Abstract
Cyclin D1 expression represents one of the key mitogen regulated events during the G1 phase of the cell cycle, while Cyclin D1 over-expression is frequently associated with human malignancy. Here we describe a novel mechanism regulating Cyclin D1 levels. We find that SNIP1, previously identified as a regulator of Cyclin D1 expression, does not, as previously thought, primarily function as a transcriptional coactivator for this gene. Rather, SNIP1 plays a critical role in co- or post-transcriptional Cyclin D1 mRNA stability. Moreover, we demonstrate that the majority of nucleoplasmic SNIP1 is present within a previously undescribed complex containing SkIP, THRAP3, BLCALF1 and Pinin, all proteins with reported roles in RNA processing and transcriptional regulation. We find that this complex, which we have termed the SNIP1/SkIP associated RNA-processing (SNARP) complex, is co-ordinately recruited to both the 3′ end of the Cyclin D1 gene and Cyclin D1 RNA. Significantly, SNIP1 is required for the further recruitment of the RNA processing factor U2AF65 to both the Cyclin D1 gene and RNA. This study demonstrates a novel mechanism regulating Cyclin D1 expression and offers new insight into the role of SNIP1 and associated proteins as regulators of proliferation and cancer.
Keywords: Cyclin D1, RNA processing, RNA splicing, transcription
Introduction
Cyclin D1, an important regulator of G1 phase of the cell cycle, is controlled at multiple levels, including gene transcription, protein stability and subcellular localisation (1). Moreover, its aberrant expression is frequently associated with human malignancy (1). Previously, Cyclin D1 expression has been shown to be regulated, at least in part, by SMAD nuclear interacting protein-1 (SNIP1) (2), a widely expressed and evolutionarily conserved factor originally identified by a yeast two-hybrid screen searching for SMAD interacting proteins (3). SNIP1 is a 396 amino acid protein that contains a nuclear localisation sequence, coiled-coil motif and, within its C-terminus, a forkhead-associated (FHA) domain which are recognised phospho-serine/threonine binding motifs (4). The majority of nucleoplasmic SNIP1 exists in a high molecular weight complex (2) of which neither the function nor composition has been previously described.
A number of functions have been ascribed to SNIP1, suggesting an ability to interact with multiple cellular partners. Although the N-terminus of SNIP1 shares homology with the RNA binding and splicing factors, SRrp86 and p54 (BLAST-link database), the majority of functions described to date have involved transcriptional regulation. When over-expressed, SNIP1 inhibits transactivation by SMADs and the RelA(p65) NF-κB subunit, by preventing their interaction with p300 (5). Conversely, SNIP1 has a positive effect on c-Myc activity, facilitating p300 recruitment to c-Myc regulated promoters, while also increasing c-Myc stability through inhibition of Skp-2 mediated ubiquitination (6). An additional role for SNIP1 has also been suggested as a regulator of ATR (ATM and Rad3 related) checkpoint kinase pathways (7). SNIP1 expression is upregulated in a wide range of tumours and, when over-expressed, SNIP1 can enhance c-Myc and H-Ras induced cell transformation (6). This suggests that SNIP1 could function as an oncogene.
Consistent with this putative oncogenic function, SNIP1 levels accumulate after serum stimulation and promote cell cycle progression through G1, which, at least in part, is accomplished through regulation of Cyclin D1 expression (2). We previously demonstrated that, in variety of cell lines, siRNA mediated down-regulation of SNIP1 inhibits both Cyclin D1 mRNA and protein levels (2). As SNIP1 has been shown to regulate transcription factor activity, it was previously assumed that this was a transcriptional effect. However, in this study we have further analysed the ability of SNIP1 to regulate the endogenous Cyclin D1 gene and surprisingly, find that the primary mechanism of SNIP1 control of Cyclin D1 expression results from specific regulation of Cyclin D1 RNA stability. We identify components of a novel SNIP1-containing complex that all have reported or suspected roles in RNA processing and transcription and show these also have a role in Cyclin D1 regulation. This study therefore represents both a novel function for SNIP1 as well as a new mechanism of regulation of this key proto-oncogene.
Methods
Cells and DNA/siRNA transfection
U-2 OS osteosarcoma, HEK-293 embryonic kidney and HeLa cervical carcinoma cell lines were grown in 10% foetal calf serum (Gibco)/DMEM (Lonza) for no more than 30 passages. DNA transfections were performed using PEI (Polysciences) and siRNA duplex oligonucleotides were synthesised by MWG and transfected using Interferin (Polyplus) as per manufacturers instruction.
Mass-spectroscopy
HEK293 cells were transfected with GST or SNIP1-GST expression plasmids, GST pulldowns performed and bound proteins eluted by boiling in LDS-sample buffer (Invitrogen) prior to separation on 4-12% pre-cast gels (Novex) and colloidal coomassie staining (Invitrogen). Bands of interest were excised and proteins subjected to in-gel trypsin digestion. Protein mass fingerprint data was obtained by MALDI-Tof-Tof (MS/MS) analysis using a 4700 Proteomic Analyser (Applied Biosystems) or by nanoLC-ESI (MS/MS) using the Ultimate 3000 nLC system (Dionex) coupled to a 4000 QTrap (Applied Biosystems). Peak list files generated from resultant data analysis was submitted to a MASCOT search engine for protein identification.
Plasmids
YFP-SNIP1 and SNIP1-YFP expression plasmids were constructed by inserting SNIP1 cDNA into pEYFPN1/C1 backbones (Clontech). Mammalian expression vectors for THRAP3, BCLAF1, SkIP and Pinin were constructed by the University of Dundee, College of Life Sciences cloning service, by reverse transcription from cellular mRNA and insertion into pCMV5-HA. THRAP3 (aa 345-509), BCLAF1 (aa 287-450) and Pinin (aa 1-132) fragments were cloned into pQE30 (Qiagen) for bacterial expression, his-tag purification and antibody production.
Antibodies
The SNIP1 rabbit polyclonal antibodies used for immunoprecipitation and mouse monoclonal SNIP1 antibodies used for western blotting were described previously in Roche et al, 2004. Polyclonal rabbit antibodies against THRAP3 (aa 345-509), BCLAF1 (aa 287-450) and Pinin (aa 1-132) were raised by Diagnostics Scotland using purified, recombinant His-tagged protein fragments. Other antibodies used in this manuscript were: anti-Cyclin D1 (cat# 556470, Pharmingen), anti-β-actin (cat# A5441, Sigma), anti-haemagglutinin (12CA5, CRUK), anti-GST (cat#27457701, Amersham-Pharmacia), anti-SkIP (ab23331, Abcam), anti-U2AF65 (cat# 32-4700, Zymed), anti-γ tubulin (cat# T6557, Sigma), anti-HDAC1 (sc-6298, Santa Cruz), anti-B23 (cat# 18-7288, Zymed) and anti-cyclin B1 (V152, CRUK). Secondary antibodies used for immunofluorescence were α-rabbit Rhodamine Red-X conjugate (cat# 711295152, Jackson ImmunoResearch) and α-mouse Cy5 conjugate (cat# 115175072, Jackson ImmunoResearch).
RACE-PAT PCR
RACE-PAT (Rapid amplification of cDNA ends - Poly(A) test) PCR was performed essentially as described in (8). Briefly, RNA was prepared from U2-OS cells, genomic DNA removed, RNA precipitated and cDNA synthesised using AMV reverse transcriptase and an oligo(dT) anchor primer. PCRs were then performed using the reverse oligo(dT) anchor primer and a forward Cyclin D1 primer sitting 200 nucleotides upstream of the transcript end. PCR products were then run on 2% agarose gels.
RNA-Protein crosslinking
RNA-protein cross-linking was performed as described in (9). Breifly, an actively growing U2-OS cell monolayer was cross-linked with 1% formaldehyde, cross-linking stopped by 0.25M glycine and cells lysed in RIPA buffer (50mM Tris pH 7.5, 1% NP-40, 0.5% sodium deoxycholate, 0.05% SDS, 1mM EDTA, 150mM NaCl, 40 units RNAsin and protease inhibitors). Lyseates were then sonicated, insoluble material removed by centrifugation, precleared with protein-A sepharose and immunoprecipitations performed. Immunoprecipitates were then washed 5x in high stringency RIPA buffer (50mM Tris pH 7.5, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1mM EDTA, 1M NaCl, 1M urea, 40 units RNAsin and protease inhibitors). Protein was then eluted (50mM Tris pH 7, 5mM EDTA, 10mM DTT, 1% SDS), cross-links reversed (70°C / 45 minutes), RNA extracted by trizol, treated with DNAseI (DNA-free kit, Ambion) and bound RNAs analysed by RT-PCR.
Nucleo-cytoplasmic RNA distribution
The cellular distribution of RNA was analysed as described in (10). Briefly, U2-OS cells were trypsinised, washed in cold PBS and resuspended by slow pipetting in 10mM Tris pH 8.4, 140mM NaCl, 1.5mM MgCl2, 0.5% NP-40, 1mM DTT and RNAsin. After centrifugation (1000rpm/3min), the supernatant was taken as the cytoplasmic fraction. Pellets were then suspended in 1/10th volume of 3.3% SDS and 6.6% Tween-20, vortexed, incubated on ice, centrifuged as above and the resulting supernatant discarded. RNA from the remaining nuclear pellets (and the above cytoplasmic fraction) were than extracted with triazol, treated with DNAse and analysed by RT-PCR.
Other experimental procedures
Western blots, protein extractions, immunoprecipitations, immunofluorescence and gel filtration procedures were performed as previously described ((2) and references therein). For GST pulldowns in Fig. 1A, whole cell lysates were prepared and 30U DNAseI was added for 30 minutes, followed by solid urea to a final concentration of 1M. Extracts were then thoroughly vortexed, Triton-X added to a final concentration of 1% and left at 4°C for a further 30 minutes. This was then snap frozen in a dry ice bath, thawed and centrifuged to generate a soluble extract which was then diluted 10 fold into pulldown buffer (20mM Hepes pH 7.9, 20% glycerol, 75mM NaCl, 1mM DTT, protease and phosphatase inhibitors). Detailed protocols for ChIP assays, cell elutriation and FACS analysis are described elsewhere (11). RNA was extracted using either Trizol (Sigma) or a Nucleospin RNA II kit (Machinary-Nagel) and cDNA synthesised using a Quantitect RT kit (Qiagen) as per manufacturers instruction.
Figure 1. SNIP1 regulates Cyclin D1 RNA processing.
(A) Cyclin D1 protein expression is SNIP1 dependent. Protein extracts were prepared from U-2 OS cells treated with SNIP1 and control siRNAs. SDS-PAGE and western blot analysis was performed using antibodies directed against Cyclin D1, Cyclin E, SNIP1, IκBα and β-actin.
(B - D) SNIP1 is required for the production of mature Cyclin D1 mRNA. U-2 OS cells were treated with a SNIP1 siRNA. The relative levels of pre-spliced and post-spliced RNAs were then assessed by semi-quantitative PCR. The pre-spliced transcript is detected using primers within intron 1 and the post-spliced transcript detected using primers situated within exons 3 and 5. The same results are also seen with primers directed to different regions of Cyclin D1 RNA (for example, within intron 3 for pre-spliced message, or within exons 1 and 3 or 1 and 5 for post-spliced message). Also shown are the levels of Cyclin D2 and CDK4 mRNAs. In (C) analysis included the alternately spliced Cyclin D1 transcript. In (D) a no reverse-transcriptase control was used to ensure the PCR signal from pre-spliced Cyclin D1 results from RNA and not DNA contamination.
Primers for PCR analysis
PCR primers used for RT-PCR analysis are as follows:
SNIP1 (f): 5′ GAAGAAGCAAGTCTCCTCGC
SNIP1 (r): 5′ GGGCTTCACTCTTCGGCC
GAPDH (f): 5′ GGTCGTATTGGGCGCCTGGTCACC
GAPDH (r): 5′ CACACCCATGACGAACATGGGGGC
Cyclin D1 (exon f): 5′ GAGAACAAACAGATCATCCGCA 3′
Cyclin D1 (exon r): 5′ GCTTCGATCTGCTCCTGG 3′
Cyclin D1 (intron f): 5′ GAGCACATTTTCAGACCTTCGG
Cyclin D1 (intron r): 5′ CCTGAAAATGACCCTCGGGC
Cyclin D2 (f): 5′ CTACCTTCCGCAGTGCTCCTA
Cyclin D2 (r): 5′ CCCAGCCAAGAAACGGTCC
rpLP32a (f): 5′ TGTGAAGCCCAAGATCGTCA
rpLP32a (r): 5′ GTCAATGCCTCTGGGTTTCC
The following primers were used in ChIP assays covering regions of the Cyclin D1 promoter and gene:
1f: 5′ GCAGAGCTGCCATAGCACGT
1r: 5′ GTGCAGGGACCAGTCCCGA
2f: 5′ CAACGTGGTTACCCTCCCGA
2r: 5′ CCTCTGGGCTTTTGAGGGTC
3f: 5′ GCTGTGAGCACCCTGGCGA
3r: 5′ GTGTTCGTGGTTACATGAGAG
4f: 5′ GTCTCCCCAGGCTGCGTGT
4r: 5′ GCCCAAAAGCCATCCCTGAG
5f: 5′ GGAAGATCGTCGCCACCTGG
5r: 5′ GGCACTGGCTTCTCCCGAAG
6f: 5′ GAGCACATTTTCAGACCTTCGG
6r: 5′ CCTGAAAATGACCCTCGGGC
7f: 5′ CTCCGTAGGTCTGCGAGGAAC
7r: 5′ CCAGTGGTTACCAGCAGCTC
8f: 5′ CCCTGATGGCCGCTCACC
8r: 5′ CTTCCCAGACAGCACCCAG
9f: 5′ GTGACTTACGGCTGCTTAAAG
9r: 5′ CTCCAGGATTTGACCCTATCC
10f: 5′ CGTGATAACATTCACAAGGCTCG
10r: 5′ GGTATCTGAGGCTCCACATCC
11f: 5′ CTGCCTCAGATGTGAAGTTCA
11r: 5′GCACCAGCCTCGGCATTTCC
12f: 5′ CCTGGGATGACTGTGTCTTTC
12r: 5′ AGCATCTGCTGAGACCCGTG
13f: 5′ CTCAGGTTCAGAGGAGGCAG
13r: 5′ CACGTCGGTGGGTGTGCAAG
14f: 5′ CTCCAGAACACGGCTCACGC
14r: 5′ GTCACAGGACAGACTCCGCTG
siRNA oligonucleotides
The SNIP and scrambled siRNAs have been published previously (2). The sequences of other siRNAs used are as follows (sense strand only):
THRAP3 (A): 5′ CAGGAGUUUCGUUCCAUUUTT
THRAP3 (B): 5′ GGAGGAGAUGGAUGAUCAATT
BCLAF1 (A): 5′ GCCCACUUAGGAUCAAAAUTT
BCLAF1 (B): 5′ UCAGGAAGCUCUAGAUUACTT
SkIP (A): 5′ GGGUAUGGACAGUGGAUUUTT
SkIP (B): 5′ UCCUUGAACUUCCAAUGAATT
Results
SNIP1 regulates Cyclin D1 RNA-processing
Previously, we demonstrated that SNIP1 regulates Cyclin D1 expression (2). SNIP1 has been shown to function as a regulator of transcription, although its N-terminus shares homology with the RNA binding and splicing factors, SRrp86 and p54. We therefore wished to confirm whether its effect on Cyclin D1 was primarily transcriptional in nature. Consistent with our previous data, we found that an siRNA targeting endogenous SNIP1, caused a significant decrease in Cyclin D1 protein and mRNA (Fig. 1A & B). However, using primers specifically designed to amplify pre-spliced message or post-spliced message, we surprisingly found that depletion of SNIP1 had no effect on the levels of pre-spliced Cyclin D1 RNA (Fig. 1B). This result did not represent an effect on alternative splicing, since SNIP1 was also required for production of the Cyclin D1 “b” transcript in which the retention of intron 4 results in a protein with an alternate C-terminal sequence (Fig. 1C) (12). A ‘no reverse transcriptase’ control confirmed that PCR signals did not result from contaminating genomic DNA (Fig. 1D).
We next determined which step in Cyclin D1 RNA processing is SNIP1-dependent. RACE-PAT PCR analysis demonstrated that polyadenylation of Cyclin D1 RNA was not affected by SNIP1 depletion (Fig 2A). Similarly, SNIP1 depletion equally reduced both nuclear and cytoplasmic levels of mature Cyclin D1 mRNA, indicating SNIP1 does not regulate nucleo-cytoplasmic mRNA shuttling (Fig 2B). These results suggested that the primary effect on Cyclin D1 expression could be at the level of co-transcriptional splicing or the stability of the nascent transcript. Therefore, we examined the effect of inhibiting transcription using actinomycin D. A very rapid decrease in pre-spliced Cyclin D1 RNA was observed in the first hour of actinomycin D treatment, consistent with loss of signal as Cyclin D1 gene transcription is inhibited and pre-spliced message is converted into spliced mRNA (Fig. 2C). Depletion of SNIP1 by siRNA had no effect on this process. By contrast, analysis of the spliced Cyclin D1 mRNA, revealed a relatively steady decline in its levels upon transcriptional inhibition, indicative of its stability within cells. Here, depletion of SNIP1 had a significant effect on Cyclin D1 mRNA degradation kinetics, with a rapid decline in post-spliced message being seen within the first hour of actinomycin D treatment. However, after this point, the rate of Cyclin D1 mRNA depletion was identical to that seen in control cells. Consistent with our previous results (Fig. 1B), no effect of SNIP1 depletion was observed on Cyclin D2 mRNA stability. Taken together, this data reveals SNIP1 to be a critical post-transcriptional regulator of Cyclin D1 RNA within the nucleus.
Figure 2. SNIP1 is required for the co-transcriptional stability of Cyclin D1 mRNA.
(A) SNIP1 is not required for Cyclin D1 polyadenylation. U2-OS cells were transfected with control or SNIP1 siRNA and RACE-PAT (rapid amplification of cDNA ends-polyA test) PCR was performed to analyse the potential for SNIP1 to regulate Cyclin D1 polyadenylation.
(B) SNIP1 does not regulate Cyclin D1 nucleo-cytoplasmic RNA shuttling. U2-OS cells were transfected with control or SNIP1 siRNAs and separated into nuclear and cytoplasmic fractions prior to RNA extraction, DNAseI treatment and RT-PCR.
(C) SNIP1 is required for the co-transcriptional Cyclin D1 stability. U2-OS cells were transfected with control or SNARP-targeted siRNA oligonucleotides, then treated with 10μg/ml actinomycin D prior to RNA extraction at the time points indicated. Transcript levels were then analysed by qRT-PCR and normalised to rpLP32, a transcript with a half-life in excess of 25h.
SNIP1 exists in a high molecular weight complex containing multiple transcription and RNA processing factors
Previously, we have demonstrated that the majority of SNIP1 is present within an unidentified high molecular weight protein complex of approximately 1.5MDa (2). To learn more about SNIP1’s cellular function, we wished to identify components of this complex. To achieve this, a GST-SNIP1 fusion protein was expressed in HEK 293 cells and affinity purified using glutathione-agarose. Several proteins reproducibly co-purified with GST-SNIP1 (Fig. 3A). These were identified by in-gel trypsin digestion and MALDI-Tof-Tof (MS/MS) to be the thyroid hormone receptor associated protein 150kDa (THRAP3 or TRAP150), the THRAP3-related protein Bcl-2 associated transcription factor (BCLAF1 or Btf), Pinin and the Ski-interacting protein (SkIP). Each of these proteins have known or suspected roles in RNA processing and transcription (13-21). Interestingly, THRAP3 has been reported to bind SRrp86 (17), a splicing factor sharing homology with SNIP1’s N-terminus. Additionally, SkIP has been previously reported to bind another FHA-domain containing factor, CHES1/FOXN3 (22) as well as the known SNIP1 interactors p300 (23) and the SMADs (24).
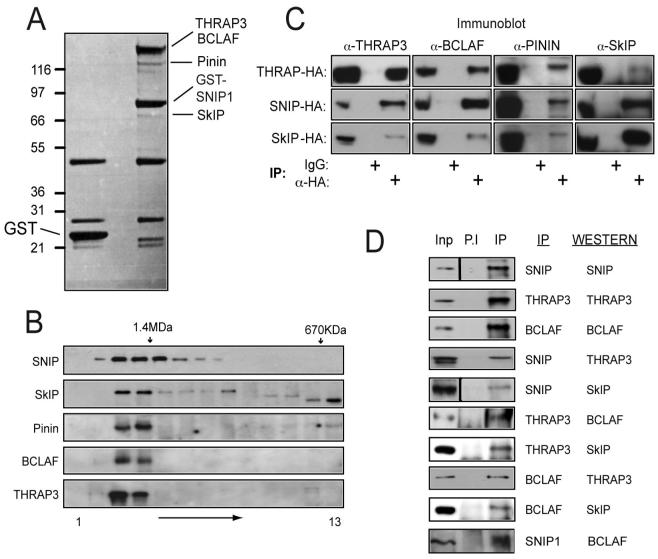
Figure 3. Identification of SNIP1 associated proteins.
(A) Purification of SNIP1 associated proteins. 10μg of expression plasmids encoding GST-SNIP1 or GST alone were transfected into HEK 293 cells and extracts were prepared prior to purification using glutathione agarose, SDS-PAGE and colloidal coomassie staining. Bands specifically present in the SNIP1-GST lane were excised and proteins identified by MALDI-Tof-Tof (MS/MS).
(B) SNIP1 associated proteins are all in a high molecular weight complex of similar size. HeLa nuclear extract was resolved by Superose 6 gel filtration and fractions separated by SDS-PAGE and western blotted using the antibodies indicated. The positions of molecular weight markers used during column calibration are shown.
(C) Confirmation of SNIP1 associated proteins by western blot analysis. 293 cells were transfected with THRAP3, SNIP1 or SkIP-HA expression vectors and anti-HA immunoprecipitations performed prior to SDS-PAGE and western blotting for endogenous proteins as indicated.
(D) Co-immunoprecipitation of endogenous SNIP1 associated proteins. 200μg of U-2 OS extract was analysed by immunoprecipitation, SDS-PAGE and western blotting using the indicated antibodies. Inputs represent 5% of total protein used in the assay.
Endogenous SkIP, Pinin, THRAP3 and BCLAF1 all co-eluted in the same high molecular weight gel filtration fractions as SNIP1, suggesting that they are components of the same complex (Fig. 3B). To confirm these interactions, HA-tagged SNIP1, THRAP3 or SkIP were expressed in 293 cells and α-HA immunoprecipitations performed prior to western blotting for endogenous SNIP1-interacting proteins (Fig. 3C). Importantly, interactions between all the identified SNIP1-associated proteins were detected, indicating these proteins are not simply isolated SNIP1-interacting factors, but rather form a larger complex of which SNIP1 is a constituent. This was further demonstrated by co-immunoprecipitations of endogenous proteins (Fig. 3D). RNAse treatment also had no effect on the ability to co-immunoprecipitate these proteins, indicating complex formation is not a result of independent RNA interactions (Supp. Fig. 1A). Taken together, these results reveal a previously undescribed, high molecular weight complex, consisting of SNIP1, SkIP, Pinin, THRAP3 and BCLAF. Due to the likely function of these proteins, we have named this complex the SNIP1/SkIP associated RNA-processing (SNARP) complex. Many components of the SNARP containing complex have known roles in RNA binding and splicing (14, 16-20) and SkIP has been previously identified in 35S and activated 45S spliceosomes (18). However, we conclude SNARP is distinct from spliceosomal complexes as we are unable to demonstrate interactions between SNIP1 and other spliceosomal factors (not shown). Furthermore, whereas SNARP components all uniformly elute in a high molecular weight fraction (Fig. 3B), spliceosome proteins were generally found distributed across a much wider molecular weight range (not shown).
Although we have previously used monoclonal antibodies raised against SNIP1 to investigate its subnuclear localisation (7), we observed that these only poorly immunoprecipitated endogenous SNIP1, suggesting that we were not detecting the location of the majority of the endogenous protein (data not shown). Therefore, to determine SNIP1 localisation, U-2 OS cell lines were constructed stably expressing SNIP1-YFP C- and N-terminal fusion proteins. Importantly, using these cells, SNIP1 was found to colocalise with other members of the SNARP complex in nuclear speckles (Supp Fig. 1), which, although are not thought to be sites of transcription or RNA processing can couple these processes by serving as sites of splicing and transcription factor storage, assembly and modification (reviewed in (25)). The identification of these bodies as nuclear speckles was confirmed by their characteristic increase in size and staining intensity after transcriptional inhibition (Supp Fig. 1D), together with the colocalisation of both SNIP1 and THRAP3 with various nuclear speckle associated splicing factors (data not shown). In contrast to other SNARP components, these cells also revealed nucleolar localisation of SNIP1, a result confirmed with the endogenous protein by sub-cellular fractionation (Supp. Fig. 2C). The function of nucleolar SNIP1 is unknown. However, the nucleolus can sequester nucleoplasmic proteins and this has been previously reported for factors associated with cell cycle progression and stress responses (26). Nucleolar localisation may therefore act as a reservoir of available SNIP1 or alternatively could contribute to other functions, such as its role as regulator of the ATR dependent DNA damage responses (Roche et al, 2007).
SNIP1-associated proteins are cell cycle regulated and control Cyclin-D1 expression
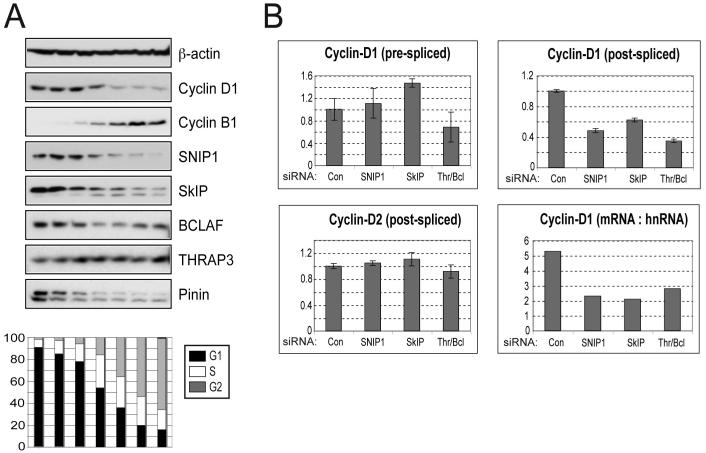
Previously we have demonstrated that SNIP1 contributes to progression through G1 phase of the cell cycle and, in particular, to Cyclin D1 expression (2). This suggested that the SNARP complex might become active during G1. To test this hypothesis, we prepared extracts from pools of cells enriched for different phases of the cell cycle by elutriation. We found significantly higher levels of SNIP1 and the other SNARP-complex proteins, SkIP, BCLAF1 and Pinin, in early G1 phase, coinciding with an increase of Cyclin D1 protein (Fig. 4A). By contrast, THRAP3 was found at all stages of the cell cycle, implying additional roles. FACS analysis and western blotting for Cyclin B1 known to be upregulated at later cell cycle stages confirmed the successful separation of cells. This result indicated that the activity of SNIP1 and its associated proteins is likely to be cell cycle regulated, with a specific role during G1 phase.
Figure 4. SNARP complex proteins are upregulated in G1 phase and are required for Cyclin D1 mRNA production.
(A) Cell cycle analysis of SNIP1 associated proteins. U-2 OS cells were fractionated by centrifugal elutriation, whole cell extracts prepared and equivalent protein amounts separated by SDS-PAGE and subjected to western blot analysis using the indicated antibodies. Cells from each fraction were also stained with propidium iodide for analysis by FACS and cell cycle percentages of each fraction are shown graphically.
(B) Analysis of RNA transcript levels following depletion of SNARP complex components. U-2 OS cells were treated with siRNAs directed against SNIP1, SkIP and THRAP3/BCLAF1 (knocked down together due to their sequence similarity and possible functional redundancy). The relative levels of pre-spliced and post-spliced RNAs were then assessed by qRT-PCR, normalised to GAPDH levels. The mRNA (post-spliced) : hnRNA (pre-spliced) ratio for the Cyclin D1 transcript is also presented. The pre-spliced transcript is detected using primers within intron 1 and the post-spliced transcript detected using primers situated within exons 3 and 5.
Although SNIP1 can regulate Cyclin D1 expression, the ability of other SNARP complex components to perform this function was unknown. Consistent with the components of this complex functioning in a coordinate manner, the production of mature Cyclin D1 mRNA was strongly dependent upon SNIP1, SkIP and THRAP3/BCLAF1 (Fig. 4B). Importantly, depletion of these factors had little effect on the expression of other SNARP components, although a small generally inhibitory effect was noted with THRAP3/BCLAF1 reduction (Supp. Fig. 2 and data not shown). We then examined the relative levels of pre- and post-spliced Cyclin D1 mRNA after siRNA mediated depletion of SNARP complex components by Q-PCR. Significantly, when the effects of knocking down SNIP1, SkIP and THRAP3/BCLAF1 were normalised by comparing the ratio of post-spliced to pre-spliced Cyclin D1 RNA (mRNA:hnRNA), all SNARP complex components displayed similar inhibition of Cyclin D1 mRNA processing (Fig. 4B). Cyclin D1 specificity was demonstrated by the failure of SNARP knockdown to affect Cyclin D2 mRNA production (Fig. 4B), as well as the transcript levels of many other genes examined, including Skp2, GADD45α, Rb, PUMA and E2F4 (not shown). The fact that SNIP1 depletion does not lead to the accumulation of pre-spliced Cyclin D1 RNA, argues against a requirement for SNIP1 in Cyclin D1 mRNA splicing. Coupled with previous data regarding the effect of SNIP1 on Cyclin D1 degradation after transcriptional blockage (Fig. 2C), this data strongly supports SNIP1 being required for stabilisation of newly spliced mRNA within the nucleus, prior to export.
The SNARP complex is recruited to the Cyclin D1 gene
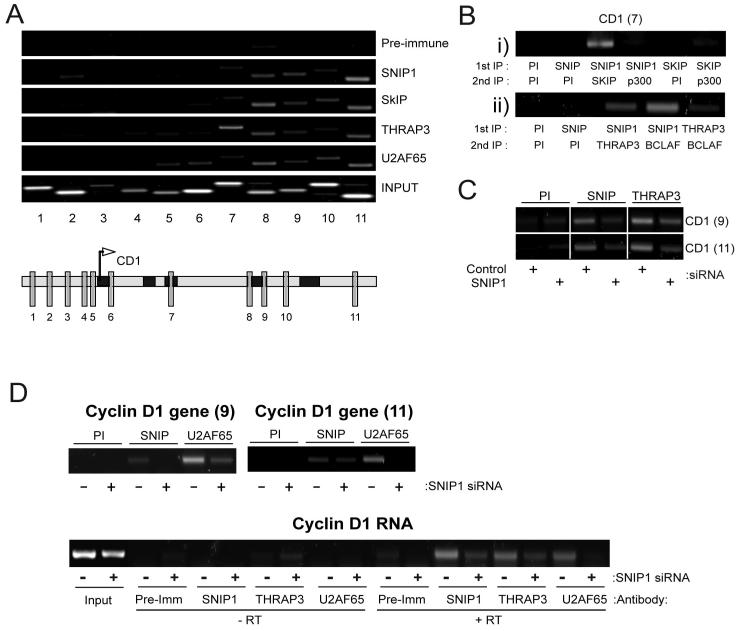
Previously we found that SNIP1 can be recruited to the promoters of c-Myc-regulated genes (6). Significantly, chromatin immunoprecipitation (ChIP) analysis revealed that SNIP1 and the SNARP complex proteins SkIP, THRAP3 and BCLAF1 are all recruited to sites within the Cyclin D1 gene, downstream of the promoter region (Fig 5A and data not shown). The recruitment of THRAP3 and BCLAF1 to genes has not previously been described, although SkIP recruitment to the 24-hydroxylase and HIV promoters has been reported (20, 27). It is noteworthy that high levels of THRAP3 were also seen binding to region 7 (Fig 5A), where other SNARP proteins are only weakly detectable. This could indicate an additional non-SNARP role for THRAP3, a possibility also suggested by its cell-cycle independent expression (Fig 4A). Significantly, serial immunoprecipitation and ChIP (Re-ChIP) analysis demonstrated co-recruitment of SNIP1 with SkIP, THRAP3 and BCLAF1 (Fig 5B), indicating the presence of the SNARP complex rather than individual protein components. Despite the reported interaction of the transcriptional coactivator p300 with both SNIP1 and SkIP (5, 23), the presence of p300 was only weakly detectable with these proteins in re-ChIP analysis (Fig 5B). SNIP1 depletion by siRNA also reduced THRAP3 recruitment to the Cyclin D1 gene (Fig 5C), providing further evidence for the recruitment of the intact SNARP complex to the Cyclin D1 gene.
Figure 5. SNARP complex components bind to the Cyclin D1 gene and RNA and are required for recruitment of U2AF65.
(A) Recruitment of SNARP complex components and splicing factors to the Cyclin D1 gene. ChIP analysis was performed on U-2 OS cells using antibodies directed against SNIP1, the SNARP complex proteins SkIP and THRAP3 together with the RNA processing factor U2AF65. PCR analysis was performed with primers directed at various regions within the Cyclin D1 promoter and along the length of the Cyclin D1 gene as indicated.
(B) SNARP complex proteins are co-recruited to the Cyclin D1 gene. Re-ChIP assays were performed as indicated, to detect co-occupancy of SNARP components on specific Cyclin D1 gene regions. Data is presented from a region in Fig. 4A which recruited the SNARP proteins. SNIP1 displays co-occupancy with SkIP, THRAP3 and BCLAF1 at this site.
(C) THRAP3 recruitment to the Cyclin D1 gene is SNIP1 dependent. ChIP assays were performed using U-2 OS cells in which SNIP1 had been transiently depleted using siRNA. α-SNIP1 and THRAP3 immunoprecipitations were performed and binding to the indicated regions of the Cyclin D1 gene detected by PCR.
(D) SNIP1 dependent binding of U2AF65 to the Cyclin D1 gene and endogenous Cyclin D1 mRNA. Upper panel: ChIP assays were performed using U-2 OS cells in which SNIP1 had been transiently depleted using siRNA. α-SNIP1 and U2AF65 immunoprecipitations were performed and binding to the indicated regions of the Cyclin D1 gene detected by PCR. Lower panel: Protein/RNA cross-linking was performed using U2-OS cells treated with control or SNIP1-targeted siRNAs. Immunoprecipitations were performed using the SNIP1, THRAP3 or U2AF65 antibodies and PCR analysis was used to detect the presence of the Cyclin D1 transcript after RNA extraction and DNAseI treatment.
SNIP1 regulates U2AF65 recruitment to the Cyclin D1 gene and RNA
In yeast, many RNA processing factors are associated with transcribed regions of genomic DNA, because of their participation in co-transcriptional mRNA processing, (28, 29). U2AF65, an RNA processing factor best characterised for its role in 3′ splice site selection, is one of the few such proteins currently demonstrated to bind along the length of mammalian genes in ChIP assays (30). Moreover, U2AF65 has also been postulated to play additional roles in RNA processing indicated by its continued association with many cellular mRNAs, of which a high percentage represent known cell cycle regulators (31). SkIP, BCLAF, THRAP3 and Pinin have also been reported in similar post-splicing RNA/protein complexes (32). We therefore investigated the ability of SNIP1 to regulate the recruitment of U2AF65 to the Cyclin D1 gene. Significantly, we were able to detect a similar pattern of U2AF65 association with the 3′ region of Cyclin D1 gene to SNARP proteins (Fig. 5A). Moreover, siRNA mediated depletion of SNIP1 strongly reduced recruitment of U2AF65 to the Cyclin D1 gene (Fig. 5D), indicating that the SNARP complex provides a link between transcription and RNA processing factors. This effect is likely to be indirect as we have not been able to demonstrate a direct SNIP1/U2AF65 interaction (data not shown).
We next determined whether SNIP1 and other SNARP complex components were also associated with Cyclin D1 RNA and whether the connection with U2AF65 binding was still observed. Importantly, we found that endogenous SNIP1 and THRAP3 both interacted with the 3′ untranslated region of endogenous Cyclin D1 RNA (Fig. 5D). Furthermore, in parallel with recruitment to the Cyclin D1 gene, depletion of SNIP1 also inhibited the recruitment of THRAP3 and U2AF65 to Cyclin D1 mRNA (Fig. 5D) to a far great extent than that simply attributable to decreased Cyclin D1 mRNA levels with SNIP1 siRNA treatment. Quantitation of these results (not shown) revealed that even after adjusting for the reduction in Cyclin D1 mRNA levels seen with SNIP1 siRNA treatment, depletion of SNIP1 resulted in a 70% decrease in THRAP3 binding, while U2AF65 association decreased by greater than 90%. Taken together this data suggests a role for SNIP1 and the SNARP complex as a link between Cyclin D1 transcription and RNA processing, aiding the recruitment of other established RNA-processing factors, such as U2AF65, to stabilise Cyclin D1 RNA within the nucleus.
Discussion
In this report we identify an important new regulatory step controlling Cyclin D1. We demonstrate that the newly identified SNARP complex is required for the production of mature Cyclin D1 mRNA within cells but is not necessary for production of the pre-spliced message. Moreover, SNARP complex components associate with both the Cyclin D1 gene and mRNA and are required for the further recruitment of the RNA processing factor U2AF65. We found that SNARP complex proteins and U2AF65 bind to the 3′ end of the Cyclin D1 gene and mRNA (Fig. 5D). At present, the mechanism required for SNIP1 recruitment is unclear. However, analysis of genome data (UCSC Genome Bioinformatics Site1) reveals significant blocks of evolutionarily conserved sequence within the 3′ untranslated region of the cyclin D1 gene, where SNARP and U2AF65 recruitment is strongest. We speculate that these may play a role in recruitment of the SNARP complex, although how this targeting might be accomplished is still unclear.
Inhibiting transcription using actinomycin D, followed by analysis of Cyclin D1 RNA degradation, indicated a role for the SNARP complex immediately after transcription (Fig. 2C). Furthermore, the observation that pre-spliced mRNA does not accumulate with SNIP1 depletion indicates that the splicing of the RNA itself is unlikely to be the stage at which SNIP1 is required. However, it has recently been shown that RNA polymerase II synthesised RNAs containing functional splicing sites are protected from nuclear degradation, presumably since the increased association of the splicing machinery will exclude nucleases (33). Our data is therefore consistent with the SNARP complex stabilising Cyclin D1 mRNA by regulating the recruitment of RNA processing factors, such as U2AF65, to nascent mRNA transcripts, thus protecting them from premature degradation. In this study, U2AF65 was chosen to examine SNIP1 dependent recruitment as it co-localises with SNIP1 in nuclear speckles (not shown), is known to be recruited to target genes in mammalian cells (30) and remains associated with the mRNAs of a number of genes after splicing, including many cell cycle regulators (31). However, it is likely that the recruitment of other factors to Cyclin D1 RNA will also be SNIP1 dependent.
The RNA polymerase II C-terminal domain heptad repeat (CTD) is thought to play a significant role in recruiting RNA processing factors, although additional mechanisms can facilitate and regulate protein recruitment (reviewed (34, 35)). Whether the SNARP complex provides a further link between the CTD and RNA-processing or represents an RNA-polymerase II independent mechanism is not currently known, although, interestingly, we have observed that SNIP1 co-immunoprecipitates with endogenous RNA polymerase II (not shown). Although our results have revealed a new role for SNIP1 as being primarily a regulator of Cyclin D1 RNA stability, these do not override previous data that suggested a transcriptional role at this and other genes. Indeed, taken together, both the previous and new observations imply a dual role for SNIP1 as a regulator of gene expression, where its relative contribution to these processes may vary depending upon the gene or cellular context.
Our results have important implications for the role for SNIP1 in tumourigenesis. SNIP1 is required for Cyclin D1 expression and augments c-Myc function in multiple cell lines (2, 6). SNIP1 and c-Myc, are over-expressed in tumours and SNIP1 co-operates with both c-Myc and H-Ras to cooperatively induce foci formation in an in vitro transformation assay (6). Interestingly, Cyclin D1 is also frequently overexpressed in cancer and like SNIP1, also co-operates with c-Myc and H-Ras in cell transformation (36, 37). Our results imply that these SNIP1 effects are mediated, at least in part, by the SNARP complex. It will be of interest to investigate whether SNARP complex proteins are co-ordinately over-expressed in tumours and whether this correlates with Cyclin D1 and c-Myc target gene expression. Alternatively, if SNIP1 levels are limiting in normal cells, its over-expression alone might be sufficient to drive tumorigenesis.
Supplementary Material
Acknowledgements
We thank Moto Ono for preparation of nucleolar extracts, the CLS cloning service for cDNA isolation and plasmid construction, Douglas Lamont and Kenneth Beattie for their assistance with mass spectrometry and all members of the NDP lab for their help and assistance. We also thank Angus Lamond, Julian Blow and Sonia Rocha for their critical reading of the manuscript and helpful suggestions. CPB is funded by a Cancer Research UK programme grant (C1443/A5435). BB is funded by a project grant from the Association of International Cancer Research.
Footnotes
References
- 1.Tashiro E, Tsuchiya A, Imoto M. Functions of cyclin D1 as an oncogene and regulation of cyclin D1 expression. Cancer Sci. 2007;98:629–35. doi: 10.1111/j.1349-7006.2007.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roche KC, Wiechens N, Owen-Hughes T, Perkins ND. The FHA domain protein SNIP1 is a regulator of the cell cycle and cyclin D1 expression. Oncogene. 2004;23:8185–95. doi: 10.1038/sj.onc.1208025. [DOI] [PubMed] [Google Scholar]
- 3.Kim RH, Wang D, Tsang M, et al. A novel smad nuclear interacting protein, SNIP1, suppresses p300-dependent TGF-beta signal transduction. Genes Dev. 2000;14:1605–16. [PMC free article] [PubMed] [Google Scholar]
- 4.Durocher D, Jackson SP. The FHA domain. FEBS Lett. 2002;513:58–66. doi: 10.1016/s0014-5793(01)03294-x. [DOI] [PubMed] [Google Scholar]
- 5.Kim RH, Flanders KC, Birkey Reffey S, et al. SNIP1 inhibits NF-κB signaling by competing for its binding to the C/H1 domain of CBP/p300 transcriptional co-activators. J Biol Chem. 2001;276:46297–304. doi: 10.1074/jbc.M103819200. [DOI] [PubMed] [Google Scholar]
- 6.Fujii M, Lyakh LA, Bracken CP, et al. SNIP1 is a candidate modifier of the transcriptional activity of c-Myc on E box-dependent target genes. Mol Cell. 2006;24:771–83. doi: 10.1016/j.molcel.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Roche KC, Rocha S, Bracken CP, Perkins ND. Regulation of ATR-dependent pathways by the FHA domain containing protein SNIP1. Oncogene. 2007 doi: 10.1038/sj.onc.1210233. [DOI] [PubMed] [Google Scholar]
- 8.Salles FJ, Richards WG, Strickland S. Assaying the polyadenylation state of mRNAs. Methods. 1999;17:38–45. doi: 10.1006/meth.1998.0705. [DOI] [PubMed] [Google Scholar]
- 9.Niranjanakumari S, Lasda E, Brazas R, Garcia-Blanco MA. Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods. 2002;26:182–90. doi: 10.1016/S1046-2023(02)00021-X. [DOI] [PubMed] [Google Scholar]
- 10.Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci U S A. 1996;93:1065–70. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barre B, Perkins ND. A cell cycle regulatory network controlling NF-κB subunit activity and function. Embo J. 2007;26:4841–55. doi: 10.1038/sj.emboj.7601899. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Betticher DC, Thatcher N, Altermatt HJ, Hoban P, Ryder WD, Heighway J. Alternate splicing produces a novel cyclin D1 transcript. Oncogene. 1995;11:1005–11. [PubMed] [Google Scholar]
- 13.Baudino TA, Kraichely DM, Jefcoat SC, Jr., Winchester SK, Partridge NC, MacDonald PN. Isolation and characterization of a novel coactivator protein, NCoA-62, involved in vitamin D-mediated transcription. J Biol Chem. 1998;273:16434–41. doi: 10.1074/jbc.273.26.16434. [DOI] [PubMed] [Google Scholar]
- 14.Figueroa JD, Hayman MJ. The human Ski-interacting protein functionally substitutes for the yeast PRP45 gene. Biochem Biophys Res Commun. 2004;319:1105–9. doi: 10.1016/j.bbrc.2004.05.096. [DOI] [PubMed] [Google Scholar]
- 15.Kasof GM, Goyal L, White E. Btf, a novel death-promoting transcriptional repressor that interacts with Bcl-2-related proteins. Mol Cell Biol. 1999;19:4390–404. doi: 10.1128/mcb.19.6.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Lin RI, Lai MC, Ouyang P, Tarn WY. Nuclear Pnn/DRS protein binds to spliced mRNPs and participates in mRNA processing and export via interaction with RNPS1. Mol Cell Biol. 2003;23:7363–76. doi: 10.1128/MCB.23.20.7363-7376.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Hawkins IC, Harvey CD, Jennings JL, Link AJ, Patton JG. Regulation of alternative splicing by SRrp86 and its interacting proteins. Mol Cell Biol. 2003;23:7437–47. doi: 10.1128/MCB.23.21.7437-7447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makarov EM, Makarova OV, Urlaub H, et al. Small nuclear ribonucleoprotein remodeling during catalytic activation of the spliceosome. Science. 2002;298:2205–8. doi: 10.1126/science.1077783. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Lou PJ, Leu S, Ouyang P. Modulation of alternative pre-mRNA splicing in vivo by pinin. Biochem Biophys Res Commun. 2002;294:448–55. doi: 10.1016/S0006-291X(02)00495-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Dowd DR, Staal A, et al. Nuclear coactivator-62 kDa/Ski-interacting protein is a nuclear matrix-associated coactivator that may couple vitamin D receptor-mediated transcription and RNA splicing. J Biol Chem. 2003;278:35325–36. doi: 10.1074/jbc.M305191200. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S, Fujimuro M, Hsieh JJ, Chen L, Hayward SD. A role for SKIP in EBNA2 activation of CBF1-repressed promoters. J Virol. 2000;74:1939–47. doi: 10.1128/jvi.74.4.1939-1947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott KL, Plon SE. CHES1/FOXN3 interacts with Ski-interacting protein and acts as a transcriptional repressor. Gene. 2005;359:119–26. doi: 10.1016/j.gene.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Leong GM, Subramaniam N, Issa LL, et al. Ski-interacting protein, a bifunctional nuclear receptor coregulator that interacts with N-CoR/SMRT and p300. Biochem Biophys Res Commun. 2004;315:1070–6. doi: 10.1016/j.bbrc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Leong GM, Subramaniam N, Figueroa J, et al. Ski-interacting protein interacts with Smad proteins to augment transforming growth factor-beta-dependent transcription. J Biol Chem. 2001;276:18243–8. doi: 10.1074/jbc.M010815200. [DOI] [PubMed] [Google Scholar]
- 25.Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nature reviews. 2003;4:605–12. doi: 10.1038/nrm1172. [DOI] [PubMed] [Google Scholar]
- 26.Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI. The multifunctional nucleolus. Nature reviews. 2007;8:574–85. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- 27.Bres V, Gomes N, Pickle L, Jones KA. A human splicing factor, SKIP, associates with P-TEFb and enhances transcription elongation by HIV-1 Tat. Genes Dev. 2005;19:1211–26. doi: 10.1101/gad.1291705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gornemann J, Kotovic KM, Hujer K, Neugebauer KM. Cotranscriptional spliceosome assembly occurs in a stepwise fashion and requires the cap binding complex. Mol Cell. 2005;19:53–63. doi: 10.1016/j.molcel.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Lacadie SA, Rosbash M. Cotranscriptional spliceosome assembly dynamics and the role of U1 snRNA:5’ss base pairing in yeast. Mol Cell. 2005;19:65–75. doi: 10.1016/j.molcel.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Listerman I, Sapra AK, Neugebauer KM. Cotranscriptional coupling of splicing factor recruitment and precursor messenger RNA splicing in mammalian cells. Nat Struct Mol Biol. 2006;13:815–22. doi: 10.1038/nsmb1135. [DOI] [PubMed] [Google Scholar]
- 31.Gama-Carvalho M, Barbosa-Morais NL, Brodsky AS, Silver PA, Carmo-Fonseca M. Genome-wide identification of functionally distinct subsets of cellular mRNAs associated with two nucleocytoplasmic-shuttling mammalian splicing factors. Genome Biol. 2006;7:R113. doi: 10.1186/gb-2006-7-11-r113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merz C, Urlaub H, Will CL, Luhrmann R. Protein composition of human mRNPs spliced in vitro and differential requirements for mRNP protein recruitment. Rna. 2007;13:116–28. doi: 10.1261/rna.336807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hicks MJ, Yang CR, Kotlajich MV, Hertel KJ. Linking splicing to Pol II transcription stabilizes pre-mRNAs and influences splicing patterns. PLoS Biol. 2006;4:e147. doi: 10.1371/journal.pbio.0040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornblihtt AR, de la Mata M, Fededa JP, Munoz MJ, Nogues G. Multiple links between transcription and splicing. Rna. 2004;10:1489–98. doi: 10.1261/rna.7100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neugebauer KM. On the importance of being co-transcriptional. J Cell Sci. 2002;115:3865–71. doi: 10.1242/jcs.00073. [DOI] [PubMed] [Google Scholar]
- 36.Lovec H, Grzeschiczek A, Kowalski MB, Moroy T. Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. Embo J. 1994;13:3487–95. doi: 10.1002/j.1460-2075.1994.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovec H, Sewing A, Lucibello FC, Muller R, Moroy T. Oncogenic activity of cyclin D1 revealed through cooperation with Ha-ras: link between cell cycle control and malignant transformation. Oncogene. 1994;9:323–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.