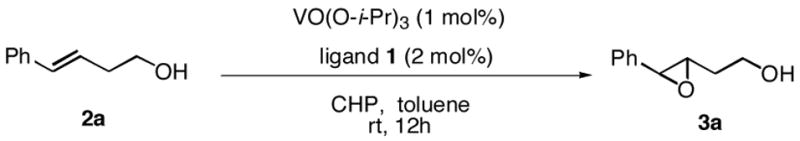
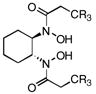
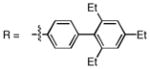
Catalytic asymmetric epoxidation of olefins is very useful for the synthesis of enantiomerically enriched epoxides, which are versatile building blocks for the synthesis of natural products and biologically active substances. There are many efficient protocols to mediate the epoxidation of allylic alcohols to provide satisfactory yields and enantioselectivities.1 However, very limited catalyst systems can be used for the asymmetric epoxidation of homoallylic alcohols and those substrates in which olefin is located more far away from the hydroxy group. Sharpless asymmetric epoxidation, which was efficient for allylic alcohols, could not provide homoallylic alcohols with satisfactory enantioselectivities.2,3 The protocol reported by our group, which used vanadium and a-amino acid-based hydroxamic acid ligands to perform the asymmetric epoxidation of homoallylic alcohols, was found to be efficient.4,5 Unfortunately, however, the enantioselectivities of trans and cis-substituted olefins were not satisfactory. Thus, there has been no truly efficient catalytic asymmetric epoxidation of homoallylic alcohols reported. Recently, we reported a vanadium-catalyzed epoxidation of allylic alcohols with newly designed bishydroxamic acid (BHA) ligands (1a and 1b),6 which has the following features: 1) high enantioselectivity for a wide scope of allylic alcohols, 2) less than 1mol% catalyst loading, 3) mild reaction conditions, and 4) use of aqueous tert-butyl hydroperoxide (TBHP) as an achiral oxidant instead of anhydrous TBHP.7,8,9 (Scheme 1) Herein, we report a new modified BHA ligand that is suitable for highly enantioselective vanadium-catalyzed epoxidation of homoallylic alcohols.
Scheme 1.
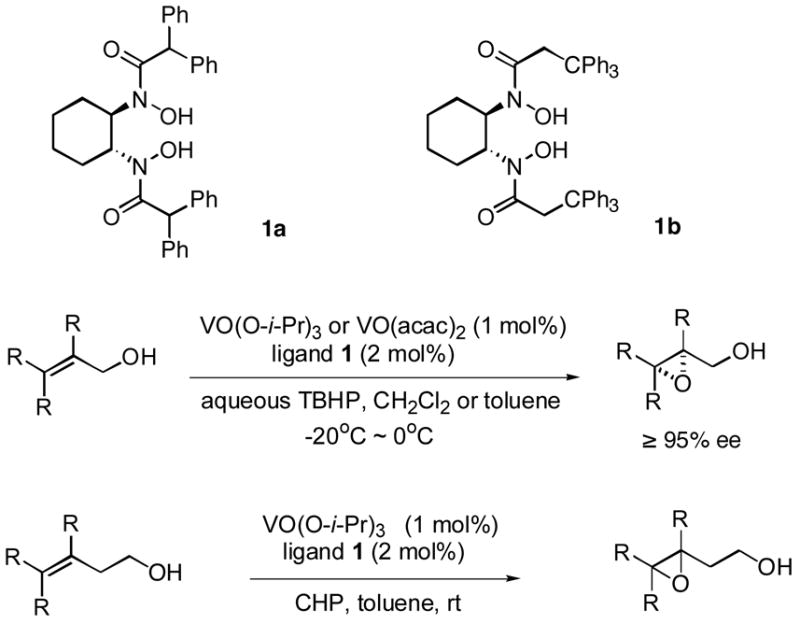
Asymmetric epoxidation of allylic alcohols and homoallylic alcohols by vanadium and BHA complex.
Initial experimental modification showed that cumene hydroperoxide (CHP) was better than TBHP to facilitate and complete the transformation and toluene was used as solvent to inhibit cyclization of the produced epoxide to the corresponding tetrahydrofuran by-product. (Scheme 1) One mol% catalyst loading was enough to perform the reaction at room temperature. Reaction proceeded smoothly and moderate enantioselectivity as well as good yield was achieved on 2 a when ligand 1b was used. (Table 1. entry 1) With this promising result in hand, new ligands based on 1b were synthesized. The enantioselectivity was increased to 90%ee with 1c. Finally, 1d, which was introduced with a more hindered substituted phenyl group, was found to be excellent for the reaction; 96% ee was obtained on 2a while the rate of the reaction was also facilitated. (Table 1.)
Table 1.
Screening of ligands.
All reactions were carried out in toluene in the presence of 1.5 equiv. of cumene hydroperoxide (CHP) (88%) unless otherwise indicated.
Isolated yield after chromatographic purification.
Ee values were determined by chiral HPLC (AD-H) and the detailed information is provided in the supporting information.
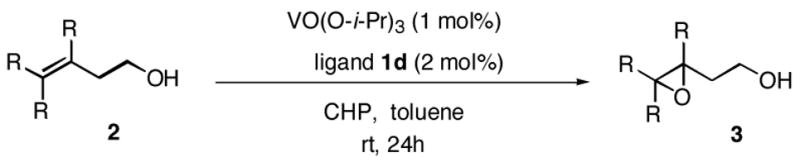
The scope of the reaction was investigated with 1d under the modified conditions. Gratifyingly, both trans- and cis-substituted epoxides were achieved with virtually complete enantioselectivities and satisfactory yields.
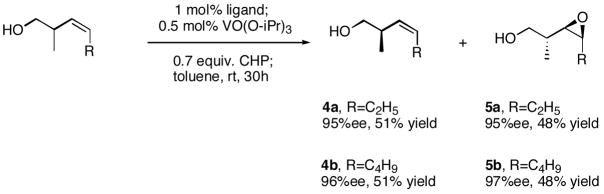
With the successful results of the asymmetric epoxidation of homoallylic alcohols, this catalyst system was applied to the kinetic resolution of these alcohols with outstanding selectivities (4a, 4b). (Scheme 2) Both the starting homoallylic alcohols and epoxy alcohols were obtained with satisfactory enantiopurity.10 It should also be noted that this kinetic resolution gave us an opportunity to generate asymmetric carbon in a completely new scheme. In fact, starting homoallylic alcohols can be synthesized efficiently using preexisting chemistry of allylic anions.11
Scheme 2.
Kinetic resolution of homoallylic alcohols.
The absolute configurations of 3c and 3f were determined as (3R, 4R) and (3R, 4S) respectively by comparison of reported optical rotation.2b,3b,12
In summary, we have designed a new chiral bis-hydroxamic acid ligand, which has been shown to be excellent for the vanadium-catalyzed asymmetric epoxidation and kinetic resolution of homoallylic alcohols. Further studies focusing on broader application of our chiral vanadium-hydroxamic acid complexes to wider scope are ongoing.
Supplementary Material
Representative experimental procedures and spectral data for 1c–1d. This material is available free of charge via the Internet at (http://pubs.acs.org/instruct/jacsat.pdf).
Table 2.
Scope of substrates.
 | |||
|---|---|---|---|
| entrya | HAA | epoxy alcohol | %yield b, %eec, config. |
| 1 |
2a |
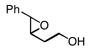 3a 3a
|
90, 96 |
| 2 |
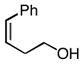
2b |
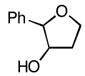 3b 3b
|
85, 99 |
| 3 |
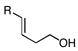
2c–e |
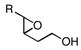
3c, R=C2H5 3d, R=C5H11 3e, R=C6H13 |
85, 93 (3R, 4R) |
| 4 | 89, 96 | ||
| 5 | 92, 98 | ||
| 6 |
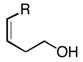
2f–i |
3f, R=C2H5 3g, R=C3H7 3h, R=C4H9 3i, R=C5H11 |
92, 95 (3R, 4S) |
| 7 | 90, 97 | ||
| 8 | 91, 99 | ||
| 9 | 90, 99 | ||
All reactions were carried out in toluene in the presence of 1.5 equiv. of cumene hydroperoxide (CHP) (88%) unless otherwise indicated.
Isolated yield after chromatographic purification.
Ee values were determined by either chiral HPLC or chiral GC and the detailed information is provided in the supporting information.
Acknowledgments
Support for this research was provided by the SORST project of the Japan Science and Technology Agency (JST), National Institutes of Health (NIH) GM068433-01, Merck Research Laboratories and a starter grant from the University of Chicago.
References
- 1.For recent reviews, see Katsuki T. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 2. Springer; Berlin: 1999. p. 621.Katsuki T, Ojima I. Org React. 1996;48:1.Keith JM, Larrow JF, Jacobsen EN. Adv Synth Catal. 2001;343:5.Adam W, Saha-Möller CR, Ganeshpure PA. Chem Rev. 2001;101:3499. doi: 10.1021/cr000019k.Adam W, Malisch W, Roschmann KJ, Saha-Möller CR, Schenk WA. J Organomet Chem. 2002;661:3.Lattanzi A, Scettri A. J Organomet Chem. 2006;691:2072.Shi Y. Acc Chem Res. 2004;37:488. doi: 10.1021/ar030063x.Shi Y, Frohn M. Synthesis. 2000;14:1979.
- 2.(a) Katsuki T, Sharpless KB. J Am Chem Soc. 1980;102:5974. [Google Scholar]; (b) Rossiter BE, Sharpless KB. J Org Chem. 1984;49:3707. [Google Scholar]; (c) Gao Y, Hanson RM, Klunder JM, Ko SY, Masamune H, Sharpless KB. J Am Chem Soc. 1987;109:5765. [Google Scholar]
- 3.For Zr catalyzed epoxidation of homoallylic alcohols, see Ikegami S, Katsuki T, Yamaguchi M. Chem Lett. 1987:83.Okashi T, Murai N, Onaka M. Org Lett. 2003;5:85. doi: 10.1021/ol027261t.
- 4.Makita N, Hoshino Y, Yamamoto H. Angew Chem Int Ed. 2003;42:941. doi: 10.1002/anie.200390250. [DOI] [PubMed] [Google Scholar]
- 5.For a related epoxidation of allylic alcohols, see Murase Y, Hoshino Y, Oishi M, Yamamoto H. J Org Chem. 1999;64:338.Hoshino Y, Murase N, Oishi M, Yamamoto H. Bull Chem Soc Jpn. 2000;73:1653.Hoshino Y, Yamamoto H. J Am Chem Soc. 2000;122:10452.
- 6.Zhang W, Basak A, Kosugi Y, Hoshino Y, Yamamoto H. Angew, Chem Int Ed. 2005;44:4389. doi: 10.1002/anie.200500938. [DOI] [PubMed] [Google Scholar]
- 7.For recent reports on chiral vanadium catalyst for epoxidation, see Bryliakov KP, Talsi EP. Kinetics and Catalysis (Translation of Kinetika i Kataliz) 2003;44:334.Bolm C, Kuhn T. Synlett. 2000:899.Traber B, Jung YG, Park TK, Hong JI. Bull Korean Chem Soc. 2001;22:547.Wu HL, Uang BJ. Tetrahedron: Asymmetry. 2002;13:2625.Bourhani Z, Malkov AV. Chem Commun. 2005:4592. doi: 10.1039/b509436d.Lattanzi A, Piccirillo S, Scettri A. Eur J Org Chem. 2005:1669.
- 8.For recent reviews on vanadium, see Hirao T. Chem Rev. 1997;97:2707. doi: 10.1021/cr960014g.Bolm C. Coord Chem Rev. 2003;237:245.Ligtenbarg AG, Hage R, Feringa BL. Coord Chem Rev. 2003;237:89.
-
9.For mechanistic study on vanadium-hydroxamic acid oxidation, see Bryliakov KP, Talsi EP. Kinetics and Catalysis (Translation of Kinetika i Kataliz) 2003;44:334.Bryliakov KP, Talsi EP, Kuehn T, Bolm C. New Journal of Chemistry. 2003;27:609.Adam W, Beck AK, Pichota A, Saha-Möller CR, Seebach D, Vogl N, Zhang R. Tetrahedron: Asymmetry. 2003;14:1355.
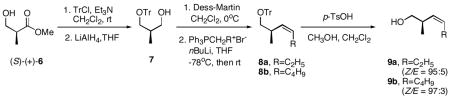
- 10.Ee values were determined by chiral GC analysis and the detailed information is provided in supporting information. The absolute configurations of 4a and 4b were determined as (R) and (R) respectively by comparison of retention times (GC analysis) of the absolute configuration known compounds (9a, 9b), which were prepared by the following reported sequence: Ehrlich G, Kalesse M. Synlett. 2005;4:655. Detailed information is provided in supporting information. For the stereoselective epoxidation of homoallylic alcohols, see Mihelich ED, Daniels K, Eickhoff DJ. J Am Chem Soc. 1981;103:7690.
- 11.Kende AS, Toder BH. J Org Chem. 1982;47:167. [Google Scholar]
- 12.To explain these enantioselectivities, as well as those of kinetic resolutions, we proposed a possible model of the asymmetric epoxidation of homoallylic alcohol catalyzed by the complex of vanadium and ligand 1d, which is provided in the supporting information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative experimental procedures and spectral data for 1c–1d. This material is available free of charge via the Internet at (http://pubs.acs.org/instruct/jacsat.pdf).