Abstract
A highly stereoselective synthesis of 11-acetoxy-4-deoxyasbestinin D (1) has been completed in 26 linear steps. The synthesis hinges on a selective glycolate aldol addition to establish the C-2 stereocenter, a ring-closing metathesis reaction to complete the oxonene, and an intramolecular Diels-Alder cycloaddition to establish the relative configuration at C-1, C-10, and C14. This initial total synthesis of an asbestinin also serves to confirm the absolute configuration of this sub-class of the C2-C11-cyclized cembranoid natural products.
Synthesis of C2–C11 cyclized cembranoids has intensified over the past decade, due to their fascinating molecular topology and interesting biological properties.1 Various approaches to the syntheses of the cladiellins and briarellins have been implemented;2 however, none of the asbestinins have been prepared by total synthesis, leaving some doubt regarding their absolute configuration and biosynthetic origin.1b,3 In 1990, Rodríguez and co-workers isolated 11-acetoxy-4-deoxyasbestinin D (1) from Briareum asbestinum, making note of its particular cytotoxicity against CHO-K1 cells (ED50=4.82 μg/mL) and strong anti-microbial activity against Klebsiella pneumoniae.3 The tetracyclic framework of 11-acetoxy-4-deoxyasbestinin D includes nine contiguous stereocenters and a fully-substituted tetrahydrofuran, rendering it a formidable and intriguing target for chemical synthesis.
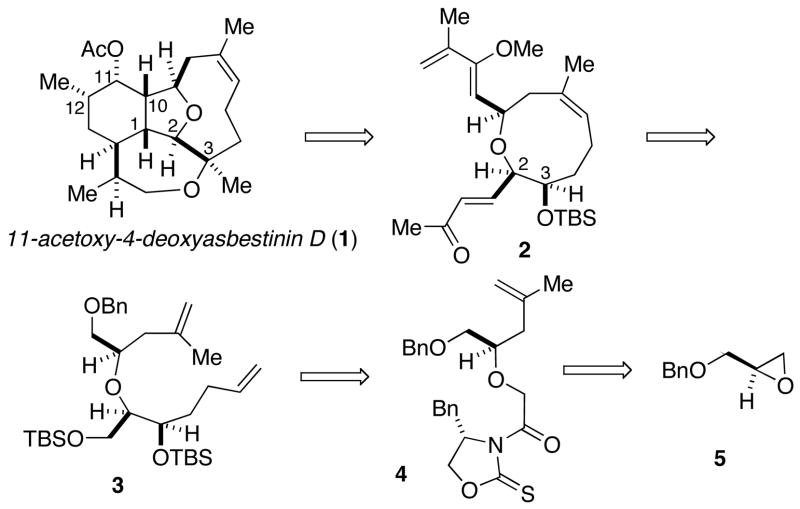
We recently reported a successful strategy for the synthesis of members of the eunicellin class of cembranoids involving the construction of the oxonene ring through ring-closing metathesis,4–6 followed by stereoselective formation of the hydroisobenzofuran via an intramolecular Diels-Alder cycloaddition.2k The total synthesis of 11-acetoxy-4-deoxyasbestinin D (1) was undertaken with the intent of validating the intramolecular Diels-Alder approach for the synthesis of the asbestinins.7 Our synthetic plan hinged on an asymmetric glycolate aldol reaction of oxazolidinethione 4 to assemble the diene 3, a precursor of the oxonene 2 (Scheme 1). This report describes the first total synthesis of an asbestinin verifying the absolute configuration of the sub-class.
Scheme 1.
Retrosynthetic Analysis of 1
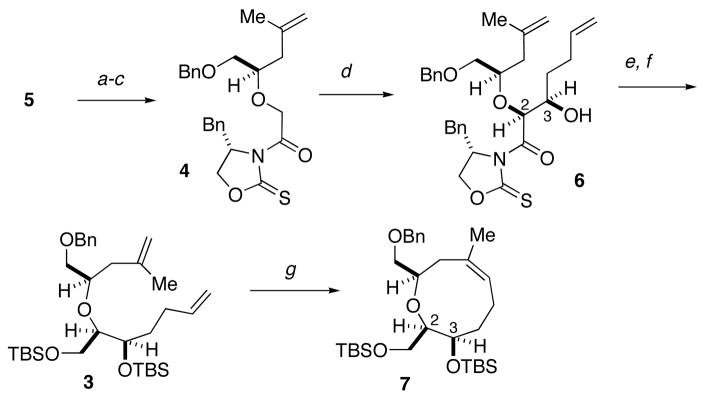
The synthesis of the oxonene core began with the addition of isoprenylmagnesium bromide to (R)-benzyl glycidyl ether (5) to afford a secondary alcohol,8 which was O-alkylated with sodium bromoacetate (Scheme 2). The resultant glycolic acid was coupled with (S)-4-benzyloxazolidinethione to deliver thioimide 4. Addition of 4-pentenal,9 to the chlorotitanium enolate of thioimide 4 in the presence of NMP,5c,7 gave syn-aldol adduct 6 in good yield and diastereoselectivity (70%, >95:5 dr).10 Reductive removal of the chiral auxiliary and protection of the diol afforded diene 3. As anticipated, based on previous successful metathesis reactions to form medium ring ethers,4–6 treatment of diene 3 with the Grubbs catalyst11 led to facile formation of oxonene 7 (99% yield).
Scheme 2.
Synthesis of the Oxonene Ringa
a(a) CH2=C(CH3)MgBr, CuI, THF, −40 °C, 99%; (b) NaH, BrCH2CO2H, THF, DMF, 95%; (c) (S)-benzyl-1,3-oxazolidine-2-thione, DCC, DMAP, CH2Cl2, 86%; (d) TiCl4, i-Pr2NEt, NMP, 4-pentenal, CH2Cl2, −78 °C, 70%; (e) LiBH4, MeOH, Et2O, 0 °C, 95%; (f) TBSCl, imid., DMAP, DMF, 50 °C, 87%; (g) Cl2(Cy3P)(IMes)Ru=CHPh, CH2Cl2, 40 °C, 99%.
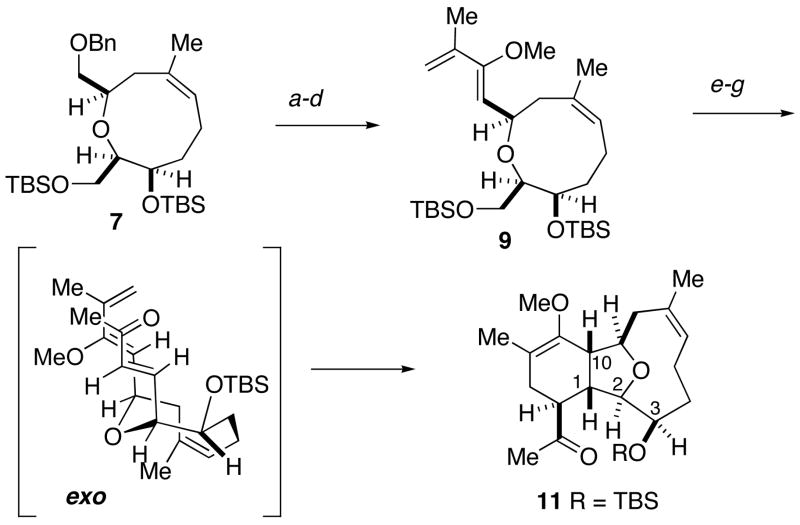
With the oxonene 7 in hand, efforts focused on construction of the required Diels-Alder precursor. To this end, the benzyl ether was reductively cleaved, and the resultant alcohol was oxidized to the aldehyde under Swern conditions (Scheme 3).12 The aldehyde was subjected to successive Wittig reactions, first using phosphorane 813, then methylene triphenylphosphorane to yield the requisite diene 9. Selective deprotection of the primary TBS ether was carefully carried out in the presence of the labile enol ether.14 Oxidation12 of the alcohol provided an aldehyde, which was treated with phosphorane 1 015 at elevated temperature. Subsequent to the Wittig reaction, a spontaneous Diels-Alder cycloaddition ensued through the more favorable exo transition state providing adduct 11 in 80% yield as a single diastereomer. Earlier work in related systems had demonstrated the importance of both the C-3 configuration and the C3 hydroxyl protecting group in controlling the diastereoselectivity of the Diels-Alder reaction.2k,16
Scheme 3.
Intramolecular Diels-Alder Cycloadditiona
a(a) Na, NH3, THF, −78 °C, 86%; (b) (COCl)2, DMSO, Et3N, CH2Cl2, −78 °C to 0 °C, 94%; (c) Ph3P=C(OMe)C(O)Me (8), PhCH3, 110 °C, 84%; (d) Ph3PCH3Br, t-BuOK, THF, 0 °C, 87%; (e) NH4F, MeOH, 79%; (f) (COCl)2, DMSO, Et3N, CH2Cl2, −78 °C to 0 °C, 93%; (g) Ph3P=CHC(O)Me (10), PhCH3, 110 °C, 80%.
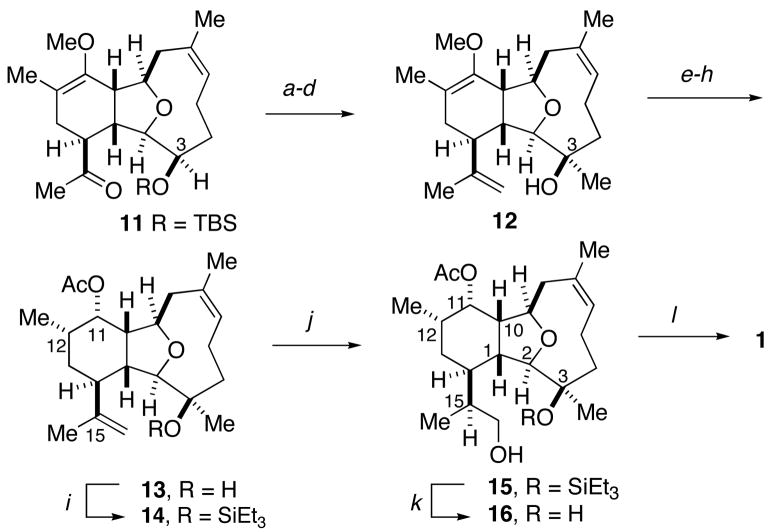
Ketone 11 was converted to an alkene to introduce a handle to establish the C-15 stereocenter (Scheme 4). Deprotection and oxidation17 of the C3 secondary alcohol, followed by addition of methylmagnesium chloride to the resultant ketone provided the tertiary alcohol 12 in excellent yield as a single isomer.
Scheme 4.
Completion of 11-Acetoxy-4-deoxyasbestinin Da
a(a) Ph3PCH3Br, KO-t-Bu, THF, 85%; (b) n-Bu4NF, THF, 95%; (c) Dess-Martin periodinane, pyridine, CH2Cl2, 98%; (d) MeMgCl, THF, 0 °C, 98%; (e) HCl, CHCl3, 96%, 10:1 dr; (f) NaH, MeOH, 99%, 1:1.2 dr; (g) L-Selectride®, THF, −78 °C, 94%; (h) Ac2O, Et3N, DMAP, CH2Cl2, 99%; (i) TESOTf, 2,6-lutidine, CH2Cl2, 0 °C, 80%; (j) (+)-Ipc2BH, THF; NaOH, H2O2; (k) n-Bu4NF, THF, 64% (two steps); (l) Tf2O, 2,6-lutidine, CHCl3, 0 °C to 25 °C, 66%.
With the required tricyclic core in place, refunctionalization of the cyclohexane ring was undertaken. Acidic hydrolysis of the enol ether yielded the α-methyl ketone (10:1 dr); however, 1H NMR analysis (COSY, nOeSY) indicated the undesired isomer was the major product. This problem was easily corrected by base catalyzed equilibration to the desired isomer, to provide 84% of the desired ketone after two recycles. Reduction of the ketone with L-Selectride® yielded the secondary alcohol as a single diastereomer, which was esterified to give acetate 13.
The final stage of the synthesis required introduction of the C-15 stereocenter and formation of the oxapane. The regioselective and stereoselective hydroboration of the 1,1-disubstituted olefin of diene 13 proved to be a challenge. Regioselective hydroboration occurred in high yield with 9-BBN, but the reaction was not stereoselective.18 It was speculated that increasing the steric bulk at C-3 could impede addition from the undesired face of the alkene, improving the diastereoselection. Accordingly, the C-3 hydroxyl was protected as triethylsilyl ether 1 4, but only moderate improvement in the diastereoselectivity (2:1 dr) was observed.
Fortunately, the use of (+)-diisopinocampheylborane delivered the desired alcohol 15 as a single isomer after oxidative workup.19,20 The triethylsilyl ether was subsequently cleaved to deliver the diol 16 in 64% yield over two steps. Taking advantage of the conditions employed by Overman in the syntheses of briarellins E and F,2i the diol 16 was treated with triflic anhydride and 2,6-lutidine to deliver 11-acetoxy-4-deoxyasbestinin D (1) in 66% yield. Spectroscopic data for synthetic 1 matched the reported data for the natural product in all regards.3 Of particular note, the specific rotation of synthetic 1 and a purified sample of natural 1 were identical ([α]D26; CHCl3; = −15) when measured under the conditions.
In summary, a highly stereoselective synthesis of 11-acetoxy-4-deoxyasbestinin D has been completed in 26 linear steps, hinging on a selective glycolate aldol addition to establish the C-2 stereocenter, a ring-closing metathesis reaction to complete the oxonene, and an intramolecular Diels-Alder cycloaddition to establish the relative configuration at C-1, C-10, and C14. This initial total synthesis of an asbestinin also serves to confirm the absolute configuration of this family of natural products.
Supplementary Material
Experimental procedures as well as 1H and 13C NMR spectra for all new compounds and synthetic 11-acetoxy-4-deoxyasbestinin D. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgments
This work was supported by a research grant from The National Institutes of Health (GM60567). We acknowledge a generous gift of (R)-benzyl glycidyl ether from Daiso, Inc. We also are grateful to Dr. Abimael D. Rodríguez (University of Puerto Rico, Río Piedras) for his donation of an authentic sample of the natural product.
References
- 1.For reviews see: Rodríguez AD. Tetrahedron. 1995;51:4571. doi: 10.1016/0040-4020(95)00216-U.Bernardelli P, Paquette LA. Heterocycles. 1998;49:531.Sung PS, Chen MC. Heterocycles. 2002;57:1705.
- 2.(a) MacMillan DWC, Overman LE. J Am Chem Soc. 1995;117:10391. [Google Scholar]; (b) Paquette LA, Moradei OM, Bernardelli P, Lange T. Org Lett. 2000;2:1875. doi: 10.1021/ol000083o. [DOI] [PubMed] [Google Scholar]; (c) Overman LE, Pennington LD. Org Lett. 2000;2:2683. doi: 10.1021/ol006236p. [DOI] [PubMed] [Google Scholar]; (d) Gallou F, MacMillan DWC, Overman LE, Paquette LA, Pennington LD, Yang J. Org Lett. 2001;3:135. doi: 10.1021/ol000345m. [DOI] [PubMed] [Google Scholar]; (e) Bernardelli P, Moradei OM, Friedrich D, Yang J, Gallou F, Dyck BP, Doskotch RW, Lange T, Paquette LA. J Am Chem Soc. 2001;123:9021. doi: 10.1021/ja011285y. [DOI] [PubMed] [Google Scholar]; (f) MacMillan DWC, Overman LE, Pennington LD. J Am Chem Soc. 2001;123:9033. doi: 10.1021/ja016351a. [DOI] [PubMed] [Google Scholar]; (g) Chai Y, Vicic DA, McIntosh MC. Org Lett. 2003;5:1039. doi: 10.1021/ol034052f. [DOI] [PubMed] [Google Scholar]; (h) Corminboeuf O, Overman LE, Pennington LD. Org Lett. 2003;5:1543. doi: 10.1021/ol034384k. [DOI] [PubMed] [Google Scholar]; (i) Corminboeuf O, Overman LE, Pennington LD. J Am Chem Soc. 2003;125:6650. doi: 10.1021/ja035445c. [DOI] [PubMed] [Google Scholar]; (j) Molander GA, StJean DJ, Jr, Haas J. J Am Chem Soc. 2004;126:1642. doi: 10.1021/ja0398464. [DOI] [PubMed] [Google Scholar]; (k) Crimmins MT, Brown BH. J Am Chem Soc. 2004;126:10264. doi: 10.1021/ja046574b. [DOI] [PubMed] [Google Scholar]
- 3.Morales JJ, Lorenzo D, Rodríguez AD. J Nat Prod. 1991;54:1368. doi: 10.1021/np50077a021. [DOI] [PubMed] [Google Scholar]
- 4.(a) Crimmins MT, Emmitte KA. Synthesis. 2000:899. [Google Scholar]; (b) Crimmins MT, DeBaillie AC. Org Lett. 2003;5:3009. doi: 10.1021/ol034923l. [DOI] [PubMed] [Google Scholar]
- 5.(a) Crimmins MT, Emmitte KA. Org Lett. 1999;1:2029. doi: 10.1021/ol991201e. [DOI] [PubMed] [Google Scholar]; (b) Crimmins MT, Choy AL. J Am Chem Soc. 1999;121:5653. [Google Scholar]; (c) Crimmins MT, Tabet EA. J Am Chem Soc. 2000;122:5473. [Google Scholar]; (d) Crimmins MT, Cleary PA. Heterocycles. 2003;61:87. [Google Scholar]
- 6.(a) Crimmins MT, Emmitte KA. J Am Chem Soc. 2001;123:1533. doi: 10.1021/ja005892h. [DOI] [PubMed] [Google Scholar]; (b) Crimmins MT, Emmitte KA, Choy AL. Tetrahedron. 2002;58:1817. [Google Scholar]; (c) Crimmins MT, Powell MT. J Am Chem Soc. 2003;125:7592. doi: 10.1021/ja029956v. [DOI] [PubMed] [Google Scholar]
- 7.(a) Crimmins MT, King BW, Tabet EA. J Am Chem Soc. 1997;119:7883. [Google Scholar]; (b) Crimmins MT, Choy AL. J Org Chem. 1997;62:7548. doi: 10.1021/jo9716688. [DOI] [PubMed] [Google Scholar]; (c) Crimmins MT, King BW, Tabet EA, Chaudhary K. J Org Chem. 2001;66:894. doi: 10.1021/jo001387r. [DOI] [PubMed] [Google Scholar]; (d) Crimmins MT, She J. Synlett. 2004:1371. [Google Scholar]
- 8.(a) Lachance H, Lu X, Gravel M, Hall DG. J Am Chem Soc. 2003;125:10160. doi: 10.1021/ja036807j. [DOI] [PubMed] [Google Scholar]; (b) Hubbs JL, Heathcock CH. J Am Chem Soc. 2003;125:12836. doi: 10.1021/ja030316h. [DOI] [PubMed] [Google Scholar]
- 9.(a) McNeill AH, Thomas EJ. Tetrahedron Lett. 1993;34:1669. [Google Scholar]; (b) Farquhar D, Cherif A, Bakina E, Nelson JA. J Med Chem. 1998;41:965. doi: 10.1021/jm9706980. [DOI] [PubMed] [Google Scholar]
- 10.The N-acyloxazolidinone derivative of glycolate 4 gave a slightly higher yield, but with diminished diastereoselection (78%, >90:10 dr)
- 11.Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 12.Mancuso AJ, Huang SL, Swern D. J Org Chem. 1978;43:2480. [Google Scholar]
- 13.Guiney D, Gibson CL, Suckling CJ. Org Biomol Chem. 2003;1:664. doi: 10.1039/b211564f. [DOI] [PubMed] [Google Scholar]
- 14.Schinzer D, Bohm OM, Altmann KH, Wartmann M. Synlett. 2004:1375. [Google Scholar]
- 15.Kuroda H, Hanaki E, Izawa H, Kano M, Itahashi H. Tetrahedron. 2004;60:1913. [Google Scholar]
- 16.Davidson JEP, Gilmour R, Ducki S, Davies JE, Green R, Burton JW, Holmes AB. Synlett. 2004:1434. [Google Scholar]
- 17.Dess DB, Martin JC. J Am Chem Soc. 1991;113:7277. [Google Scholar]
- 18.(a) Brown HC, Midland MM, Levy AB, Kramer GW. Organic Synthesis via Boranes. Wiley; New York: 1975. [Google Scholar]; (b) Cheng D, Zhu S, Yu Z, Cohen T. J Am Chem Soc. 2001;123:30. doi: 10.1021/ja0029782. [DOI] [PubMed] [Google Scholar]; (c) Ebel H, Polborn K, Steglich W. Eur J Org Chem. 2002:2905. [Google Scholar]; (d) Miyaoka H, Honda D, Mitome H, Yamada Y. Tetrahedron Lett. 2002;43:7773. [Google Scholar]
- 19.Brown HC, Desai MC, Jadhav PK. J Org Chem. 1982;47:5065. [Google Scholar]
- 20.Masamune S, Lu LDL, Jackson WP, Kaiho T, Toyoda T. J Am Chem Soc. 1982;104:5523. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures as well as 1H and 13C NMR spectra for all new compounds and synthetic 11-acetoxy-4-deoxyasbestinin D. This material is available free of charge via the internet at http://pubs.acs.org.