124 CPAP studies show significant improvement in insulin sensitivity and left ventricular function with a corresponding decrease in blood pressure.116
Figure 4.
Benefits of sleep apnea treatment, which may include CPAP/bilevel PAP therapy (sometimes used in combination with behavior modification), use of oral-dental devices, or surgical procedures (e.g., UPPP and LAUP).
The Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) identifies the metabolic syndrome as a potential secondary target of risk-reduction therapy.125 It is recommended that patients meeting criteria for the syndrome receive adequate, tailored treatment aimed at reducing obesity through lifestyle modifications including increased physical activity and improved dietary habits.129 Evidence from randomized controlled trials suggests that weight loss among overweight and obese individuals reduces blood pressure, improves lipid profiles, and improves blood glucose levels.130,131 Pharmacotherapy is recommended only for individuals who do not respond positively to behavioral therapies. As gradual sleep loss in the population (be it a sequela of sleep apnea or behaviorally determined) is a strong determinant of insulin resistance,107–109 individuals at risk for developing cardiovascular disease should be encouraged not to curtail their sleep time.
Future Directions and Clinical Implications
The epidemiologic and clinical studies summarized in this paper provide a framework for future research in the underpinnings of the metabolic syndrome and related morbidities. Current studies have raised many important issues that require empirical testing, although initial questions regarding relationships between obstructive sleep apnea and cardiovascular morbidity or between obstructive sleep apnea and the metabolic syndrome have been addressed to some extent. Whether the metabolic syndrome represents a mediating factor in the link between obstructive sleep apnea and cardiovascular disease remains to be determined. Alternatively, the metabolic syndrome itself might potentiate the effects of obstructive sleep apnea on cardiovascular disease.
Future research is necessary to rank individuals with regard to their risk of sudden cardiac death and target them for educational and medical intervention. Research is also needed to establish noninvasive markers with adequate sensitivity and specificity in predicting which patients are at greater risk. For example, patients with sleep apnea showing depressed LV function should be considered at greater risk for ventricular arrhythmias and other cardiovascular events.132 Identification and treatment of patients with overlapping syndromes (e.g., CHF and COPD with sleep apnea) is paramount in the fight to reduce cardiovascular morbidity.
While awaiting answers to those important questions, many interventions can be envisaged to improve the management of existing metabolic disorders. First, as sleep apnea is highly prevalent among patients with diabetes, hypertension, and dyslipidemia, it seems prudent to administer a sleep apnea screening questionnaire to those at-risk patients. Likewise, such questionnaires should be given to patients with increased adiposity in the neck area and/or who present with abdominal (visceral) obesity. Second, patients meeting criteria for sleep apnea should be referred to a sleep clinic for a detailed laboratory study. Third, tailored behavioral interventions should be developed to ensure adherence to CPAP or bilevel PAP treatment recommendations. Fourth, individually tailored weight management programs should be designed to assist overweight/obese patients, as weight reduction helps diminish the severity of sleep apnea, thereby improving overall health, daytime performance, cognitive ability, and well-being.
It is evident that the recent rise in metabolic disorders comprising the metabolic syndrome affects persons of differing age groups, of both genders, and across geographic regions. However, public health advocates have been particularly concerned about individuals living in at-risk, underserved communities that are traditionally underrepresented in the health care industry. In black communities, for instance, cardiovascular risk factors are disproportionately high (see Figure 5). In the public health literature, many have argued in favor of greater access to health care,133,134 reasoning that with greater access there will be a commensurate decline in morbidity and mortality. Unfortunately, even when blacks have adequate insurance coverage, they are not as likely as their white counterparts to utilize available services.135 This suggests that physicians practicing in those communities may have to develop novel strategies to encourage participation of black patients in healthcare practices, as they often endorse a disproportionate disease burden.68,90,91,136,137
ACKNOWLEDGMENTS
This research was supported by funding from the NIH (1R24MD001090 and HL085042).
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Partinen M, Guilleminault C. Obstructive sleep apnea syndrome. New York: Raven Press; 1990. Evolution of obstructive sleep apnea syndrome. In: Partinen M, Guilleminault C, eds; pp. 15–23. [Google Scholar]
- 2.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400, patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–4. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 3.Hoffstein V. Blood pressure, snoring, obesity, and nocturnal hypoxaemia. Lancet. 1994;344:643–5. doi: 10.1016/s0140-6736(94)92084-2. [DOI] [PubMed] [Google Scholar]
- 4.Hoffstein V, Mateika S. Cardiac arrhythmias, snoring, and sleep apnea. Chest. 1994;106:466–71. doi: 10.1378/chest.106.2.466. [DOI] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 6.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 7.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 8.Aartz M, Young T, Finn L, et al. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto JM, Garpestad E, Weiss JW, et al. Hemodynamic changes associated with obstructive sleep apnea followed by arousal in a porcine model. J Appl Physiol. 1993;75:1439–43. doi: 10.1152/jappl.1993.75.4.1439. [DOI] [PubMed] [Google Scholar]
- 10.Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patil SP, Schneider H, Schwartz AR, et al. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132:325–37. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudgel DW. Mechanisms of obstructive sleep apnea. Chest. 1992;101(2):541–549. doi: 10.1378/chest.101.2.541. [DOI] [PubMed] [Google Scholar]
- 13.Narkiewicz K, Montano N, Cogliati C, et al. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98:1071–7. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- 14.Netzer NC, Hoegel JJ, Loube D, et al. Prevalence of symptoms and risk of sleep apnea in primary care. Chest. 2003;124:1406–14. doi: 10.1378/chest.124.4.1406. [DOI] [PubMed] [Google Scholar]
- 15.Teculescu D, Benamghar L, Hannhart B, et al. [Habitual snoring. Prevalence and risk factors in a sample of the French male population] Rev Mal Respir. 2007;24(3, Pt 1):281–7. doi: 10.1016/s0761-8425(07)91059-1. [DOI] [PubMed] [Google Scholar]
- 16.Findley LJ, Weiss JW, Jabour ER. Drivers with untreated sleep apnea. A cause of death and serious injury. Arch Intern Med. 1991;151:1451–52. [PubMed] [Google Scholar]
- 17.Stoohs RA, Guilleminault C, Itoi A, et al. Traffic accidents in commercial long-haul truck drivers: the influence of sleep-disordered breathing and obesity. Sleep. 1994;17:619–23. [PubMed] [Google Scholar]
- 18.El Ad B, Lavie P. Effect of sleep apnea on cognition and mood. Int Rev Psychiatry. 2005;17:277–82. doi: 10.1080/09540260500104508. [DOI] [PubMed] [Google Scholar]
- 19.Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–11. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 20.Young T, Finn L. Epidemiological insights into the public health burden of sleep disordered breathing: sex differences in survival among sleep clinic patients. Thorax. 1998;53(Suppl 3):S16–9. doi: 10.1136/thx.53.2008.s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omnibus Sleep in America Poll. National Sleep Foundation. 2005:1–51. [Google Scholar]
- 22.Phillipson EA. Sleep apnea--a major public health problem. N Engl J Med. 1993;328:1271–3. doi: 10.1056/NEJM199304293281712. [DOI] [PubMed] [Google Scholar]
- 23.The National Commission on Sleep Disorders Research. Wake up America: a national sleep alert. Washington DC: US Government Printing Office; 2002. [Google Scholar]
- 24.American Heart Association. Dallas, TX: American Heart Association; 2007. Heart Disease and Stroke Statistics — 2007, Update; pp. 9–17. [Google Scholar]
- 25.Coccagna G, Mantovani M, Brignani F, et al. Tracheostomy in hypersomnia with periodic breathing. Bull Physiopathol Respir. 1972;8:1217–27. [PubMed] [Google Scholar]
- 26.Coccagna G, Mantovani M, Brignani F, et al. Continuous recording of the pulmonary and systemic arterial pressure during sleep in syndromes of hypersomnia with periodic breathing. Bull Physiopathol Respir. 1972;8:1159–72. [PubMed] [Google Scholar]
- 27.Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med. 1976;27:484. doi: 10.1146/annurev.me.27.020176.002341. [DOI] [PubMed] [Google Scholar]
- 28.Stradling J. Sleep apnea does not cause cardiovascular disease. Am J Respir Crit Care Med. 2004;169:148–9. doi: 10.1164/rccm.2310012. [DOI] [PubMed] [Google Scholar]
- 29.Kiely JL, McNicholas WT, Zgierska A, et al. Cardiovascular risk factors in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2000;16:128–33. doi: 10.1034/j.1399-3003.2000.16a23.x. [DOI] [PubMed] [Google Scholar]
- 30.Somers VK, Gami AS, Olson LJ. Treating sleep apnea in heart failure patients: promises but still no prizes. J Am Coll Cardiol. 2005;45:2012–4. doi: 10.1016/j.jacc.2005.02.081. [DOI] [PubMed] [Google Scholar]
- 31.Somers VK. Sleep--a new cardiovascular frontier. N Engl J Med. 2005;353:2070–73. doi: 10.1056/NEJMe058229. [DOI] [PubMed] [Google Scholar]
- 32.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gami AS, Pressman G, Caples SM. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;27:367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher EC. Cardiovascular disease associated with obstructive sleep apnea. Monaldi Arch Chest Dis. 2003;59:254–61. [PubMed] [Google Scholar]
- 35.Fletcher EC. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep. 2003;26:15–19. doi: 10.1093/sleep/26.1.15. [DOI] [PubMed] [Google Scholar]
- 36.Parker JD, Brooks D, Kozar LF, et al. Acute and chronic effects of airway obstruction on canine left ventricular performance. Am J Respir Crit Care Med. 1999;160:1888–96. doi: 10.1164/ajrccm.160.6.9807074. [DOI] [PubMed] [Google Scholar]
- 37.Lavie L, Lotan R, Hochberg I, et al. Haptoglobin polymorphism is a risk factor for cardiovascular disease in patients with obstructive sleep apnea syndrome. Sleep. 2003;26(5):592–595. doi: 10.1093/sleep/26.5.592. [DOI] [PubMed] [Google Scholar]
- 38.Lavie L. Obstructive sleep apnoea syndrome--an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 39.Zwillich C, Devlin T, White D, et al. Bradycardia during sleep apnea. Characteristics and mechanism. J Clin Invest. 1982;69:1292. doi: 10.1172/JCI110568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazarus A, Varin J, Jauvert G, et al. Relationship between cardiac arrhythmias and sleep apnoea in permanently paced patients with type I myotonic dystrophy. Neuromuscul Disord. 2007;17:392–9. doi: 10.1016/j.nmd.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Shalaby A, Atwood C, Hansen C, et al. Feasibility of automated detection of advanced sleep disordered breathing utilizing an implantable pacemaker ventilation sensor. Pacing Clin Electrophysiol. 2006;29:1036–43. doi: 10.1111/j.1540-8159.2006.00496.x. [DOI] [PubMed] [Google Scholar]
- 42.Dyken ME, Somers VK, Yamada T, et al. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27:401–7. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 43.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–71. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 44.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007;3:409–15. [PMC free article] [PubMed] [Google Scholar]
- 45.Kasasbeh E, Chi DS, Krishnaswamy G. Inflammatory aspects of sleep apnea and their cardiovascular consequences. South Med J. 2006;99:58–67. doi: 10.1097/01.smj.0000197705.99639.50. [DOI] [PubMed] [Google Scholar]
- 46.Lavie L. Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach. Neurol Clin. 2005;23:1059–75. doi: 10.1016/j.ncl.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1391–6. doi: 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- 48.Nanduri J, Nanduri RP. Cellular mechanisms associated with intermittent hypoxia. Essays Biochem. 2007;43:91–104. doi: 10.1042/BSE0430091. [DOI] [PubMed] [Google Scholar]
- 49.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6, in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 50.Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–98. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 51.Fletcher EC. Cardiovascular effects of continuous positive airway pressure in obstructive sleep apnea. Sleep. 2000;23(Suppl 4):S154–7. [PubMed] [Google Scholar]
- 52.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 53.Malone S, Liu PP, Holloway R, et al. Obstructive sleep apnoea in patients with dilated cardiomyopathy: effects of continuous positive airway pressure. Lancet. 1991;338:1480–4. doi: 10.1016/0140-6736(91)92299-h. [DOI] [PubMed] [Google Scholar]
- 54.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 55.Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467–72. [PMC free article] [PubMed] [Google Scholar]
- 57.Kylin E. Studien uber das Hypertonie-Hyperglykamie-Hyperurikamiesyndrome. Zentralblatt fur innere Medizin. 1923;44:127. [Google Scholar]
- 58.Wilcox I, McNamara SG, Collins FL, et al. ≪Syndrome Z≫: the interaction of sleep apnoea, vascular risk factors and heart disease. Thorax. 1998;53(Suppl 3):S25–8. [PMC free article] [PubMed] [Google Scholar]
- 59.Zimmet P, Magliano D, Matsuzawa Y, et al. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 60.Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25:2243–4. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 61.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 62.Grundy SM. Metabolic syndrome: therapeutic considerations. Handb Exp Pharmacol. 2005:107–33. doi: 10.1007/3-540-27661-0_3. [DOI] [PubMed] [Google Scholar]
- 63.Grundy SM. A constellation of complications: the metabolic syndrome. Clin Cornerstone. 2005;7:36–45. doi: 10.1016/s1098-3597(05)80066-3. [DOI] [PubMed] [Google Scholar]
- 64.Alexander CM. The coming of age of the metabolic syndrome. Diabetes Care. 2003;26:3180–1. doi: 10.2337/diacare.26.11.3180. [DOI] [PubMed] [Google Scholar]
- 65.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am. 2004;33:283–303. doi: 10.1016/j.ecl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–23. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]
- 67.Reaven GM. Insulin resistance, cardiovascular disease, and the metabolic syndrome: how well do the emperor's clothes fit? Diabetes Care. 2004;27:1011–2. doi: 10.2337/diacare.27.4.1011. [DOI] [PubMed] [Google Scholar]
- 68.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 69.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–9. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 70.Alberti KG. The costs of non-insulin-dependent diabetes mellitus. Diabet Med. 1997;14:7–9. doi: 10.1002/(SICI)1096-9136(199701)14:1<7::AID-DIA321>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 71.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 72.Reilly DF, Westgate EJ, FitzGerald GA. Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1694–1705. doi: 10.1161/ATVBAHA.107.144923. [DOI] [PubMed] [Google Scholar]
- 73.Ivanov PC, Hu K, Hilton MF, et al. Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics. Proc Natl Acad Sci U S A. 2007;104:20702–7. doi: 10.1073/pnas.0709957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tofler GH, Brezinski D, Schafer AI, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–8. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 75.Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–7. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 76.Kario K, Shimada K, Pickering TG. Abnormal nocturnal blood pressure falls in elderly hypertension: clinical significance and determinants. J Cardiovasc Pharmacol. 2003;41(Suppl 1):S61–6. [PubMed] [Google Scholar]
- 77.Wilcox I, Grunstein RR, Collins FL, et al. Circadian rhythm of blood pressure in patients with obstructive sleep apnea. Blood Press. 1992;1:219–22. doi: 10.3109/08037059209077666. [DOI] [PubMed] [Google Scholar]
- 78.Bravo ML, Serpero LD, Barcelo A, et al. Inflammatory proteins in patients with obstructive sleep apnea with and without daytime sleepiness. Sleep Breath. 2007;11:177–85. doi: 10.1007/s11325-007-0100-7. [DOI] [PubMed] [Google Scholar]
- 79.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 80.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 81.National Center for Health Statistics Health. United States. 2006:1–543. [PubMed] [Google Scholar]
- 82.Kumanyika SK, Gary TL, Lancaster KJ, et al. Achieving healthy weight in African-American communities: research perspectives and priorities. Obes Res. 2005;13:2037–47. doi: 10.1038/oby.2005.251. [DOI] [PubMed] [Google Scholar]
- 83.Grunstein R, Wilcox I, Yang TS, et al. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17:533–40. [PubMed] [Google Scholar]
- 84.Kushida CA, Efron B, Guilleminault C. A predictive morphometric model for the obstructive sleep apnea syndrome. Ann Intern Med. 1997;127(8, Pt 1):581–7. doi: 10.7326/0003-4819-127-8_part_1-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 85.Punjabi NM, Sorkin JD, Katzel LI, et al. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 86.Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 87.Coughlin SS, Calle EE, Teras LR, et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–7. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 88.Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 89.Makinodan K, Yoshikawa M, Fukuoka A, et al. Effect of serum leptin levels on hypercapnic ventilatory response in obstructive sleep apnea. Respiration. 2008;75:257–64. doi: 10.1159/000112471. [DOI] [PubMed] [Google Scholar]
- 90.Ancoli-Israel S, Klauber MR, Stepnowsky C, et al. Sleep-disordered breathing in African-American elderly. J Gerontol. 1989;44:M18–21. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 91.Redline S, Tishler P, Hans M, et al. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 92.Palmer LJ, Buxbaum SG, Larkin EK, et al. Whole genome scan for obstructive sleep apnea and obesity in African-American families. Am J Respir Crit Care Med. 2004;169(12):1314–1321. doi: 10.1164/rccm.200304-493OC. [DOI] [PubMed] [Google Scholar]
- 93.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 94.Fletcher EC. The relationship between systemic hypertension and obstructive sleep apnea: facts and theory. Am J Med. 1995;98:118–28. doi: 10.1016/S0002-9343(99)80395-7. [DOI] [PubMed] [Google Scholar]
- 95.Mayer J, Becker H, Brandenburg U, et al. Blood pressure and sleep apnea: results of long-term nasal continuous positive airway pressure therapy. Cardiology. 1991;79:84–92. doi: 10.1159/000174864. [DOI] [PubMed] [Google Scholar]
- 96.Hoffstein V, Chan CK, Slutsky AS. Sleep apnea and systemic hypertension: a causal association review. Am J Med. 1991;91:190–6. doi: 10.1016/0002-9343(91)90014-o. [DOI] [PubMed] [Google Scholar]
- 97.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320:479–82. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohayon MM, Guilleminault C, Priest RG, et al. Is sleep-disordered breathing an independent risk factor for hypertension in the general population (13, 057 subjects)? J Psychosom Res. 2000;48:593–601. doi: 10.1016/s0022-3999(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 99.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 100.Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21(Suppl 3):C11–4. doi: 10.2337/diacare.21.3.c11. [DOI] [PubMed] [Google Scholar]
- 101.Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2, diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–14. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ip MS, Lam B, Ng MM, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 103.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 104.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 105.Spiegel R, Knudtson K, Leproult R , et al. Sleep loss: a novel risk factor for insulin resistance and Type II diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 106.Punjabi NM, Ahmed MM, Polotsky VY, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiol Neurobiol. 2003;136(2-3):167–178. doi: 10.1016/s1569-9048(03)00079-x. [DOI] [PubMed] [Google Scholar]
- 107.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 108.Ayas NT, White DP, Al Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 109.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 110.Sanders MH, Givelber R. Sleep disordered breathing may not be an independent risk factor for diabetes, but diabetes may contribute to the occurrence of periodic breathing in sleep. Sleep Med. 2003;4:349–50. doi: 10.1016/s1389-9457(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 111.Jean-Louis G, Kripke DF, Ancoli-Israel S, et al. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry. 2000;47:921–7. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- 112.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 113.Jean-Louis G, Kripke DF, Ancoli-Israel S. Sleep and quality of well-being. Sleep. 2000;23:1115–21. [PubMed] [Google Scholar]
- 114.Kripke DF, Simons RN, Garfinkel L, et al. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–16. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 115.Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 116.Harsch IA, Hahn EG, Konturek PC. Insulin resistance and other metabolic aspects of the obstructive sleep apnea syndrome. Med Sci Monit. 2005;11:RA70–5. [PubMed] [Google Scholar]
- 117.Coughlin SR, Mawdsley L, Mugarza JA, et al. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29:720–7. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 118.Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222, primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 1986;256:2823–8. [PubMed] [Google Scholar]
- 119.Goff DC, Jr, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113:647–56. doi: 10.1161/CIRCULATIONAHA.105.552737. [DOI] [PubMed] [Google Scholar]
- 120.O'Meara JG, Kardia SL, Armon JJ, et al. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med. 2004;164:1313–8. doi: 10.1001/archinte.164.12.1313. [DOI] [PubMed] [Google Scholar]
- 121.Kiely JL, McNicholas WT. Cardiovascular risk factors in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2000;16:128–33. doi: 10.1034/j.1399-3003.2000.16a23.x. [DOI] [PubMed] [Google Scholar]
- 122.Zgierska A, Gorecka D, Radzikowska M, et al. Obstructive sleep apnea and risk factors for coronary artery disease. Pneumonol Alergol Pol. 2000;68:238–46. [PubMed] [Google Scholar]
- 123.Tan KC, Chow WS, Lam JC, et al. HDL dysfunction in obstructive sleep apnea. Atherosclerosis. 2006;184:377–82. doi: 10.1016/j.atherosclerosis.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 124.Borgel J, Sanner BM, Bittlinsky A, et al. Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J. 2006;27:121–7. doi: 10.1183/09031936.06.00131304. [DOI] [PubMed] [Google Scholar]
- 125.Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 126.Lee PY, Yun AJ, Bazar KA. Acute coronary syndromes and heart failure may reflect maladaptations of trauma physiology that was shaped during pre-modern evolution. Med Hypotheses. 2004;62:861–7. doi: 10.1016/j.mehy.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 127.Mansfield DR, Gollogly NC, Kaye DM, et al. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–6. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 128.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–34. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 129.Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8:iii-182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 130.Thoolen B, De Ridder D, Bensing J, et al. The effectiveness of a self-management intervention in patients with screen-detected type-2, diabetes. Diabetes Care. 2007;30:2832–7. doi: 10.2337/dc07-0777. [DOI] [PubMed] [Google Scholar]
- 131.Erdman DM, Cook CB, Greenlund KJ, et al. The impact of outpatient diabetes management on serum lipids in urban African-Americans with type 2 diabetes. Diabetes Care. 2002;25:9–15. doi: 10.2337/diacare.25.1.9. [DOI] [PubMed] [Google Scholar]
- 132.Okuda N, Ito T, Emura N, et al. Depressed myocardial contractile reserve in patients with obstructive sleep apnea assessed by tissue Doppler imaging with dobutamine stress echocardiography. Chest. 2007;131:1082–9. doi: 10.1378/chest.06-2444. [DOI] [PubMed] [Google Scholar]
- 133.Penn NE, Kar S, Kramer J, et al. Ethnic minorities, health care systems, and behavior. Health Psychol. 1995;14:641–6. doi: 10.1037//0278-6133.14.7.641. [DOI] [PubMed] [Google Scholar]
- 134.Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57(Suppl 1):108–45. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]
- 135.McBean AM, Gornick M. Differences by race in the rates of procedures performed in hospitals for Medicare beneficiaries. Health Care Financ Rev. 1994;15:77–90. [PMC free article] [PubMed] [Google Scholar]
- 136.Meetze K, Gillespie MB, Lee FS. Obstructive sleep apnea: a comparison of black and white subjects. Laryngoscope. 2002;112(7, Pt 1):1271–4. doi: 10.1097/00005537-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 137.Lackland DT, Lin Y, Tilley BC, et al. An assessment of racial differences in clinical practices for hypertension at primary care sites for medically underserved patients. J Clin Hypertens. 2004;6:26–31. doi: 10.1111/j.1524-6175.2004.03089.x. [DOI] [PubMed] [Google Scholar]