Abstract
Many cyanobacterial strains are able to grow at a pH range from neutral to pH 10 or 11. Such alkaline conditions favor cyanobacterial growth (e.g., bloom formation), and cyanobacteria must have developed strategies to adjust to changes in CO2 concentration and ion availability. Synechocystis sp. strain PCC 6803 exhibits similar photoautotrophic growth characteristics at pH 10 and pH 7.5, and we examined global gene expression following transfer from pH 7.5 to pH 10 to determine cellular adaptations at an elevated pH. The strategies used to develop homeostasis at alkaline pH had elements similar to those of many bacteria, as well as components unique to phototrophic microbes. Some of the response mechanisms previously identified in other bacteria included upregulation of Na+/H+ antiporters, deaminases, and ATP synthase. In addition, upregulated genes encoded transporters with the potential to contribute to osmotic, pH, and ion homeostasis (e.g., a water channel protein, a large-conductance mechanosensitive channel, a putative anion efflux transporter, a hexose/proton symporter, and ABC transporters of unidentified substrates). Transcriptional changes specific to photosynthetic microbes involved NADH dehydrogenases and CO2 fixation. The pH transition altered the CO2/HCO3− ratio within the cell, and the upregulation of three inducible bicarbonate transporters (BCT1, SbtA, and NDH-1S) likely reflected a response to this perturbed ratio. Consistent with this was increased transcript abundance of genes encoding carboxysome structural proteins and carbonic anhydrase. Interestingly, the transition to pH 10 resulted in increased abundance of transcripts of photosystem II genes encoding extrinsic and low-molecular-weight polypeptides, although there was little change in photosystem I gene transcripts.
Cyanobacteria are among the most alkaliphilic microbes, and they frequently dominate alkaline environments, such as soda lakes and microbial mats (30, 36). In addition to pH, key parameters that include nutrient availability and temperature influence the population composition of phytoplankton communities (18). However, cyanobacterial bloom formation is usually accompanied by an elevated pH that results from increased photosynthesis that depletes CO2. Many cyanobacterial strains are alkali tolerant and grow at pHs ranging from neutral to 10 to 11, so that cyanobacteria both generate and thrive in alkaline conditions. There have been numerous reports of habitats where photosynthetic rates are high (such as shallow lakes), pH values exceed pH 10, and cyanobacteria become the major phytoplankton species (7, 27). Such populations of cyanobacteria are frequently associated with the production of a range of secondary metabolites, including nuisance and toxic compounds (5). This has led to experiments aimed at reducing cyanobacterial populations; e.g., it has been established for a long time that adding carbon dioxide or acid to lower the pH of lake samples can increase the abundance of green algae relative to that of cyanobacteria (42).
One reason that cyanobacteria have an advantage over other phytoplankton species at high pH is that the carbon-concentrating mechanism of cyanobacteria is better able to utilize bicarbonate than the mechanism in green algae (21). It is anticipated that cyanobacteria must employ additional mechanisms to maintain pH homeostasis in order to flourish at high pH. Many nonphotosynthetic bacteria are able to survive or grow at alkaline pH, and they respond to increased pH using a variety of mechanisms to maintain homeostasis within the cell; the best characterized of these strategies is the increased expression and activity of monovalent cation/proton antiporters (33). These transmembrane proteins maintain the intracellular pH through the uptake of protons, utilizing outward monovalent cation gradients. Multiple cation/proton antiporters have been identified in cyanobacteria, and their involvement in pH homeostasis has been suggested by gene knockout studies with different strains exhibiting altered pH- and NaCl-sensitive phenotypes (3, 14, 57). Additional bacterial responses aimed at regulating intracellular pH include elevated metabolic acid production (via amino acid deaminases and sugar fermentation), increased ATP synthase activity (H+ entry coupled to ATP generation), and altered cell surface properties (33, 52). However, the extent to which these strategies are employed and their impact in different bacterial strains remain to be determined.
Compared to other alkaliphilic bacteria, cyanobacteria have two additional complexities, photosynthetic (thylakoid) membranes and the presence of ATP synthase in both thylakoid and plasma membranes (43). Compartments within the cell are maintained at different pHs, and the thylakoid lumen has a pH that is ∼2 units lower than the pH of the cytosol (2). Changes in the external pH have been shown to alter both the cytoplasmic and thylakoid lumen pHs, with an increase in the external pH of 2 pH units resulting in an increase of ∼0.2 pH unit (2, 40). Therefore, growth of cyanobacteria in alkaline environments requires maintenance of pH gradients across multiple membrane systems, regulation of inorganic carbon uptake, and adjustment to changes in the abundance of different ions.
We investigated the impact of a pH transition from pH 7.5 to pH 10 in Synechocystis sp. strain PCC 6803. This freshwater cyanobacterium is a halo- and alkali-tolerant strain which exhibits similar growth at pH 7.5 and pH 10 (8). Six genes have been annotated as genes that encode sodium/proton antiporters in Synechocystis sp. strain PCC 6803 (19). It is likely that these proteins have overlapping functions, and this has made it difficult to define their role in pH homeostasis (59). In addition, Synechocystis sp. strain PCC 6803 accumulates acetolactate under alkaline conditions, and it has been suggested that this is a mechanism for pH homeostasis (28).
The importance of external pH has been demonstrated in Synechocystis sp. strain PCC 6803 by identification of a number of pH-sensitive photosystem II (PSII) mutants that are able to grow photoautotrophically at pH 10 but not at pH 7.5 (8, 54). Each of these pH-sensitive strains contains two mutations in PSII, including the absence of either the PsbO or PsbV luminal protein. The cellular adaptations that occur during changes in the external pH that make the differential growth possible have not been identified. We examined global gene expression in Synechocystis sp. strain PCC 6803 following a transition to high pH by establishing a time course to identify genes that showed pH-dependent expression at 1 h (t1), 2 h (t2), and 6 h (t6) following transfer from pH 7.5 to pH 10. This study revealed that the response of Synechocystis sp. strain PCC 6803 to alkaline conditions was cell-wide and included strategies typical of many bacteria, as well as strategies specific to phototrophic microbes. The levels of transcripts of a number of the components involved in acclimation to alkaline pH in other bacteria, such as monovalent cation/proton antiporters and ATP synthase, were elevated at pH 10. We observed increased abundance of transcripts of additional transporters with the potential to contribute to osmotic, pH, and ion homeostasis. Changes specific to photosynthesis included the upregulation of genes encoding three bicarbonate transport systems, probably in response to a perturbed CO2/HCO3− ratio within the cell. Consistent with this was increased abundance of transcripts of genes encoding carboxysome structural proteins and carbonic anhydrase. The transcripts of a number of genes encoding transcriptional regulators were differentially regulated at pH 10. Furthermore, we observed that at an elevated pH, the levels of transcripts of genes encoding PSII extrinsic and low-molecular-weight polypeptides were increased.
MATERIALS AND METHODS
Growth conditions.
The glucose-tolerant organism Synechocystis sp. strain PCC 6803 (60) was grown at 30 ± 2°C using cool white fluorescent light at an intensity of ∼30 microeinsteins m−2 s−1 with shaking at 125 rpm in BG-11 medium (4). The pH of the BG-11 medium was maintained by addition of either 25 mM HEPES (pH 7.5) or 25 mM 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS) (pH 10) (8). The cell densities of the cultures were determined by measuring the optical density at 750 nm (OD750) as previously described (6, 29).
RNA isolation.
Total RNA was extracted and purified using phenol-chloroform extraction and CsCl gradient purification as previously described (39, 48).
Microarray design and analysis.
The microarray platform used and construction of this platform were described previously by Postier et al. (38), and the cDNA labeling, prehybridization, and hybridization protocols were described in detail by Singh et al. (47). The microarray experiment involved a loop design that allowed comparison of Synechocystis sp. strain PCC 6803 at different time points following a transition from pH 7.5 to pH 10 by using an analysis of variance model (26, 47). Cells were grown in BG-11 medium at pH 7.5 until the OD750 was ∼0.2 (approximately 8 × 107 cells/ml) before they were harvested by centrifugation (5,000 × g for 5 min) and transferred to pH 10 at an OD750 of ∼0.2. Samples were collected immediately after transfer to pH 10 (t0) and at t1, t2, and t6 after transfer.
Data acquisition and analysis were performed as described by Singh et al. (47); this included an analysis of variance model approach to test the null hypothesis that a particular gene's expression level was not different over time, and a P value was calculated. We used a false discovery rate (FDR) of 5% to control the proportion of significant results that were type I errors (false rejection of the null hypothesis) as described previously (55). Genes that had an FDR of 0.05 (corresponding to 5% expected false positives) and that exhibited a change of at least 1.5-fold were considered interesting and retained for further analysis. The P values for these genes ranged from 6.0 × 10−3 to 1.4 × 10−13.
RESULTS AND DISCUSSION
Global transcriptional response to the transition from pH 7.5 to pH 10.
Approximately 7, 12, and 10% of the chromosomal genes were differentially regulated at t1, t2, and t6 after transfer from pH 7.5 to pH 10, respectively. Genes were divided into functional categories according to Cyanobase (http://bacteria.kazusa.or.jp/cyanobase), and the number of differentially expressed genes in each category is shown in Table 1. Excluding hypothetical and unknown genes, photosynthesis and respiration was the category with the largest number of differentially regulated genes following transfer to pH 10, and these genes were almost all upregulated. Other categories with elevated levels of transcripts after transfer to pH 10 included proteins with regulatory functions and transport and binding proteins (Table 1).
TABLE 1.
Functional categories of genes differentially regulated at pH 10 compared to pH 7.5 in Synechocystis sp. strain PCC 6803a
| General pathway | No. of genesb | No. of differentially regulated genes (no. of genes upregulated)c
|
|||
|---|---|---|---|---|---|
| pH independent (t2/t0)d | pH 10 compared to pH 7.5
|
||||
| t1/t0 | t2/t0 | t6/t0 | |||
| Amino acid biosynthesis | 97 | 6 (5) | 6 (6) | 10 (9) | 9 (8) |
| Biosynthesis of cofactors, prosthetic groups, and carriers | 124 | 11 (11) | 10 (6) | 14 (8) | 11 (8) |
| Cell envelope | 67 | 2 (1) | 2 (1) | 4 (1) | 3 (2) |
| Cellular processes | 76 | 5 (4) | 7 (6) | 8 (8) | 4 (4) |
| Central intermediary metabolism | 31 | 4 (3) | 0 (0) | 1 (1) | 1 (1) |
| DNA replication, restriction, recombination, and repair | 60 | 0 (0) | 4 (4) | 11 (4) | 9 (2) |
| Energy metabolism | 132 | 11 (10) | 14 (10) | 13 (7) | 15 (12) |
| Hypothetical | 1,076 | 40 (22) | 73 (37) | 130 (71) | 96 (62) |
| Other categories | 175e | 10 (8) | 15 (7) | 28 (8) | 27 (11) |
| Photosynthesis and respiration | 141 | 23 (23) | 23 (22) | 30 (28) | 34 (31) |
| Purines, pyrimidines, nucleosides, and nucleotides | 41 | 3 (3) | 2 (1) | 4 (2) | 5 (2) |
| Regulatory functions | 146 | 4 (2) | 14 (13) | 18 (13) | 19 (15) |
| Transcription | 30 | 3 (3) | 2 (2) | 2 (1) | 3 (2) |
| Translation | 168 | 51 (51) | 12 (11) | 15 (12) | 15 (13) |
| Transport and binding proteins | 196 | 3 (3) | 13 (10) | 19 (10) | 20 (15) |
| Unknown | 474 | 22 (9) | 19 (4) | 63 (15) | 45 (17) |
| Total | 3,165 | 198 (158) | 216 (140) | 370 (198) | 316 (205) |
Genes were considered differentially regulated when the FDR was 0.05 (change, >1.5-fold).
Total number of genes based on Kazusa annotation prior to May 2002.
The upregulated genes were the genes upregulated at pH 10 compared to pH 7.5.
Differentially regulated genes after transfer from pH 7.5 to pH 10 and after transfer from pH 10 to pH 7.5.
The number does not include genes encoding transposases.
pH-independent gene expression.
Genes that were upregulated both after transfer from pH 7.5 to pH 10 and after transfer from pH 10 to pH 7.5 were designated pH independent and are listed separately from the pH 10-responsive data set in Table 1. These genes were identified by combining data from the microarray experiment examining transfer from pH 7.5 to pH 10 with data from a similar experiment examining the transition from pH 10 to pH 7.5. There were 198 genes whose transcription was found to change independent of the direction of the pH transition, and >75% of these genes were upregulated after transfer. Upregulated genes encoding ribosomal proteins accounted for approximately one-quarter of the differentially expressed genes. Other categories with differentially expressed genes included photosynthesis and respiration, energy metabolism, and biosynthesis of cofactors, prosthetic groups, and carriers (Table 2). Increased abundance of the transcripts of a number of these genes, such as those encoding ribosomal proteins and ATP synthase, has been associated with favorable growth conditions, including light-versus-dark transition and log-phase growth versus stationary-phase growth (10, 53). However, the doubling times following the transition from pH 7.5 to pH 10 and the transition from pH 10 to pH 7.5 remained ∼12 h (data not shown). Additional pH-independent transcriptional changes likely include mechanisms to maintain cellular homeostasis and are discussed in more detail below.
TABLE 2.
Selected differentially regulated pH-independent genes in Synechocystis sp. strain PCC 6803 after transfer from pH 7.5 to pH 10 and after transfer from pH 10 to pH 7.5a
| Gene | Designation or function | Change (fold) after transfer to:
|
|||
|---|---|---|---|---|---|
| pH 10
|
pH 7.5 (t2) | ||||
| t1 | t2 | t6 | |||
| Photosynthesis and respiration | |||||
| ATP synthase | |||||
| sll1321 | Hypothetical | 1.7 | 1.4 | 1.4 | 3.0 |
| sll1322 | atpI | 1.3 | 1.2 | 1.2 | 2.9 |
| ssl2615 | atpH | 2.1 | 1.8 | 1.8 | 3.7 |
| sll1323 | atpG | 2.1 | 1.7 | 1.7 | 4.7 |
| sll1324 | atpF | 2.5 | 2.1 | 2.0 | 5.5 |
| sll1325 | atpD | 3.1 | 2.8 | 2.6 | 4.5 |
| sll1326 | atpA | 2.0 | 1.8 | 1.7 | 3.1 |
| sll1327 | atpC | 1.9 | 1.6 | 1.6 | 3.6 |
| slr1329 | atpB | 2.1 | 1.7 | 1.8 | 2.6 |
| slr1330 | atpE | 3.0 | 2.5 | 2.4 | 3.1 |
| CO2 fixation: carboxysome | |||||
| sll1028 | ccmK2 | 2.0 | 2.0 | 2.1 | 2.4 |
| sll1029 | ccmK1 | 2.4 | 2.4 | 2.6 | 2.2 |
| sll1030 | ccmL | 1.8 | 1.8 | 2.3 | 2.4 |
| sll1031 | ccmM | 2.3 | 1.9 | 2.2 | 2.1 |
| sll1032 | ccmN | 1.7 | 1.4 | 1.9 | 1.9 |
| slr1838 | ccmK3 | 1.3 | 1.8 | 1.8 | 1.3 |
| slr1839 | ccmK4 | 1.3 | 1.7 | 1.4 | 1.6 |
| NADH dehydrogenase: bicarbonate transport | |||||
| sll1732 | ndhF3 | 2.1 | 1.4 | 3.7 | 1.3 |
| sll1733 | ndhD3 | 2.8 | 2.1 | 4.6 | 1.8 |
| sll1734 | cupA | 3.3 | 2.7 | 5.3 | 2.9 |
| sll1735 | Hypothetical | 2.0 | 1.6 | 3.8 | 3.2 |
| Regulatory functions | |||||
| slr0473 | cph1 | −2.4 | −1.9 | −1.9 | −1.8 |
| slr0474 | rcp1 | −4.6 | −3.6 | −3.4 | −2.3 |
| Transport proteins: bicarbonate | |||||
| slr0040 | cmpA | 1.4 | 1.1 | 5.3 | 2.9 |
| slr0041 | cmpB | 1.4 | 1.2 | 9.4 | 2.8 |
| slr0043 | cmpC | 1.5 | 1.4 | 2.4 | 1.6 |
| slr0044 | cmpD | 1.3 | 1.5 | 3.3 | 2.0 |
| slr1512 | sbtA | 2.9 | 2.1 | 5.5 | 3.7 |
| slr1513 | sbtB | 2.5 | 2.1 | 6.5 | 2.9 |
| Gene clusters | |||||
| slr1667 | Hypothetical | −1.8 | −2.5 | −1.7 | −5.8 |
| slr1668 | Hypothetical | −1.5 | −1.7 | −1.3 | −1.5 |
| sll1077 | speB2 | 1.7 | 2.2 | −1.1 | 2.2 |
| sll1078 | hypA2 | 2.2 | 3.5 | −1.1 | 1.6 |
| sll1079 | hypB2 | 2.5 | 3.9 | −1.4 | 1.6 |
| sll1080 | Transport | 2.3 | 2.7 | −1.1 | −1.4 |
| sll1081 | Transport | 1.8 | 2.4 | −1.3 | NDb |
| sll1082 | Transport | 1.4 | 1.9 | −1.2 | −1.1 |
Genes were considered differentially regulated when the FDR was 0.05 (change, >1.5 fold) (indicated by bold type).
ND, not determined.
Photosynthesis and respiration.
Cyanobacterial NADH dehydrogenase complexes (NDH-1) are composed of a multiprotein core (NDH-1M) and NdhD and NdhF subunits. Different NdhD and NdhF subunits determine whether an NDH-1 complex functions in cyclic electron transfer around PSI and respiratory electron transfer or CO2 uptake (1). Genes encoding 7 of the 13 NDH-1M core proteins, including ndhD2,were upregulated at pH 10 (Table 3). The protein encoded by ndhD2 is hypothesized to associate with the NDH-1M core along with NdhF1 to form the NDH-1L′ complex, and gene knockout studies indicated that this subunit is involved in PSI cyclic electron transfer (32). The NdhD3 and NdhF3 subunits, together with the proteins designated CupA and CupS, associate with NDH-1M to form a low-CO2-inducible transporter (Sll1732 to Sll1735) (62). The operon consisting of sll1732 to sll1735 was upregulated both after transfer to pH 10 and after transfer to pH 7.5 (Table 2).
TABLE 3.
Selected differentially regulated genes in Synechocystis sp. strain PCC 6803 after transition from pH 7.5 to pH 10a
| Gene | Designation or function | Change (fold) at:
|
||
|---|---|---|---|---|
| t1 | t2 | t6 | ||
| Cellular processes: chaperones | ||||
| sll1514 | hspA | 1.4 | 1.9 | 1.4 |
| sll0058 | dnaK1 | 1.7 | 1.7 | 1.7 |
| sll0430 | htpG | 1.9 | 1.5 | 1.3 |
| Photosynthesis and respiration | ||||
| PSII | ||||
| Oxygen-evolving complex | ||||
| sll0427 | psbO | 1.5 | 1.9 | 1.7 |
| sll1194 | psbU | 1.5 | 1.6 | 1.7 |
| sll1418 | psbP | 1.4 | 1.5 | 1.6 |
| sll1638 | psbQ | 1.6 | 2.1 | 1.7 |
| Low-molecular-mass polypeptides | ||||
| sml0002 | psbX | 2.4 | 2.2 | 2.1 |
| sml0003 | psbM | 1.2 | 1.5 | 1.5 |
| sml0005 | psbK | 1.3 | 1.5 | 1.7 |
| ssr3451 | psbE | 1.4 | 1.8 | 1.5 |
| smr0006 | psbF | 1.4 | 1.9 | 1.6 |
| smr0007 | psbL | 1.2 | 1.7 | 1.6 |
| smr0008 | psbJ | 1.3 | 2.2 | 1.9 |
| Putative assembly protein | ||||
| sll1414 | psb29 | 1.4 | 1.8 | 1.8 |
| NADH dehydrogenase: core subunits | ||||
| sll0223 | ndhB | 1.6 | 1.4 | 1.7 |
| sll0520 | ndhI | 2.0 | 1.8 | 2.3 |
| sll0521 | ndhG | 1.8 | 2.0 | 2.3 |
| sll0522 | ndhE | 1.6 | 1.9 | 1.9 |
| slr1279 | ndhC | 1.6 | 1.5 | 1.7 |
| slr1280 | ndhK | 2.0 | 1.8 | 2.1 |
| slr1281 | ndhJ | 1.6 | 1.4 | 2.0 |
| slr1291 | ndhD2 | 2.5 | 1.6 | 1.8 |
| CO2 fixation | ||||
| slr1347 | cab | 1.6 | 1.6 | 1.5 |
| slr0436 | ccmO | 1.5 | 1.6 | 1.8 |
| sll0934 | ccmA | 1.2 | 1.5 | −1.1 |
| Regulatory functions | ||||
| sll0567 | fur | 1.6 | 1.7 | 1.6 |
| sll1937 | fur | 2.9 | 2.7 | 2.8 |
| sll1423 | ntcA | 1.2 | 1.5 | 1.8 |
| sll0594 | Tregc | 1.5 | 1.7 | 1.6 |
| sll0782 | Treg | 1.6 | 1.9 | 1.6 |
| slr0599 | spkC | 1.3 | 1.6 | 1.6 |
| slr0210 | hik9 | 1.4 | 1.9 | 1.7 |
| sll1672 | hik12 | 1.5 | 1.2 | 1.9 |
| slr1042 | rre7 | 1.7 | 1.5 | 2.7 |
| slr1594 | rre5 | 1.8 | 1.2 | −1.2 |
| Transport and binding proteins | ||||
| sll0689 | nhaS3 | 2.1 | 1.4 | 2.0 |
| sll0771 | glcP | 1.7 | 1.5 | 1.5 |
| slr0753 | Transport | 1.3 | 1.3 | 2.2 |
| slr0875 | mscL | 1.4 | 1.5 | 1.7 |
| slr2057 | apqZ | 1.9 | 2.2 | 2.9 |
| Gene cluster | ||||
| Putative cation/H+ antiporter | ||||
| slr2006 | mrpC | 1.8 | 1.2 | 1.9 |
| slr2007 | mrpD | 2.0 | 1.7 | 2.3 |
| slr2008 | mrpC | 1.6 | 1.6 | 1.8 |
| slr2009 | mrpD | 1.7 | 1.6 | 1.7 |
| slr2010 | mrpE | 2.3 | 1.8 | 1.8 |
| ssr3409 | mrpF | 1.4 | 1.4 | 1.5 |
| ssr3410 | mrpG | 1.8 | 1.7 | 1.7 |
| slr2011 | mrpA | 2.1 | 1.7 | 1.9 |
| slr2012 | mrpB | 1.7 | 1.4 | 1.5 |
| slr1501 | Other | 3.9 | 3.9 | 6.4 |
| slr1113 | Transport | 1.4 | 1.4 | 2.O |
| slr1114 | Hypothetical | 1.3 | 1.3 | 1.6 |
| sll1392 | Treg | 1.3 | 1.6 | 1.8 |
| slr0408 | Unknown | −1.4 | −2.1 | −1.9 |
| slr0142 | Hypothetical | NDb | ND | ND |
| slr0143 | hat | ND | ND | ND |
| slr0144 | 4VRd | ND | ND | ND |
| slr0145 | Unknown | −1.5 | 1.4 | 1.4 |
| slr0146 | Hypothetical | −2.4 | −1.2 | −1.1 |
| slr0147 | 4VR | −1.9 | −1.0 | −1.1 |
| slr0148 | Hypothetical | −2.6 | −2.0 | −2.0 |
| slr0149 | Hypothetical | −2.7 | −2.2 | −2.1 |
| slr0150 | petF | −1.8 | −1.6 | −1.6 |
| slr0151 | Unknown | −2.1 | −3.9 | −3.5 |
Genes were considered differentially regulated when the FDR was 0.05 (change, >1.5-fold) (indicated by bold type).
ND, not determined.
Treg, transcriptional regulator.
4VR, 4-vinyl reductase.
The transcript encoding a β-type carbonic anhydrase (slr1347) was upregulated after transfer to pH 10 (Table 3). This protein is located in the carboxysome, where it catalyzes the conversion of HCO3− to CO2 and both activates ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and compensates for the low affinity of Rubisco for CO2 (21, 51). The assembly and structural protein components of the carboxysome are encoded by the carbon-concentrating mechanism (ccm) genes; the abundance of the transcripts was increased after transfer to pH 10, and the abundance of many of them was increased after transfer to pH 7.5 (Table 2).
The genes encoding the PSII extrinsic proteins PsbO, PsbP, PsbQ, and PsbU were upregulated at pH 10. In addition, the genes encoding seven low-molecular-weight proteins implicated in assembly or stability of PSII centers were upregulated at pH 10 (44). These genes comprised the psbEFLJ operon, whose genes encode the cytochrome b559 α and β subunits, PsbL, and PsbJ, respectively, and the psbK, psbM, and psbX genes. Increased abundance was observed for the sll1414 transcript encoding the PSII-associated protein Psb29, which is thought to be involved in PSII biogenesis (22). There was no change in the abundance of transcripts for the core proteins D1, D2, CP47, and CP43, and the genes encoding these proteins were highly expressed at both pH 7.5 and pH 10. This is consistent with the similar oxygen evolution rates and PSII abundance observed at pH 10 and pH 7.5 (54).
ATP synthase genes were upregulated after transfer to both pH 7.5 and pH 10 (Table 2). As ATP synthase is associated with both the thylakoid and plasma membranes, the pH regulation of this complex may be different from that observed for nonphotosynthetic bacteria.
Regulatory genes.
Genes encoding 21 regulatory components were upregulated after transfer to pH 10, including genes that had different transcriptional kinetics (Table 3; see Table S1 in the supplemental material). Following transfer to pH 10, increased abundance was observed for the transcripts of two-component system histidine kinases (hik9 and hik12) and response regulators (rre7 and rre5), a serine/threonine kinase, the global nitrogen regulator, ntcA, and several putative transcription factors (Table 3). The response regulator rre5 gene was upregulated under inorganic carbon (Ci) limitation conditions and was suggested previously to be involved in CO2 uptake and pH homeostasis (58). In addition, sll1937 and sll0567, encoding putative ferric uptake regulation (Fur) proteins, were upregulated at pH 10 from t1 to t6 (1.7- to 2.9-fold). Fur proteins are metal ion uptake regulators, and the sll0567 product is essential for growth under normal culture conditions and is part of the iron-responsive regulation mechanism (25). The role of sll1937 is not clear, as deletion of this gene did not alter iron-stress-induced gene expression (25). Transcripts of a histidine kinase and response regulator (cph1/rcp1) exhibited strong pH-independent downregulation (Table 2). These transcripts are upregulated in the dark and are thought to be involved in regulation at light-dark transitions (49).
Stress response.
The slr1516 gene, encoding superoxide dismutase (sodB), was upregulated at pH 10 (1.5-fold). Expression of this gene, which encodes an antioxidant, is induced by various stress conditions, including temperature (high or low), salt, hydrogen peroxide, and light (57). Superoxide dismutase converts reactive oxygen species to hydrogen peroxide, which then is scavenged by catalases or peroxidases or both. The slr1992 gene, encoding glutathione peroxidase, was upregulated at t1 through t6 (1.9- to 1.8-fold). Two genes encoding thioredoxin (slr0623 and slr1139) were upregulated at t2 and t6 (1.5- to 1.7-fold). The levels of the slr0623 transcript were high, consistent with the hypothesis that it encodes the most abundant of the four Synechocystis sp. strain PCC 6803 thioredoxins. Furthermore, Slr0623 has been suggested to have a major role in supplying reducing equivalents to the antioxidant systems (13). In addition, the genes encoding the chaperones HspA, DnaK1, and HtpG were upregulated at pH 10 (Table 3), and previous reports indicated that these genes are upregulated under various stress conditions, including oxidative stress (20, 26, 34, 45, 50). Two of these genes, hspA and htpG, were upregulated in a pseudorevertant of a PSII mutant, ΔPsbO:ΔPsbU. The original ΔPsbO:ΔPsbU strain is able to grow at pH 10, although it does not grow at pH 7.5, but the pseudorevertant was able to grow at both pH 10 and pH 7.5 (53).
Monovalent cation/proton antiporters.
Six genes have been annotated as genes encoding Na+/H+ antiporters in Synechocystis sp. strain PCC 6803, including sll0689 (nhaS3), whose transcript level was increased twofold at pH 10 (Table 3). Unlike four of the Na+/H+ antiporters, sll0689 appeared to be essential for cell viability, as mutants lacking this gene could not be fully segregated (16, 59). Moreover, the partially segregated ΔSll0689 strain was sensitive to high-salt conditions at pH 9, and Sll0689 has a high affinity for both Na+ and Li+ ions (16, 59).
The eight-gene cluster containing slr2006 to slr2012 (including ssr3410) was upregulated 1.7- to 2.3-fold within 1 h at pH 10, and the transcript level remained elevated at 6 h (Table 3). Two of these genes were annotated as genes encoding NDH subunits; however, this cluster has similarity to genes encoding a putative multiprotein cation/H+ antiporter in Anabaena sp. strain PCC 7120 (3). Interruption of one of these genes in Anabaena sp. strain PCC 7120 resulted in a strain that exhibited retarded growth at elevated pH and enhanced salt sensitivity at pH 10.5 (3). Blanco-Rivero et al. (3) designated this cluster mrp (multiple resistance and pH adaptation) due to similarity to a Bacillus subtilis mrp operon involved in Na+ resistance, particularly at high pH (17). The similarity between subunits of the Mrp and NDH complexes has been described previously and may reflect a common origin and similar functions of the two complexes (12).
Other transporters.
The transfer to pH 10 resulted in differential regulation of a number of transporters that may contribute to osmotic, pH, and ion homeostasis (Table 3). This differential regulation included upregulation (1.9- to 2.9-fold) of the slr2057 transcript encoding a water channel protein (ApqZ). Water channels allow bidirectional movement of water and often highly specific movement of other compounds, although they are usually impermeable to ions (41). In cyanobacteria these channels have been postulated to have a role in CO2 uptake (56). A ΔSlr2057 strain exhibited altered cell shrinkage and altered gene expression under hyperosmotic stress conditions (41). Another channel protein that is responsive to osmotic shock had elevated transcript levels at pH 10. The gene (slr0875) encoded a high-conductance mechanosensitive channel; such channels are present in bacterial membranes and open in response to stretch forces in the lipid bilayer, preventing cell lysis (23). In addition, activation of Slr0875 was shown to result in Ca2+ release (31). Increased abundance of the transcript for this channel may be part of a response directed at maintaining ion homeostasis at high pH. The same may be true of the slr0753 gene, encoding a putative anion efflux transporter, which was upregulated >2-fold following 6 h at pH 10. Mutations in this gene removed the chloride requirement of a PSII strain lacking PsbV, suggesting that the gene encodes a chloride extrusion protein (24). Finally, the gene encoding a glucose transporter (glcP) was upregulated at pH 10. This transporter has been suggested to be a hexose/proton symporter and therefore may be involved in pH homeostasis (9).
In addition to the NDH-1-associated bicarbonate transport, two low-Ci-inducible bicarbonate uptake systems were upregulated after transfer to both pH 10 and pH 7.5. These systems are BCT1, encoded by the cmp operon, and SbtA, plus the neighboring gene sbtB. Changes in the external pH have been shown to alter the cytosolic and thylakoid lumen pH (2); thus, the transfer of cells to a different pH alters the CO2/HCO3− ratio within the cell. The upregulation of three inducible bicarbonate transporters (BCT1, SbtA, and NDH-1S) after transfer to pH 7.5 and pH 10 may reflect a response to this perturbed ratio.
Metabolic acid production.
Deaminases have been suggested to play a role in acclimation to alkaline pH in some bacteria, and this appears to be true in Synechocystis sp. strain PCC 6803 as well. Upon transfer to pH 10, the level of the transcript encoding l-threonine deaminase (slr2072) was increased. In addition, a number of genes involved in the biosynthesis of valine, leucine, and isoleucine were upregulated (see Table S1 in the supplemental material). These genes included the gene encoding acetolactate synthase, ilvB (sll1981), which may play a role in pH homeostasis (26).
Gene clusters.
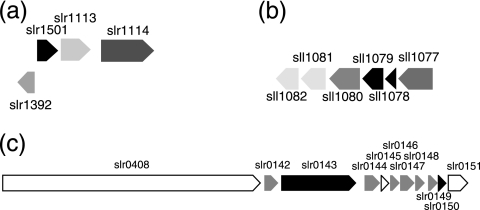
The slr1501 gene was upregulated after transfer to pH 10 and was rapidly downregulated after transfer to pH 7.5. The adjacent gene sll1392 was similarly regulated, suggesting that there may be a divergent promoter. In addition, two genes downstream of slr1501 (slr1113 and slr1114) were upregulated at pH 10, and slr1113 was also downregulated after transfer to pH 7.5 (Fig. 1a and Table 3). The designations of these genes in Cyanobase are as follows: probable acetyltransferase gene, slr1501; transcriptional regulator gene, sll1392; ABC transporter ATP-binding protein gene, slr1113; and permease gene, slr1114. The accumulation of these transcripts at pH 10 and their striking downregulation at pH 7.5 suggest that this gene cluster has a role in growth at high pH.
FIG. 1.
Coordinately regulated gene clusters of Synechocystis sp. strain PCC 6803. (a) Gene cluster upregulated at pH 10 compared to pH 7.5. (b) Gene cluster showing pH-independent regulation. (c) Gene cluster containing many genes that exhibit upregulation after transfer to pH 10.
The cluster containing genes slr0145 to slr0151 was downregulated at pH 10 (Fig. 1c and Table 3). Both slr0148 and slr0150 encode ferredoxinlike proteins, and slr0150 has been shown to be downregulated following high-light treatment (37). Two genes in this cluster (slr0144 and slr0147) contain 4-vinyl reductase motifs predicted to be involved in small-molecule binding (no data were available for slr0144 from this experiment due to a high P value). Another two genes (slr0146 and slr0149) encode proteins containing bilin-binding domains. This cluster was previously shown to be downregulated in iron-deficient media and in the presence of hydrogen peroxide, and it was suggested that the proteins may be involved in PSI function and assembly (46). This function would be consistent with the downregulation of this cluster and the upregulation of a number of PSII genes at pH 10. The slr0408 gene is upstream of this cluster and was upregulated at pH 10 (Fig. 1c and Table 3). This large gene encodes an unknown protein with a putative Ca2+ expulsion domain.
The slr1667 and slr1668 genes encoding a hypothetical protein and an unknown protein, respectively, were downregulated after transfer to pH 10 and pH 7.5 (Table 2). These genes may be regulated through 3′,5′-cyclic AMP (cAMP) signaling as the levels of both transcripts were decreased in a strain lacking a cAMP receptor protein encoded by sycrp1 (sll1371) (61). In addition, the adenylyl cyclase Cya1 (Slr1991), which synthesizes cAMP, is activated by CO2 (11). This raises the possibility that Cya1 may sense the altered HCO3−/CO2 ratio, resulting in the downregulation of slr1667 and slr1668.
Genes in the cluster consisting of sll1077 to sll1082 were upregulated for the first 2 h of the transition to pH 10, and sll1077, sll1078, and sll1079 were upregulated after transfer to pH 7.5 (Fig. 1b and Table 2). The sll1077 gene product is annotated as an agmatinase (EC 3.5.3.11), an enzyme that catalyzes the conversion of agmatine to putrescine and urea. The polyamine putrescine forms a necessary component of the outer membrane of some gram-negative bacteria during biofilm formation (35). The sll1078 and sll1079 genes were annotated as genes encoding hydrogenase formation proteins. However, deletion of these proteins did not alter hydrogenase activity, leading to the suggestion that they are metallochaperones of the protein encoded by sll1077 and not hydrogenases (14). The sll1080, sll1081, and sll1082 genes are predicted to encode the substrate-binding, permease, and ATP-binding subunits of an ABC transporter, respectively. The coordinated regulation of genes sll1077 to sll1082 at pH 10 suggests that this transporter may be involved in putrescine transport to the outer membrane.
Validation of microarray results.
The microarray data were validated by semiquantitative reverse transcription-PCR, and good correspondence was observed for all genes examined (see Fig. S1 in the supplemental material).
Summary.
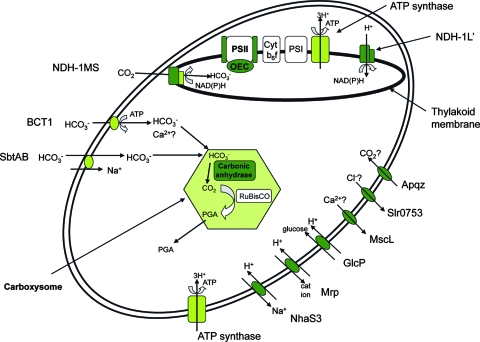
Figure 2 illustrates the upregulation of transcripts encoding structural proteins that may be involved in the maintenance of cellular homeostasis following a pH transition. The response of Synechocystis sp. strain PCC 6803 to high pH had similarities to the response reported for other bacteria. This included the pH 10 upregulation of genes encoding two cation/H+ antiporters (NhaS3 and Mrp), ATP synthase (also upregulated after transfer to pH 7.5), and at least one amino acid deaminase. One important difference between the response of Synechocystis sp. strain PCC 6803 and the response of other bacteria may be related to the lack of transcript level changes for genes involved in the cell surface; however, the cluster containing the agmatinase gene was upregulated, indicating that the cell may produce polyamines destined for the outer membrane. Furthermore, the cell envelope of cyanobacteria has characteristics associated with both gram-negative and gram-positive bacteria, as well as cyanobacterium-specific characteristics, which result in distinct cell wall properties (15). In addition to the general bacterial response, we identified other transporters that may be involved in maintaining pH and ion homeostasis following a transition to pH 10. These include a putative chloride extrusion protein (Slr0753), a mechanosensitive channel that may also act as a calcium channel (MscL), a hexose/proton symporter (GlcP) (Fig. 2), and several ABC transporter subunits for unidentified substrates that are not shown in Fig. 2. A major result of these changes is that they permit the cell to more readily dissipate a buildup of protons in the cytoplasm.
FIG. 2.
Model of a Synechocystis sp. strain PCC 6803 cell showing the transcriptional response to pH change. Genes upregulated after transfer to pH 10 are indicated by dark green, and genes upregulated after transfer to both pH 10 and pH 7.5 are indicated by light green. Genes that are not differentially regulated are indicated by open boxes (e.g., Rubisco). The upregulated genes include the genes that encode two cation/proton antiporters (NhaS3 and Mrp), a putative chloride extrusion protein (Slr0753), a mechanosensitive channel that may also act as a calcium channel (MscL), a hexose/proton symporter (GlcP), and a water channel (ApqZ). In addition, genes encoding subunits of NADH dehydrogenase (NDH) and PSII, including the oxygen evolving center (OEC), are upregulated. PGA, phosphoglyceric acid; Cyt b6f, cytochrome b6 f complex.
The most important cyanobacterium-specific findings were related to transcriptional changes involved in the maintenance of photosynthetic capability. This study focused on NADH dehydrogenases and the carbon-concentrating mechanism, including the genes encoding carboxysome components and carbonic anhydrase (Fig. 2). In cyanobacteria, changes in the external pH alter the intracellular pH, and an increase in the external pH of 2 pH units results in an increase of ∼0.2 pH unit in both the cytosol and the thylakoid lumen (2). Such changes alter the CO2/HCO3− ratio within the cell, and regulation of CO2/HCO3− concentration is essential for maintaining the carboxylase activity of Rubisco. The upregulation of three inducible bicarbonate transporters (BCT1, SbtA, and NDH-1S) and many of the transcripts encoding the structural components of the carboxysome after transfer to pH 7.5 and pH 10 may reflect a response to this perturbed ratio. The pH 10-specific regulation may be a response to increased external pH that decreases CO2 levels in the cell (e.g., upregulation of the transcripts encoding the carboxysome β-type carbonic anhydrase and the water channel protein [ApqZ] that has been implicated in CO2 import).
The transcriptional response to the transition from pH 7.5 to pH 10 was not the same as the response to Ci limitation. The levels of a number of genes that were upregulated under Ci limitation conditions were unchanged by the pH transition, and a number of genes that were downregulated by Ci were upregulated by the pH transition (e.g., many of the genes encoding low-molecular-weight PSII polypeptides [Table 3]) (58). The ATP synthase, β-type carbonic anhydrase, and ribosomal genes were downregulated at low Ci levels but upregulated at pH 10. In contrast, the flavoprotein-encoding transcripts slr0217 and slr0219 were upregulated at low Ci levels but were downregulated at high pH. These differences may reflect the decreased growth that was observed after transition to a low Ci level but that was not observed at high pH. The pH change had another impact specific to photosynthesis, namely, the pH 10 upregulation of genes encoding the extrinsic and low-molecular-weight intrinsic proteins of PSII, including the psbO and psbU transcripts. This was surprising as photoautotrophic growth of a ΔPsbO:ΔPsbU strain at pH 10 but not at pH 7.5 had been interpreted as indicating that PsbO and PsbU may be more important at lower pH. Furthermore, this upregulation was specific to the PSII genes, and very little change in the abundance of the PSI gene transcripts was observed. The mutant lacking luminal proteins may require quantitatively greater enhancement of some of the components highlighted in Fig. 2, and comparisons to examine this possibility will be the objective of future experiments.
Supplementary Material
Acknowledgments
This research was funded by grant DE-FG02-99ER20342 from the Department of Energy.
Footnotes
Published ahead of print on 7 July 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Battchikova, N., and E. M. Aro. 2007. Cyanobacterial NDH-1 complexes: multiplicity in function and subunit composition. Physiol. Plant. 131:22-32. [DOI] [PubMed] [Google Scholar]
- 2.Belkin, S., and L. Packer. 1988. Determination of pH gradients in intact cyanobacteria by electron spin resonance spectroscopy. Methods Enzymol. 167:677-685. [DOI] [PubMed] [Google Scholar]
- 3.Blanco-Rivero, A., F. Leganes, E. Fernandez-Valiente, P. Calle, and F. Fernandez-Pinas. 2005. mrpA, a gene with roles in resistance to Na+ and adaptation to alkaline pH in the cyanobacterium Anabaena sp. PCC7120. Microbiology 151:1671-1682. [DOI] [PubMed] [Google Scholar]
- 4.Castenholz, R. W. 1988. Culturing methods for cyanobacteria. Methods Enzymol. 167:68-93. [Google Scholar]
- 5.Codd, G. A., S. G. Bell, K. Kaya, C. J. Ward, K. A. Beattie, and J. S. Metcalf. 1999. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 34:405-415. [Google Scholar]
- 6.Colon-Lopez, M. S., D. M. Sherman, and L. A. Sherman. 1997. Transcriptional and translational regulation of nitrogenase in light-dark- and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 179:4319-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Figueiredo, D. R., M. J. Pereira, A. Moura, L. Silva, S. Barrios, F. Fonseca, I. Henriques, and A. Correia. 2007. Bacterial community composition over a dry winter in meso- and eutrophic Portuguese water bodies. FEMS Microbiol. Ecol. 59:638-650. [DOI] [PubMed] [Google Scholar]
- 8.Eaton-Rye, J. J., J. A. Shand, and W. S. Nicoll. 2003. pH-dependent photoautotrophic growth of specific photosystem II mutants lacking lumenal extrinsic polypeptides in Synechocystis PCC 6803. FEBS Lett. 543:148-153. [DOI] [PubMed] [Google Scholar]
- 9.Flores, E., and G. Schmetterer. 1986. Interaction of fructose with the glucose permease of the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 166:693-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, J. S., A. K. Singh, L. J. Rothschild, and L. A. Sherman. 2007. Growth-phase dependent differential gene expression in Synechocystis sp. strain PCC 6803 and regulation by a group 2 sigma factor. Arch. Microbiol. 187:265-279. [DOI] [PubMed] [Google Scholar]
- 11.Hammer, A., D. R. W. Hodgson, and M. J. Cann. 2006. Regulation of prokaryotic adenylyl cyclases by CO2. Biochem. J. 396:215-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiramatsu, T., K. Kodama, T. Kuroda, T. Mizushima, and T. Tsuchiya. 1998. A putative multisubunit Na+/H+ antiporter from Staphylococcus aureus. J. Bacteriol. 180:6642-6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hishiya, S., W. Hatakeyama, Y. Mizota, N. Hosoya-Matsuda, K. Motohashi, M. Ikeuchi, and T. Hisabori. 2008. Binary reducing equivalent pathways using NADPH-thioredoxin reductase and ferredoxin-thioredoxin reductase in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Cell Physiol. 49:11-18. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann, D., K. Gutekunst, M. Klissenbauer, R. Schulz-Friedrich, and J. Appel. 2006. Mutagenesis of hydrogenase accessory genes of Synechocystis sp. PCC 6803. Additional homologues of hypA and hypB are not active in hydrogenase maturation. FEBS J. 273:4516-4527. [DOI] [PubMed] [Google Scholar]
- 15.Hoiczyk, E., and A. Hansel. 2000. Cyanobacterial cell walls: news from an unusual prokaryotic envelope. J. Bacteriol. 182:1191-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inaba, M., A. Sakamoto, and N. Murata. 2001. Functional expression in Escherichia coli of low-affinity and high-affinity Na+(Li+)/H+ antiporters of Synechocystis. J. Bacteriol. 183:1376-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito, M., A. A. Guffanti, B. Oudega, and T. A. Krulwich. 1999. mrp, a multigene, multifunctional locus in Bacillus subtilis with roles in resistance to cholate and to Na+ and in pH homeostasis. J. Bacteriol. 181:2394-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacoby, J. M., D. C. Collier, E. B. Welch, F. J. Hardy, and M. Crayton. 2000. Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Can. J. Fish. Aquat. Sci. 57:231-240. [Google Scholar]
- 19.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:109-136. [DOI] [PubMed] [Google Scholar]
- 20.Kanesaki, Y., H. Yamamoto, K. Paithoonrangsarid, M. Shoumskaya, I. Suzuki, H. Hayashi, and N. Murata. 2007. Histidine kinases play important roles in the perception and signal transduction of hydrogen peroxide in the cyanobacterium, Synechocystis sp. PCC 6803. Plant J. 49:313-324. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan, A., and L. Reinhold. 1999. CO2 concentrating mechanisms in photosynthetic microorganisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50:539-570. [DOI] [PubMed] [Google Scholar]
- 22.Keren, N., H. Ohkawa, E. A. Welsh, M. Liberton, and H. B. Pakrasi. 2005. Psb29, a conserved 22-kD protein, functions in the biogenesis of photosystem II complexes in Synechocystis and Arabidopsis. Plant Cell 17:2768-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloda, A., E. Petrov, G. R. Meyer, T. Nguyen, A. C. Hurst, L. Hool, and B. Martinac. 2008. Mechanosensitive channel of large conductance. Int. J. Biochem. Cell Biol. 40:164-169. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi, M., H. Katoh, and M. Ikeuchi. 2006. Mutations in a putative chloride efflux transporter gene suppress the chloride requirement of photosystem II in the cytochrome c550-deficient mutant. Plant Cell Physiol. 47:799-804. [DOI] [PubMed] [Google Scholar]
- 25.Kunert, A., J. Vinnemeier, N. Erdmann, and M. Hagemann. 2003. Repression by Fur is not the main mechanism controlling the iron-inducible isiAB operon in the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol. Lett. 227:255-262. [DOI] [PubMed] [Google Scholar]
- 26.Li, H., A. K. Singh, L. M. McIntyre, and L. A. Sherman. 2004. Differential gene expression in response to hydrogen peroxide and the putative PerR regulon of Synechocystis sp. strain PCC 6803. J. Bacteriol. 186:3331-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Archilla, A. I., D. Moreira, P. Lopez-Garcia, and C. Guerrero. 2004. Phytoplankton diversity and cyanobacterial dominance in a hypereutrophic shallow lake with biologically produced alkaline pH. Extremophiles 8:109-115. [DOI] [PubMed] [Google Scholar]
- 28.Maestri, O., and F. Joset. 2000. Regulation by external pH and stationary growth phase of the acetolactate synthase from Synechocystis PCC6803. Mol. Microbiol. 37:828-838. [DOI] [PubMed] [Google Scholar]
- 29.Meunier, P. C., M. S. Colon-Lopez, and L. A. Sherman. 1997. Temporal changes in state transitions and photosystem organization in the unicellular, diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. Plant Physiol. 115:991-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mogelhoj, M. K., P. J. Hansen, P. Henriksen, and N. Lundholm. 2006. High pH and not allelopathy may be responsible for negative effects of Nodularia spumigena on other algae. Aquat. Microb. Ecol. 43:43-54. [Google Scholar]
- 31.Nazarenko, L. V., I. M. Andreev, A. A. Lyukevich, T. V. Pisareva, and D. A. Los. 2003. Calcium release from Synechocystis cells induced by depolarization of the plasma membrane: MscL as an outward Ca2+ channel. Microbiology 149:1147-1153. [DOI] [PubMed] [Google Scholar]
- 32.Ohkawa, H., H. B. Pakrasi, and T. Ogawa. 2000. Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC6803. J. Biol. Chem. 275:31630-31634. [DOI] [PubMed] [Google Scholar]
- 33.Padan, E., E. Bibi, M. Ito, and T. A. Krulwich. 2005. Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta 1717:67-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paithoonrangsarid, K., M. A. Shoumskaya, Y. Kanesaki, S. Satoh, S. Tabata, D. A. Los, V. V. Zinchenko, H. Hayashi, M. Tanticharoen, I. Suzuki, and N. Murata. 2004. Five histidine kinases perceive osmotic stress and regulate distinct sets of genes in Synechocystis. J. Biol. Chem. 279:53078-53086. [DOI] [PubMed] [Google Scholar]
- 35.Patel, C. N., B. W. Wortham, J. L. Lines, J. D. Fetherston, R. D. Perry, and M. A. Oliveira. 2006. Polyamines are essential for the formation of plague biofilm. J. Bacteriol. 188:2355-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pikuta, E. V., R. B. Hoover, and J. Tang. 2007. Microbial extremophiles at the limits of life. Crit. Rev. Microbiol. 33:183-209. [DOI] [PubMed] [Google Scholar]
- 37.Poncelet, M., C. Cassier-Chauvat, X. Leschelle, H. Bottin, and F. Chauvat. 1998. Targeted deletion and mutational analysis of the essential (2Fe-2S) plant-like ferredoxin in Synechocystis PCC6803 by plasmid shuffling. Mol. Microbiol. 28:813-821. [DOI] [PubMed] [Google Scholar]
- 38.Postier, B. L., H. L. Wang, A. Singh, L. Impson, H. L. Andrews, J. Klahn, H. Li, G. Risinger, D. Pesta, M. Deyholos, D. W. Galbraith, L. A. Sherman, and R. L. Burnap. 2003. The construction and use of bacterial DNA microarrays based on an optimized two-stage PCR strategy. BMC Genomics 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy, K. J., R. Webb, and L. A. Sherman. 1990. Bacterial RNA isolation with one hour centrifugation in a table-top ultracentrifuge. BioTechniques 8:250-251. [PubMed] [Google Scholar]
- 40.Ritchie, R. J. 1991. Membrane-potential and pH control in the cyanobacterium Synechococcus R-2 (Anacystis nidulans) PCC 7942. J. Plant Physiol. 137:409-418. [Google Scholar]
- 41.Shapiguzov, A., A. A. Lyukevich, S. I. Allakhverdiev, T. V. Sergeyenko, I. Suzuki, N. Murata, and D. A. Los. 2005. Osmotic shrinkage of cells of Synechocystis sp. PCC 6803 by water efflux via aquaporins regulates osmostress-inducible gene expression. Microbiology 151:447-455. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro, J. 1973. Blue-green algae: why they become dominant. Science 179:382-384. [DOI] [PubMed] [Google Scholar]
- 43.Sherman, D. M., T. A. Troyan, and L. A. Sherman. 1994. Localization of membrane proteins in the cyanobacterium Synechococcus sp. PCC7942 (radial asymmetry in the photosynthetic complexes). Plant Physiol. 106:251-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi, L. X., and W. P. Schroder. 2004. The low molecular mass subunits of the photosynthetic supracomplex, photosystem II. Biochim. Biophys. Acta 1608:75-96. [DOI] [PubMed] [Google Scholar]
- 45.Shoumskaya, M. A., K. Paithoonrangsarid, Y. Kanesaki, D. A. Los, V. V. Zinchenko, M. Tanticharoen, I. Suzuki, and N. Murata. 2005. Identical Hik-Rre systems are involved in perception and transduction of salt signals and hyperosmotic signals but regulate the expression of individual genes to different extents in Synechocystis. J. Biol. Chem. 280:21531-21538. [DOI] [PubMed] [Google Scholar]
- 46.Singh, A. K., H. Li, and L. A. Sherman. 2004. Microarray analysis and redox control of gene expression in the cyanobacterium Synechocystis sp. PCC 6803. Physiol. Plant. 120:27-35. [DOI] [PubMed] [Google Scholar]
- 47.Singh, A. K., L. M. McIntyre, and L. A. Sherman. 2003. Microarray analysis of the genome-wide response to iron deficiency and iron reconstitution in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 132:1825-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh, A. K., and L. A. Sherman. 2002. Characterization of a stress-responsive operon in the cyanobacterium Synechocystis sp. strain PCC 6803. Gene 297:11-19. [DOI] [PubMed] [Google Scholar]
- 49.Singh, A. K., and L. A. Sherman. 2005. Pleiotropic effect of a histidine kinase on carbohydrate metabolism in Synechocystis sp. strain PCC 6803 and its requirement for heterotrophic growth. J. Bacteriol. 187:2368-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh, A. K., T. C. Summerfield, H. Li, and L. A. Sherman. 2006. The heat shock response in the cyanobacterium Synechocystis sp. strain PCC 6803 and regulation of gene expression by HrcA and SigB. Arch. Microbiol. 186:273-286. [DOI] [PubMed] [Google Scholar]
- 51.So, A. K., and G. S. Espie. 1998. Cloning, characterization and expression of carbonic anhydrase from the cyanobacterium Synechocystis PCC6803. Plant Mol. Biol. 37:205-215. [DOI] [PubMed] [Google Scholar]
- 52.Stancik, L. M., D. M. Stancik, B. Schmidt, D. M. Barnhart, Y. N. Yoncheva, and J. L. Slonczewski. 2002. pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J. Bacteriol. 184:4246-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Summerfield, T. C., J. Eaton-Rye, and L. A. Sherman. 2007. Global gene expression of a ΔPsbO:ΔPsbU mutant and a spontaneous revertant in the cyanobacterium Synechocystis sp. strain PCC 6803. Photosynth. Res. 94:265-274. [DOI] [PubMed] [Google Scholar]
- 54.Summerfield, T. C., J. A. Shand, F. K. Bentley, and J. J. Eaton-Rye. 2005. PsbQ (Sll1638) in Synechocystis sp. PCC 6803 is required for photosystem II activity in specific mutants and in nutrient-limiting conditions. Biochemistry 44:805-815. [DOI] [PubMed] [Google Scholar]
- 55.Summerfield, T. C., and L. A. Sherman. 2007. Role of sigma factors in controlling global gene expression in light/dark transitions in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 189:7829-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tchernov, D., Y. Helman, N. Keren, B. Luz, I. Ohad, L. Reinhold, T. Ogawa, and A. Kaplan. 2001. Passive entry of CO2 and its energy-dependent intracellular conversion to HCO3− in cyanobacteria are driven by a photosystem I-generated DmH+. J. Biol. Chem. 276:23450-23455. [DOI] [PubMed] [Google Scholar]
- 57.Ushimaru, T., Y. Nishiyama, H. Hayashi, and N. Murata. 2002. No coordinated transcriptional regulation of the sod-kat antioxidative system in Synechocystis sp. PCC 6803. J. Plant Physiol. 159:805-807. [Google Scholar]
- 58.Wang, H. L., B. L. Postier, and R. L. Burnap. 2004. Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J. Biol. Chem. 279:5739-5751. [DOI] [PubMed] [Google Scholar]
- 59.Wang, H. L., B. L. Postier, and R. L. Burnap. 2002. Polymerase chain reaction-based mutageneses identify key transporters belonging to multigene families involved in Na+ and pH homeostasis of Synechocystis sp. PCC 6803. Mol. Microbiol. 44:1493-1506. [DOI] [PubMed] [Google Scholar]
- 60.Williams, J. G. K. 1988. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 167:766-778. [Google Scholar]
- 61.Yoshimura, H., S. Yanagisawa, M. Kanehisa, and M. Ohmori. 2002. Screening for the target gene of cyanobacterial cAMP receptor protein SYCRP1. Mol. Microbiol. 43:843-853. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, P., N. Battchikova, T. Jansen, J. Appel, T. Ogawa, and E. M. Aro. 2004. Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp. PCC 6803. Plant Cell 16:3326-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.