Abstract
The polypyrimidine tract binding protein (PTB) binds pre-mRNAs to alter splice site choice. We characterized a series of spliceosomal complexes that assemble on a pre-mRNA under conditions of either PTB mediated splicing repression or its absence. In the absence of repression, exon-definition complexes assembled downstream of the regulated exon were able to progress to pre-spliceosomal A complexes and functional spliceosomes. Under PTB mediated repression, assembly was arrested at an A-like complex unable to transition to spliceosomal complexes. Trans-splicing experiments indicated that even when the U1 and U2 snRNPs are bound properly to the upstream and downstream exons, the presence of PTB prevents the interaction of the two exon complexes. Proteomic analyses of these complexes provide a new description of exon definition complexes, and indicate that splicing regulators can act on the transition between the exon definition complex and an intron-defined spliceosome.
Introduction
Alternative splicing is a common means of regulation for eukaryotic gene expression1,2,3. Splicing choices are directed by proteins that bind to specific regulatory sequences in the pre-mRNA to alter spliceosome assembly, but the interactions of these proteins with the splicing apparatus are mostly unknown4,5,6. Each intron to be excised from a pre-mRNA must be assembled into a spliceosome containing the five small ribonucleoprotein particles (snRNPs) U1, U2, U4, U5, and U6, and multiple auxiliary proteins7,8,9. In vitro, the snRNPs sequentially bind the target intron to form discrete intermediate complexes termed H, E, A, B, and C. The H complex contains the U1 snRNP bound to the 5′ splice site of the pre-mRNA, as well as many sequence specific RNA binding proteins, including members of the hnRNP group of proteins10. The subsequent E complex contains the U2 auxiliary factor (U2AF) and Splicing Factor 1 (SF1) bound to the 3′ splice site and branchpoint, respectively. The U2 snRNP is also present in this complex, but is relatively loosely associated11. In the E complex, the 5′ and 3′ splice sites are brought together via interaction between the U1 containing complex and the U2AF complex12,13,14. The first ATP dependent step in spliceosome assembly results in the stable association of the U2 snRNP to the branchpoint via RNA base-pairing, and the formation of the A complex. The binding of the U4/U6-U5 tri-snRNP then forms the B complex. Several structural rearrangements in the B complex lead to loss of the U1 and U4 snRNPs, and result in the C complex9,15,16. In this complex, the U6 snRNA is base-paired to the 5′ splice site, and the base pairing between the U4/U6 snRNAs is replaced with a U2/U6 snRNA interaction. This creates the active conformation of the spliceosome; the two-transesterification reactions of splicing occur in the spliceosomal C complex.
Our current knowledge of spliceosome assembly is mostly derived from in vitro studies of short single intron splicing substrates, where assembly is primarily driven by cross-intron interactions. In this intron definition scenario, splice site recognition and pairing are promoted by interactions between the components at the 5′ and 3′ splice sites, to form the E complex. However, most vertebrate pre-mRNAs contain multiple short exons separated by relatively long introns17,18. Initial splice site recognition in these transcripts is mediated by exon definition interactions, where the binding of splicing components to a 5′ splice site promotes U2AF recognition of the 3′ splice site upstream across the exon19,20. This leads to the formation of an exon definition complex prior to assembly of a spliceosome across the intron. The conversion from these cross-exon interactions to an intron-defined complex, where the splice sites are paired, must occur during formation of an active spliceosome. However, this transition is very poorly understood. The components of exon definition complexes have not been determined. It is not clear how they differ from an intronic E complex for example, and whether special factors might be required for their transition to intron-defined spliceosomes. Understanding these steps will be essential to understanding how regulatory proteins can alter the choice of splice sites.
The polypyrimidine tract binding protein (PTB) has served as a paradigm for a repressor of alternative splicing events in mammalian cells21,22. PTB contains four RNA binding domains of the RNA recognition motif (RRM) type, each of which binds to a short CU rich element23. CU rich sequences are clustered upstream and downstream of PTB repressed exons, and are sometimes found within the exon itself. The binding of one or more PTB proteins to these elements can block spliceosome assembly by a variety of potential mechanisms22. The binding of PTB to the polypyrimidine tract of a 3′ splice site can directly occlude binding of U2AF, or PTB binding within the exon may block exon definition interactions required for U2AF assembly24,25,26. However, PTB binding sites that flank the repressed exon and its splice sites also prevent its splicing 27,28,29,30. In this most common case, PTB must block splicing at steps later than initial recognition of the splice sites10.
PTB regulates the splicing of many muscle and neuron specific exons, such as the neuron specific N1 exon of the c-src pre-mRNA28. The mechanism of N1 splicing has been studied in some detail in an in vitro system using extracts from neuronal WERI-1 retinoblastoma cells and non-neuronal HeLa cells 31,32,30. CU rich elements flanking N1 bind to PTB and are essential for PTB mediated repression of N1 splicing in HeLa extract. In contrast, in WERI extract PTB is mostly replaced with the inactive homolog nPTB and N1 splices efficiently. We showed previously that in HeLa extract PTB does not interfere with U1 snRNP assembly on the N1 5′ splice site10. Instead, PTB blocks formation of the E complex on the intron downstream of N1. To allow characterization of this E complex, these experiments used pre-mRNA substrates that do not carry out exon definition on the downstream exon 4. However, these exon definition reactions must occur on the endogenous transcript in vivo33. To look at the role of exon definition in PTB-mediated repression, we have now characterized the exon definition complexes that assemble on exon 4. Under conditions of either splicing or PTB mediated repression, we find that the U2 snRNP can assemble on the 3′ splice site of exon 4 via exon definition interactions. However, this formation of a defined exon 4 complex downstream does not overcome the PTB mediated repression of exon N1.
Results
The Exon 4 3′ splice site complex can assemble via exon definition
Previously, we analyzed the PTB mediated repression of N1 exon splicing in HeLa extract using the single intron substrate, BS713, where splicing complex formation is driven by intron definition interactions (Figure 1a). Comparing this to spliceosome assembly in WERI extract, where PTB mediated repression is absent, we showed that PTB interfered with an intron definition interaction required for assembly a spliceosome on the intron downstream of the N1 exon10. Although PTB does not prevent the binding of the U1 snRNP at the N1 exon 5′ splice site, the binding of U2AF and the U2 snRNP downstream does not occur on this RNA, and formation of the E complex is blocked. Under normal conditions in vivo, the downstream exon 4 would carry a 5′ splice site not present in our construct. We thus wanted to test whether the 3′ splice site complex for exon 4 could assemble via exon definition interactions when the 5′ splice site was included downstream of exon 4 (Figure 1a). We could then examine the effect of this downstream exon definition complex on repression of N1 splicing by PTB.
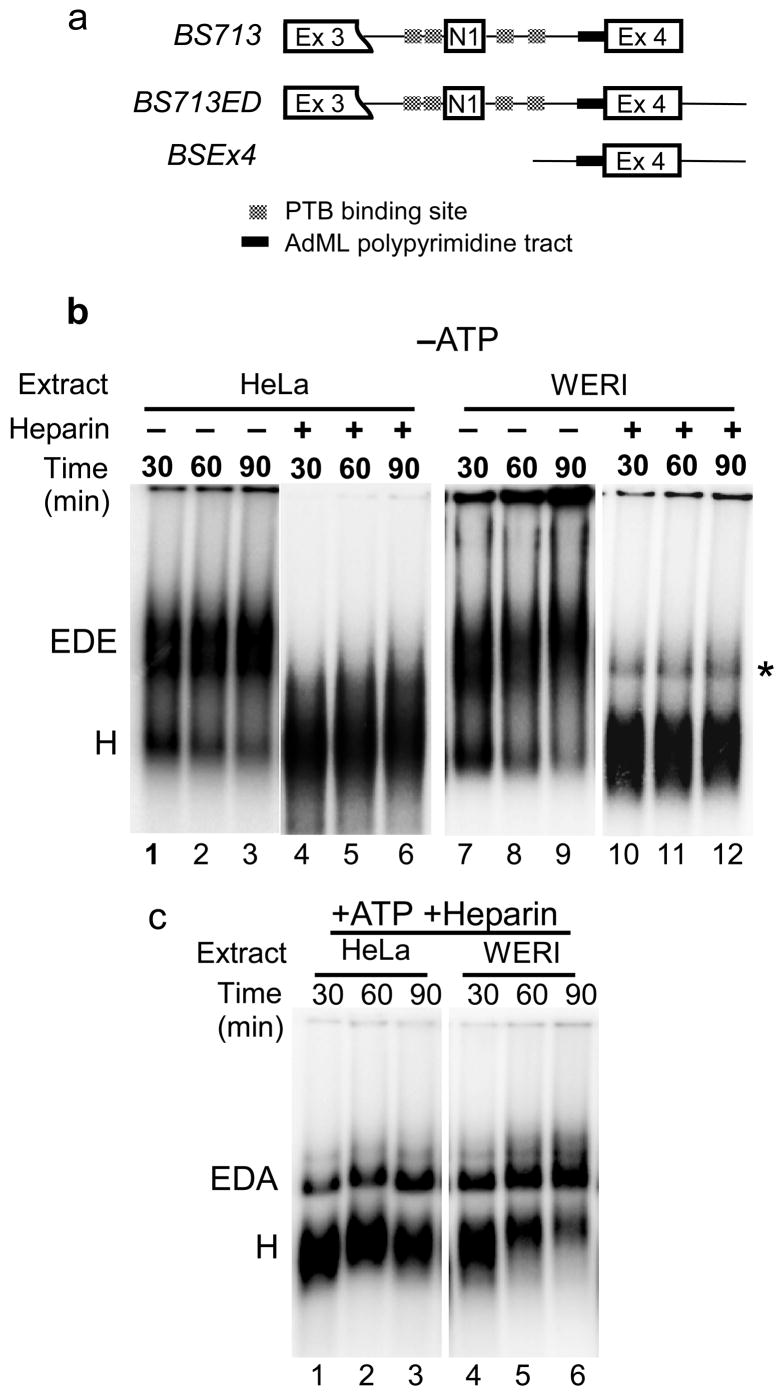
Figure 1. An exon definition complex forms in both HeLa and WERI extracts.
(a) Maps of the BS713, BS713ED, and BSEx4 constructs. BS713 has the 5′ splice site of exon 3 deleted and the AdML polypyrimidine tract introduced into the exon 4 3′ splice site. In BS713ED, 5′ splice site is included downstream of exon 4. BSEx4 has exon 4 and 50-nucleotide sequences from the upstream and downstream introns. (b) Formation of the ATP independent EDE complex. Native agarose gel analysis of complex formation on the Ex4 RNA in HeLa (lanes 1–3) and WERI (lanes 7–9) extracts in the absence of ATP. Heparin treatment dissociates the EDE complex in both HeLa (lanes 4–6) and WERI (lanes 10–12) extracts. Positions of the H and EDE complexes are indicated. A minor heparin resistant complex, indicated by an asterisk, is sometimes seen in the WERI extract, but this band is not consistently observed. (c) Formation of ATP dependent EDA complex. Native agarose gel analysis of complex formation on the Ex4 RNA in presence of ATP in HeLa (lanes1–3) and WERI (lanes 4–6) extract after heparin treatment. Positions of the H and EDA complexes are indicated.
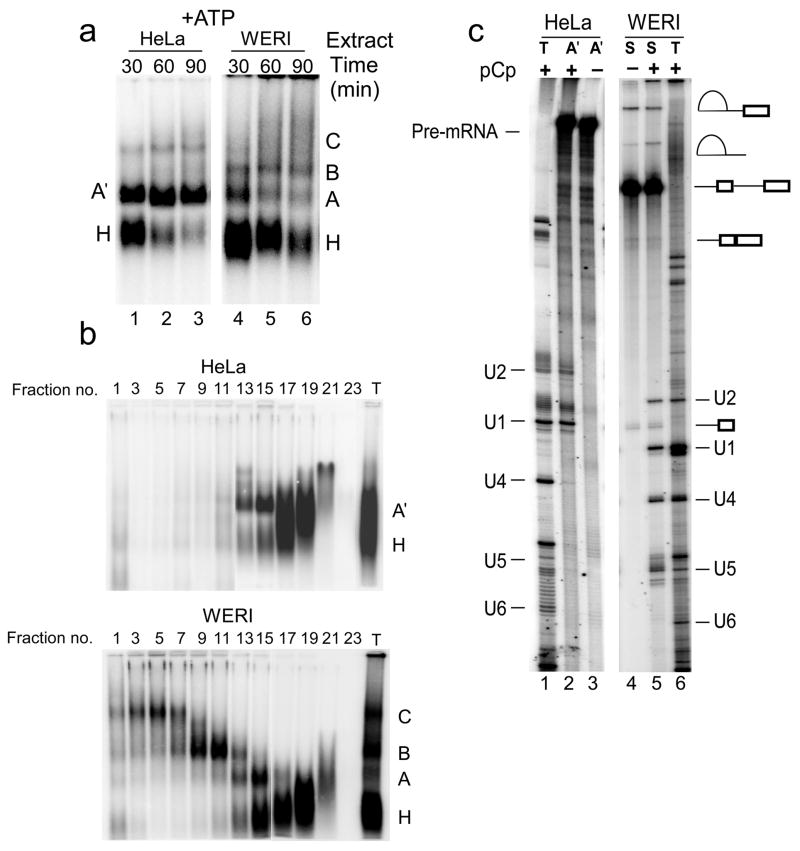
To characterize the exon definition complex under the two regulatory conditions, we used the Ex4 RNA carrying exon 4, its upstream 3′ splice site and polypyrimidine tract as modified for the splicing substrate BS713, as well as the downstream 5′ splice site (Fig. 1a). Splicing complex formation on this RNA was analyzed in the presence and absence of ATP in both HeLa and WERI extracts using native agarose gels34. For the reactions lacking ATP, extracts were depleted of endogenous ATP by preincubation at room temperature for 30 minutes. The ATP containing reactions were treated with heparin to improve the resolution of complexes. Gel analysis showed that complexes formed on the Ex4 RNA in both the presence and absence of ATP, and these complexes assembled with near equal efficiency in either HeLa or WERI extract (Fig. 1b and 1c). Heparin treatment disrupted complexes formed in the absence of ATP in both HeLa and WERI extract (Figure 1b). However in the presence of ATP, heparin resistant complexes formed in both extracts (Figure 1c). Analogous to the intron defined E and A complexes, we termed these ATP-independent and ATP-dependent complexes as the exon defined E complex (EDE) and the exon defined A complex (EDA), respectively.
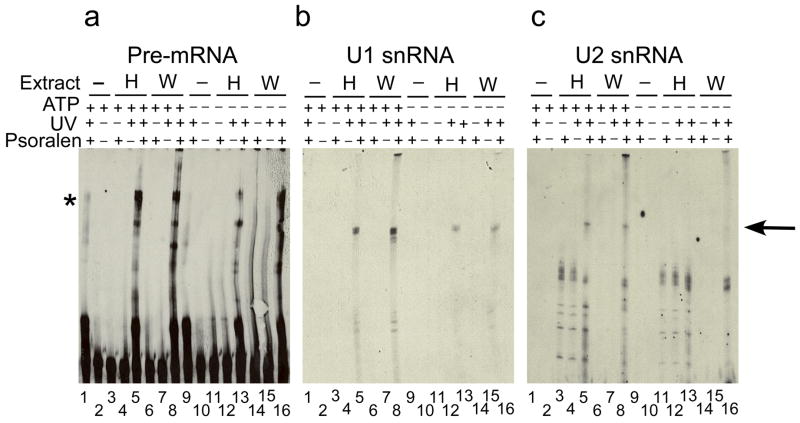
We then purified the EDE and EDA complexes using the MS2 tagged version of the Ex4 RNA (Supplementary Figure 1a). Prior to assembly of the EDE and EDA complexes, the Ex4-MS2 RNA was pre-bound to the MS2-MBP protein. The complexes were assembled on the MS2-MBP bound RNA, fractionated on glycerol density gradients, and affinity purified on amylose resin as described previously10. The purified complexes were analyzed for the bound snRNAs. The EDE and EDA complexes from both HeLa and WERI extracts contained the U1 and U2 snRNAs, but none of the other spliceosomal snRNAs (Supplementary Figure 1b and 1c). Psoralen crosslinking analysis was carried out to determine the base pairing of the U1 and U2 snRNA to the Ex4 RNA in the EDE and EDA complexes. The Ex4 RNA was incubated in HeLa and WERI extracts in the presence of ATP. 4′-Aminomethyl-4, 5′, 8-trimethylpsoralen (AMT-psoralen) was added and reactions were subjected to long wave UV crosslinking. The crosslinked products were analyzed by Northern blotting using probes against the pre-mRNA, U1, or U2 snRNA (Figure 2). No crosslinked bands were detected with any of the probes in the absence of psoralen or UV irradiation (Figure 1a, b, and c lanes 2, 3, 6, 7, 10, 11, 14, and 15). In the presence of psoralen and UV irradiation, the naked pre-mRNA showed weak intramolecular crosslinking (Figure 1a lanes 1 and 9). Several crosslinked bands were detected with the pre-mRNA specific probe in both HeLa and WERI extracts (Figure 2a lanes 5, 8, 12, and 16). One of these bands was also detected with the U1 snRNA probe in presence and absence of ATP (Figure 2b lanes 5, 8, 12, and 16). The U2 snRNA probe detected a crosslinked product in the presence of ATP, but not in its absence (Figure 2c lanes 5 and 8). Thus in the EDE complex, only the U1 snRNA is base-paired to the pre-mRNA. Whereas in the EDA complex, both the U1 and U2 snRNAs are base-paired to the pre-mRNA.
Figure 2. The U2 snRNA is basepaired to the pre-mRNA in the EDA complex.
Northern analysis of the snRNAs crosslinked to the Ex4 RNA in buffer DG (lane 1, 2, 9, and 10) or in HeLa (lanes 3–5 and lanes 11–13) or in WERI (lanes 6–8 and lanes 14–16) extract in the absence (lanes 1–8) or presence (lanes 9–16) of ATP, using probes recognizing the pre-mRNA RNA (a), U1 (b), or U2 snRNAs (c). An arrow on the right indicates the crosslinked species. Note that the crosslinked U1 and U2 snRNA bands have similar migration but do not exactly overlap. Identity of the band indicated by the asterisk in not clear.
Exon 4 Complexes from the two Extracts have Similar Components
To compare the exon definition complexes under the two splicing regulatory conditions, we determined their protein composition using mass spectrometry (MS). As exon 4 is spliced under both regulatory conditions, this would also allow us to identify factors that bind to this region of the RNA and likely do not determine the regulation. The purified complexes were RNase A treated, TCA precipitated, and digested with trypsin in solution. Peptides from each of the samples were identified by MS using the MudPIT procedure35. The EDE and EDA complexes from both HeLa and WERI extract were purified and analyzed. Proteins present in the four complexes are listed in Supplementary Table I. The compositions of the four complexes were very similar. All complexes contained the U1 and U2 snRNP specific proteins, and the Sm proteins. Not all components of a complex were detected in every MS experiment, but comparisons between these complexes, as well as additional complexes (see below), indicate the likely presence of some missing proteins. For example, the HeLa EDA and WERI EDE complexes showed no peptides for SmD2 but contained other components of the Sm core. Given that the HeLa EDE and WERI EDA complexes clearly contained SmD2, we infer its presence in the other two exon-definition complexes. The large numbers of proteins common to both the EDE and EDA complexes from both extracts are listed in Table I. The Ex4 RNA lacks the PTB binding sequences involved in the regulation of N1 exon splicing, and the PTB protein was not detected in any of the complexes. Western blot analysis of the purified EDE complexes from HeLa and WERI extracts confirmed the presence of U2AF and absence of the PTB proteins (Supplementary Figure 2).
Table I.
Proteins present in both the EDE and EDA complexes purified from HeLa and WERI extracts.
| U1 snRNP specific | hnRNP proteins | Others |
|---|---|---|
| U2 snRNP specific | hnRNP U | PUF60 |
| Sm proteins | hnRNP R | CAPER |
| Essential factors | hnRNP Q | CROP |
| CBP80 | hnRNP L | LUC7L |
| CBP20 | hnRNP K | CARP-1 |
| SF1 | FUS | ARS2b |
| U2AF65 | YB-1 | SR140 |
| U2AF35 | hnRNP G | BUB3 |
| DEAD box proteins | hnRNP F | NFAT-90 |
| DDX 5, p68 | hnRNP D | NFAT-45 |
| DDX 15, Prp43 | hnRNP C | ZNF 207 |
| DDX 17, p72 | hnRNP A/B | RBM25 |
| DDX 36 | hnRNP A3 | SPF31 |
| DDX 46, Prp5 | hnRNP A2/B1 | TAT SF1 |
| SR proteins | hnRNP A1 | fSAP18 |
| SRrp86, SFRS12 | hnRNP A0 | NELF E |
| SRp54, SFRS11 | hnRNP RALY | CCAP1 |
| SRp20, SFRS3 | HuR |
Interestingly, the proteins in the two WERI complexes were nearly identical to those in the HeLa complexes. The only protein detected in the HeLa complex but not the equivalent WERI complex was the DEAD box protein DDX1. The similarity of the two complexes indicates that the components of the exon 4 complexes are not likely to affect splicing regulation. This was not unexpected, as exon 4 must splice to an upstream exon in either of the two regulatory conditions. The addition of ATP to convert the complexes from EDE to EDA resulted in the apparent gain of some proteins such as CGI-99 and RBM39 in the HeLa complex, and hnRNP H and hnRNP A/B in both the HeLa and WERI complexes. However, their presence needs to be verified by immunoblotting.
In summary, exon definition interactions across exon 4 can induce 3′ splice site complex assembly for the intron downstream of the N1 exon. These complexes assemble with similar efficiency in HeLa and WERI extracts and are very similar in their protein composition.
PTB Represses Splicing of N1 to the Defined Exon 4 Complex
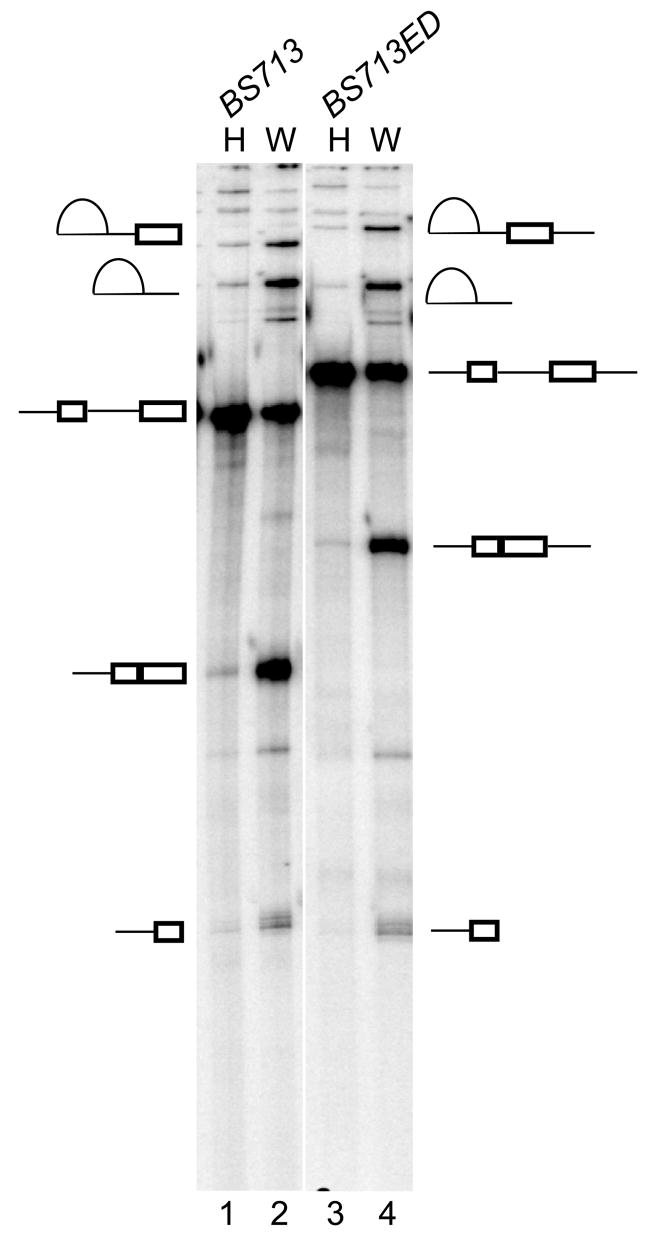
To examine splicing repression in the presence of the exon definition complex on exon 4, we made the BS713ED construct, which includes the 5′ splice site downstream of exon 4 (Figure 1a). Both the BS713 and BS713ED transcripts have one removable intron and can assemble a single functional spliceosome. Both transcripts splice efficiently in WERI extract (Figure 3 lanes 2 and 4). However, both were strongly repressed for splicing in HeLa extract, inspite of the potential for exon definition in BS713ED (Figure 3 lanes 1 and 3).
Figure 3. U2 snRNP assembly via exon definition does not overcome splicing repression.
In vitro splicing of BS713ED RNA (lanes 3 and 4) in HeLa and WERI extract is compared to the BS713 (lanes 1 and 2). Splicing intermediates and products are indicated.
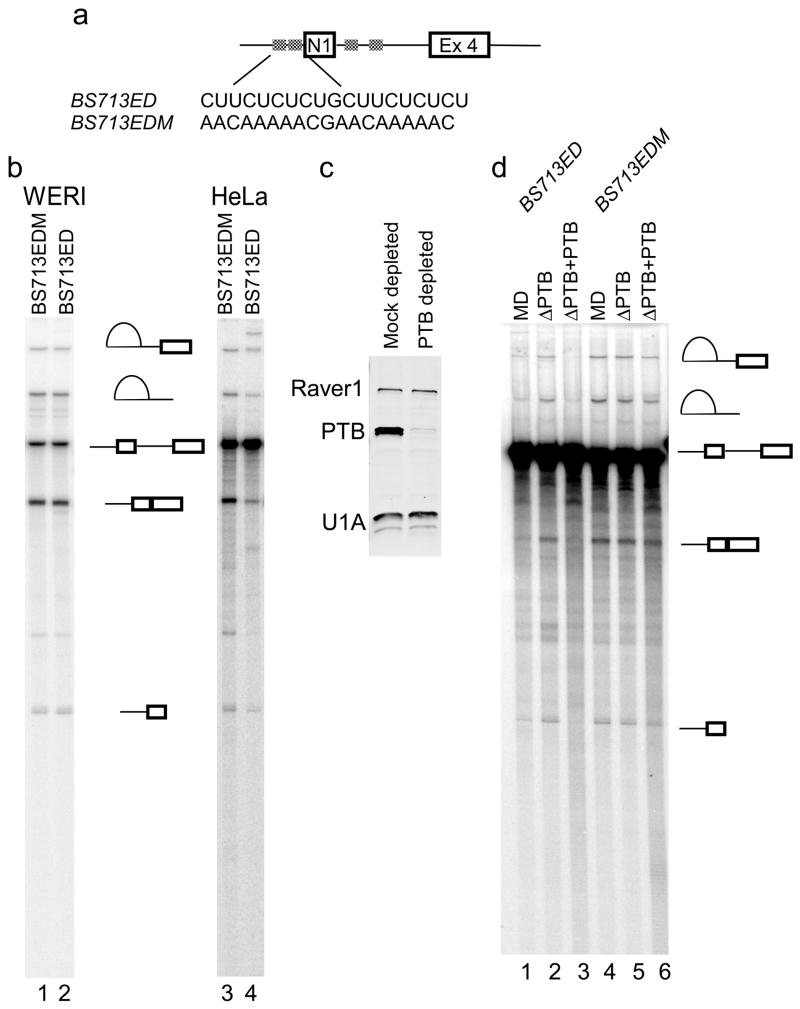
To confirm that splicing repression in HeLa extract was mediated by PTB, the PTB binding elements upstream of N1 were mutated to make BS713EDM (Figure 4a). Splicing of this mutant RNA was compared to the wild type. In WERI extract, both RNAs, spliced with equal efficiency (Figure 4b, lanes 1 and 2). But in HeLa extract, splicing of the mutant RNA was about 8-fold more efficient than the wild type RNA. PTB was also depleted from HeLa nuclear extract using the PTB specific monoclonal antibody, BB7. Western blot analysis confirmed efficient depletion of PTB in comparison to the mock-depleted extract, whereas the levels of two other proteins, Raver1 and U1A, were unaffected (Figure 4c). Splicing of the BS713ED RNA was stimulated about 5-fold in the PTB depleted extract compared to the mock depleted extract (Figure 4d, lanes 1 and 2). Add-back of recombinant PTB restored splicing repression (lane 3). Splicing of the mutant RNA was not affected significantly by PTB depletion or add-back (lanes 4–5). Thus, similar to the BS713 RNA, splicing of the BS713ED is repressed by PTB.
Figure 4. Splicing repression after exon definition in HeLa extract is PTB dependent.
(a) The BS713EDM construct carries a mutation in the N1 exon 3′ splice site as shown that eliminates PTB binding. (b) In vitro splicing of the BS713EDM transcript (lanes 1 and 3) is compared to BS713ED (lanes 2 and 4) in HeLa and WERI extracts. (c) Western analysis of the mock depleted and the PTB depleted extract using antibodies against proteins PTB, U1A, and Raver1. (d) In vitro splicing of the BS713ED (lanes 1–3) and BS713EDM (lanes 4–6) transcripts in mock depleted, PTB depleted, and PTB reconstituted HeLa extract is compared.
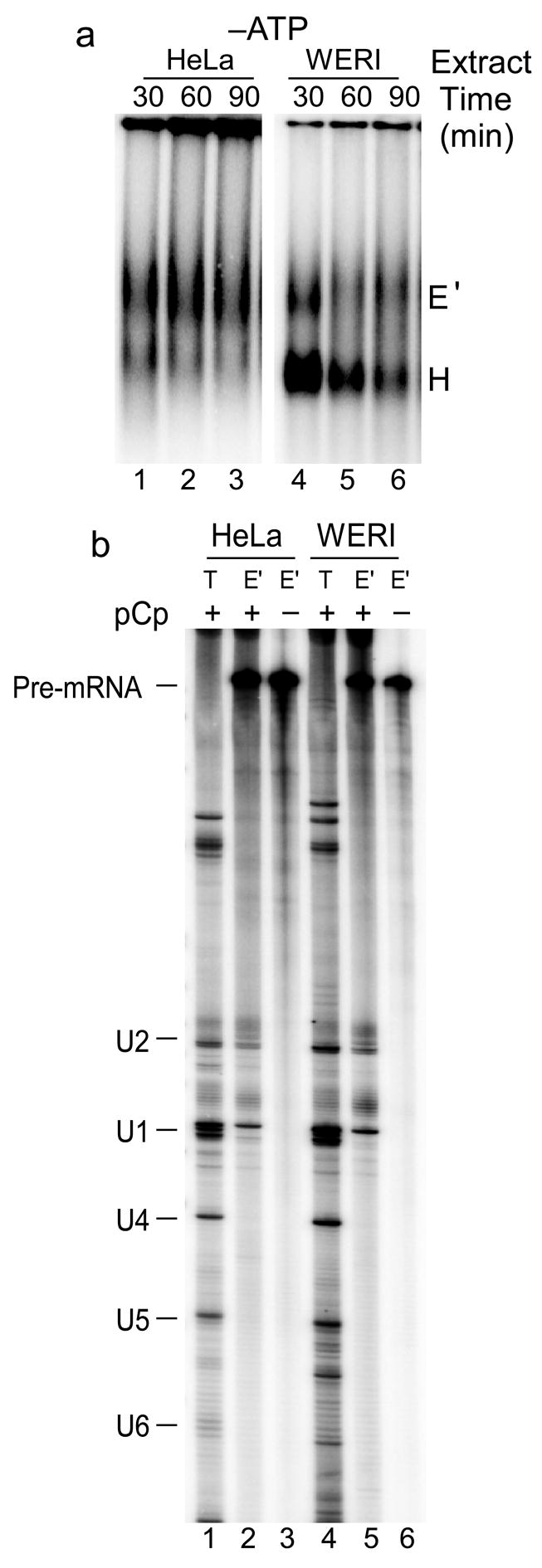
In contrast to what was seen previously with the BS713 RNA, the BS713ED RNA should form an exon definition complex on exon 4 in either extract10. Indeed, native gel analysis showed complex formation on BS713ED RNA in the absence of ATP in both HeLa (Figure 5a lanes 1–3) and WERI (Figure 5a lanes 4–6) extract. Since these complexes can potentially assemble via either intron or exon definition pathways, we refer to them as E-like (E′) to distinguish them from an intron defined E complex. These ATP-independent complexes were then purified by the MS2-affinity chromatography using the BS713ED-MS2 RNA. RNA 3′ end labeling showed that the U1 and U2 snRNAs were present in both the HeLa (Figure 5b lane 2) and WERI (Figure 5b lane 5) complexes.
Figure 5. ATP-independent E′ complex assembles in both HeLa and WERI extracts.
(a) Native agarose gel analysis of complex formation on the BS713ED RNA in absence of ATP in HeLa (lanes1–3) and WERI (lanes 4–6) extract. Positions of the H and E′ complexes are indicated. (b) 32P-pCp labeling of RNA from the purified E′ complexes. Total RNA from nuclear extract (T) was used as markers for the U snRNAs. The positions of the pre-mRNA and U snRNAs are indicated.
The addition of ATP to WERI extract caused the expected conversion of the E′ complex into splicing complexes A, B, and C (Figure 6a lanes 4–6). However, in the HeLa extract, ATP induced the conversion into a prespliceosomal A-like complex (A′), but this complex did not transition into the spliceosomal B and C complexes (Figure 6a lanes 1–3). Fractionation of these complexes on glycerol density gradients after heparin treatment showed clear separation of the A, B, and C complexes in WERI extract (Figure 6b). In HeLa extract only the H and A′ complexes were seen. The A′ complex from HeLa extract and mixed spliceosomes from WERI extract were isolated without heparin treatment as described previously10. Analysis of their RNA content showed that the WERI spliceosomes contained the expected splicing intermediates and products, as well as the U1, U2, U4, and U5 snRNAs. (Note that U6 often does not label well with pCp.) Whereas the HeLa A′ complex lacked any spliced products, and contained only the U1 and U2 snRNAs (Figure 6c). Thus, in HeLa nuclear extract, the assembly of U2 snRNP on exon 4 via exon definition interactions is not sufficient to overcome splicing repression by PTB. In WERI extract, which lacks PTB repression, the U2 snRNP bound at exon 4 is able to convert into active spliceosomal complexes.
Figure 6. ATP dependent splicing complex assembly in HeLa extract is stalled at the A.
′ complex. (a) Native agarose gel analysis of complex formation on the BS713ED RNA in presence of ATP in HeLa (lanes1–3) and WERI (lanes 4–6) extract. Positions of the H, A, A′, B, and C complexes are indicated. Note that the nature of the minor high molecular weight complex in HeLa extract is not clear. (b) Native agarose gel analysis of splicing complexes assembled on BD713ED RNA in HeLa (top panel) and WERI (bottom panel) extracts and fractionated on 15–30% glycerol density gradients. (c) 32P-pCp labeling of RNA from the purified A′ complex (A′) and from total spliceosomes (S). Total RNA from nuclear extract (T) was used as markers for the U snRNAs. The positions of the pre-mRNA and U snRNAs are indicated.
Proteomic Analysis Identifies Potential PTB Corepressors
To compare the splicing complexes that assemble under the two regulatory conditions, we determined their protein composition. The purified HeLa E′ and A′, and the WERI E′ and total spliceosomal complexes were treated with trypsin and subjected to MS analysis. All proteins identified in these complexes are listed in Supplementary Table I. The Ex4 exon definition complex proteins listed in Table I were identified in all the BS713ED complexes. These BS713ED complexes also contained proteins that presumably bind to the upstream sequence not present in the Ex4 RNA, including PTB, hnRNP E2 and Matrin 3. Unlike the Ex4 complexes, the E′ complexes from both extracts changed significantly upon addition of ATP.
In HeLa extract, conversion to the A′ complex resulted in the loss and gain of multiple proteins. Proteins present in the HeLa complexes but not in the WERI complexes are potential PTB cofactors in splicing repression. Interesting proteins in this group were CUGBP1 and 2, MBNL 1, 2, and 3, and Raver 1 and 2 (Table II). Raver 1, in particular, has been shown to cooperate with PTB in the repression of α-tropomyosin exon 336.
Table II.
Proteins present in the HeLa and WERI BS713ED complexes.
| Protein Name | Acc. No. | Sequence motifs |
|---|---|---|
| Proteins present only in the WERI spliceosomes | ||
| U5 snRNP proteins | ||
| U4/U6 snRNP proteins | ||
| LSm proteins | ||
| PRP19 complex | ||
| Tri-snRNP protein | ||
| 110k, SART1, hSnu66 | NP_005137 | RS |
| DEAD box protein | ||
| DDX39, UAP56 | NP_004631 | DEXD |
| SR proteins | ||
| ASF/SF2, SFRS1 | NP_008855 | 2 RRMs, RS |
| 9G8, SFRS7 | NP_001026854 | RRM, RS |
| hTra-2alpha | NP_037425 | RRM, RS |
| hTra-2b, SFRS10 | NP_004584 | RRM, RS |
| Non-snRNP splicing proteins | ||
| SRm160 | AAC09321 | PWI domain |
| SRm300 | NP_057417 | 2 RS, S-rich |
| EWS | NP_005234 | RRM, ZnF |
| DEK | NP_003463 | SAP, DEK |
| Smu-1 homolog | NP_060695 | WD40 |
| RED | NP_006074 | RED repeats |
| RBM22, FLJ0290 | NP_060517 | C3H1ZnF, RRM |
| RBM42, MGC10433 | NP_077297 | RRM |
| Aquarius, IBP160 | NP_055506 | Helicase SF |
| hPrp4k | NP_003904 | S/T kinase |
| RBM26, Se702 | NP_071401 | RRM, C3H1 ZnF |
| WD33 | NP_060853 | WD40 |
| PEP19 | NP_006189 | |
| BAT2 | NP_542417 | Pro-rich |
| PP1gamma | NP_002701 | PP2Ac |
| NIPP1 | NP_054829 | FHA |
| hnRNP proteins | ||
| nPTB, PTB-2 | NP_067013 | 4 RRMs |
| PSF | NP_005057 | 2 RRM, NOPS |
| p54nrb | NP_031389 | 2 RRM, NOPS |
|
| ||
| Proteins present only in the HeLa A′ complex | ||
| DEAD box proteins | ||
| DDX1, DED1 | NP_004930 | DEAD |
| DDX 36 | NP_065916 | DEAD |
| KIAA0052, SKIV2L2 | NP_056175 | DEXH |
| hnRNP proteins | ||
| FBP | NP_003893 | 3 KH |
| SAF-B | NP_002958 | RRM, SAP |
| CUGBP1 | NP_941989 | 3RRM |
| MBNL3 | NP_597846 | C3H1 ZnF |
| MBNL2 | NP_659002 | C3H1 ZnF |
| MBNL1 | NP_066368 | C3H1 ZnF |
| Raver1 | NP_597709 | 3 RRMs |
| Raver2 | NP_060681 | 3 RRMs |
Proteins detected in the WERI E′ complex but not in the HeLa E′ complex included SF2/ASF, EWS, nPTB, and FLJ38348. With ATP addition, this WERI E′ complex efficiently transitions to functional spliceosomal A, B, and C complexes. During this transition, PRP19 complex proteins, U5, U4/6, and tri-snRNP specific proteins, and several other non-snRNP splicing proteins all bind to the complex (Table II). The absence of some second step factors such as Prp17, Prp18, and Slu7 in the MS analysis could be due to the under representation of late spliceosomes in the mixture of complexes.
The A complex that assembles on an AdML intron has been studied in some detail37. A number of proteins are present in both this functional HeLa A complex and the WERI spliceosomes, but are absent from the blocked HeLa A′ complex. These different components include the Srm 160 and 300 proteins, and the proteins of the Prp19 complex and give clues to how A′ is blocked from further assembly. Notably, Prp3 and Prp4 proteins were present in the HeLa A′ complex but no other U5 snRNP, U4/U6 snRNP, or tri-snRNP specific proteins were detected.
Trans-splicing of N1 to Exon 4 Occurs in WERI but not HeLa Extract
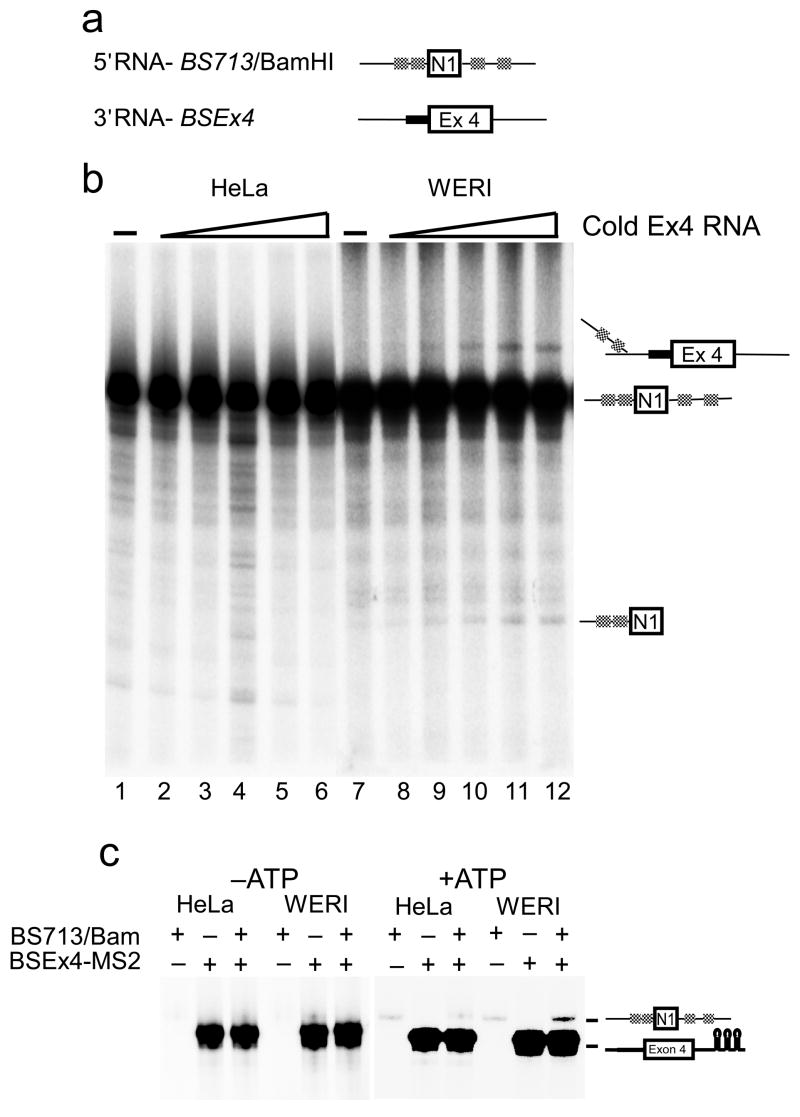
The exon definition complex assembles properly in the HeLa extract but does not convert to a spliceosome. One possibility is that PTB blocks the interaction of the upstream and downstream complexes, preventing their conversion to an intron defined spliceosome. To look directly at the interaction between the 5′ and 3′ exon complexes, we used a trans-splicing assay that has been described by others38,39,40. The BS713ED construct was split in the intron to produce 5′ and 3′ exon transcripts (Figure 7a). Labeled 5′ exon RNA and cold 3′ exon RNA were incubated in HeLa and WERI extracts. Denaturing gel analysis was used to assay formation of the Y-RNA and the detached 5′ exon that result from the first step of the trans-splicing reaction. Incubation of the 5′ exon RNA in the absence of the 3′ exon RNA did not show formation a trans-spliced product in either extract (Figure 7a lanes 1 and 7). Similarly, we have not observed trans-splicing between exon 4 complexes, perhaps due to the limited sequence downstream of the 5′ splice site in this RNA (data not shown; see also Figure 1). When both 5′ and 3′ exon RNAs were mixed in the reaction, trans-splicing was observed in WERI but not in the HeLa extract (Figure 7b). Thus, PTB assembling on the 5′ exon transcript prevents its trans-splicing to the downstream exon.
Figure 7. Trans splicing occurs in WERI but not HeLa extract.
(a) RNA substrates used in the trans splicing assay. The 5′ RNA was transcribed from BamHI cleaved BS713 and the 3′ RNA was transcribed from BSEx4. (b) Trans-splicing analysis of uniformly 32P-labeled 5′ RNA alone (lanes 1 and 7) or in presence of cold 3′ RNA in HeLa (lanes 1–6) and WERI (lanes 7–12) extract. (c) MS2-affinity tag mediated pull down of uniformly 32P-labeled 3′ RNA in the presence or absence of the 5′ RNA in HeLa and WERI extract either in the presence or absence of ATP.
It was possible that under PTB repression, the upstream exon was still pairing with the downstream exon, but could not proceed further in assembly. To examine this, we carried out affinity pull down experiments using MS2 tagged exon 4 RNA, pre-bound to the MS2-MBP protein. Uniformly labeled 5′ and 3′ exon RNAs at 5 nM concentrations were incubated in HeLa and WERI extract in the absence or presence of ATP. The reactions were incubated with amylose beads and the bound RNAs were isolated. In reactions lacking ATP, the 5′ exon RNA could not be pulled down in association with the MS2 tagged 3′ RNA (Figure 7c lanes 1–6). However, the addition of ATP induced the association of the 5′ exon RNA with the tagged 3′ exon RNA. This interaction was observed in WERI extract, but not HeLa extract (lanes 7–12). Thus, the splicing complexes that assemble on the 5′ and 3′ exons stably interact only in WERI extract and in the presence of ATP.
Discussion
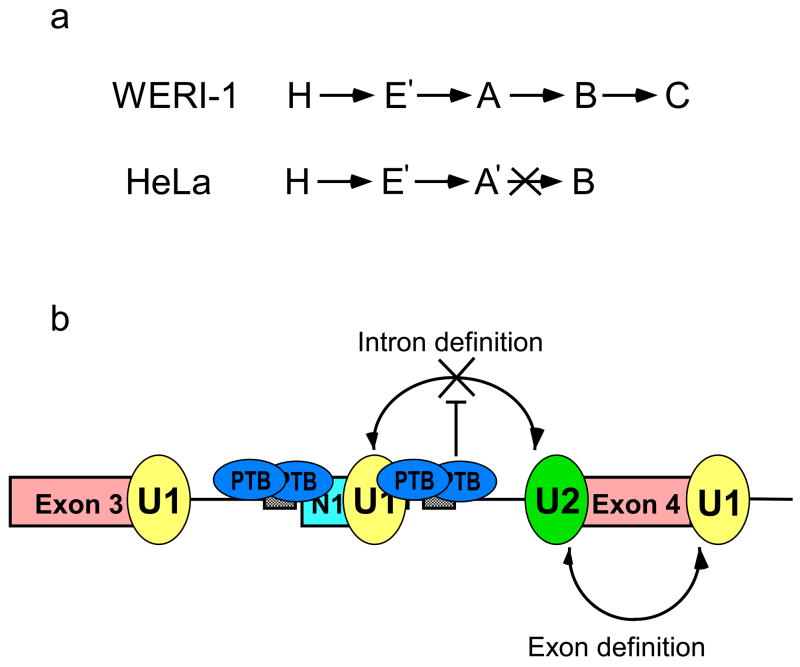
Our goal in this work is to understand how splicing regulators interact with the spliceosome during its assembly to alter splicing choices. We find that in repressing splicing of the c-src N1 exon to the constitutive exon 4 downstream, PTB prevents the interaction of the N1 5′ splice site complex with the 3′ splice site complex assembled on exon 4. This results in the accumulation of an A-like complex (A′), which contains the U2 snRNP assembled through exon definition on exon 4 and the U1 snRNP assembled at exon N1, but is blocked from further assembly (Figure 8A and 8B). These results are in agreement with earlier studies showing under conditions of intron definition that PTB blocks the assembly of U2AF, which requires an interaction with the U1 snRNP complex upstream to be incorporated into an intron defined spliceosome10. Under the exon definition conditions used here, the U2AF is stabilized by the 5′ splice site of exon 4 downstream, but its interaction with the upstream N1 5′ splice site still does not occur. Thus, PTB is acting on the 5′ splice site side of this interaction (Figure 8B).
Figure 8. Model for PTB mediated splicing repression.
(a) In WERI extract spliceosomal complexes H, E, A, B, and C form efficiently, whereas is HeLa extract assembly is stalled at an A-like complex (A′). (b) Intron bound PTB does not interfere with the binding of U1 snRNP at the N1 exon 5′ splice site or with the assembly of an exon definition complex on the downstream exon. PTB prevents the intron bound U1 and U2 snRNPs from interacting and thus prevents intron definition and spliceosome assembly.
The exon 4 complex has the same components whether PTB is present or not, allowing it to splice to the upstream exons 3 or N1, depending on the presence or absence of PTB. The transition from an exon definition complex to an intron defined spliceosome is poorly understood. The ability of PTB to prevent this step gives clues to general aspects of spliceosome assembly, as well as PTB’s specific regulatory targets.
Although they are major regulatory points in the assembly of the spliceosome, the compositions of the E and exon definition complexes (EDCs) have not been previously described. We find that both complexes contain the expected components of the U1 and U2 snRNPs, and U2AF, as well as SR proteins that presumably bind within the exon. Although PTB is not present, the EDC does contain multiple hnRNP proteins whose binding sites are unknown and which do not seem to inhibit its splicing. The EDC also contains a number of ATP dependent RNA helicases of the DEAD box family, including p68, Prp5 and others. Interestingly, when ATP is added to convert the EDE complex to an EDA complex with U2 base-paired to the branchpoint, the components of the complex change very little. Thus, one of the component DEAD-box proteins of the EDC could drive the ATP dependent transition to a base paired U2 snRNA. However, it is possible that another ATPase is involved that only transiently associates with the complex and is lost upon isolation.
Although the EDC’s were similar in the two extracts, isolation of the complexes on the longer spliceable pre-mRNA identified several proteins whose assembly depends on the regulatory conditions. As seen previously, it is clear that U2 can assemble properly at the branchpoint before the transition to intron bridging interactions. Since this bridging interaction has apparently not formed in the PTB repressed A-like (A′) complex, it is interesting to compare the A′ components to the functional A complexes studied by others37. Nearly all proteins found in the A complex assembled on the Adeno Major late intron in HeLa extract are also seen in the mixed spliceosomes assembled here in WERI extract. Proteins present in these complexes, but missing from the repressed HeLa A′ complex, are candidates for factors needed for this bridging interaction. The Prp19 complex is required for the transition to an active B complex, but is present in the Adeno A complex and may have additional earlier functions41,37. SRm160 and SRm300 are SR domain proteins shown to interact with components at both the 5′ and 3′ splice sites, and were proposed to act as the intronic bridge42,43. Interestingly, both these proteins are present in the Adeno A complex, and the WERI spliceosomes, but not in the HeLa A-like complex, consistent with a role as bridging factors37. Another protein present in the WERI spliceosomes but not the HeLa A-like complex is DEK, which was shown to be important for proofreading of the U2AF/3′ splice site interaction44. This is a step that could occur in the transition from exon definition to the intronic spliceosome. Other proteins previously proposed to mediate 5′ and 3′ splice site interactions are the Prp5 and Prp40 proteins45,46,47,48,49. These are present in all of our complexes (repressed or not). They could still be bridging factors that have lost the ability to contact one side of the intron in the repressed complex. Defining the roles of all of these proteins will require further study using depletion experiments and other approaches.
The pull-down and trans-splicing experiments indicate that PTB prevents the interaction of the 5′ splice site complex with the exon definition complex downstream. PTB could block contacts on the U1 snRNP needed for this interaction, or it could prevent a required change in U1 conformation. The A-like complex formed when splicing is repressed by PTB contains several proteins not found in the ATP-dependent exon definition complex. Some of these proteins are ATP dependent and include candidates for proteins that act as corepressors with PTB. Of these, several were previously shown to functionally interact with PTB, including CUGBP, MBNL, and Raver. CUGBP and PTB have antagonistic effects on the splicing of exons in α–actinin and α-tropomyosin27,50. Both MBNL and PTB repress the splicing of an exon in cardiac Troponin-T51. Raver-1 is a known cofactor required for proper PTB repression of α-tropomyosin exon 336. It will be interesting to test the involvement of these proteins in PTB mediated repression of N1, although initial experiments do not indicate a requirement for Raver-1 (data not shown).
The action of PTB in blocking exon defined complexes from forming functional spliceosomes is a potential mechanism for many splicing regulators. There are many intronic silencing elements that presumably recruit specific repressor proteins. Splicing repressors such as PTB, hnRNPs A1 and L can also sometimes bind in exons rather than introns52,53. For hnRNP A1 and PTB, this binding can disrupt exon definition, or SR protein-dependent splicing enhancement. This may lead to the failure to recognize the exon at all. However, for CD45 exon 4 repression by hnRNP L, the exon definition complex does form, but is not able to convert to an intronic spliceosome, similar to what is observed here54. It is interesting that these two mechanisms will allow normal exon definition on adjacent exons and their splicing, but will prevent the target exon from entering a productive assembly pathway.
Methods
Pre-mRNAs and Affinity Tags
Pre-mRNAs were transcribed in vitro from plasmids BS713, BS713ED, BS713EDM, and BSEx4 (Figure 1a). The BS713 construct has been described before31. The BS713ED construct includes the 5′ splice site of c-src exon 4 and a 50 nucleotide region of the downstream intron. In the BS713EDM construct, the PTB binding element in the N1 exon 3′ splice site is changed from CUUCUCUCUGCUUCUCUCU to AACAAAAACGAACAAAAAC. The BS713Bam RNA was transcribed from BS713 plasmid cleaved at the Bam HI site in the intron downstream of N1 exon. Constructs BS713ED-MS2 and BSEx4-MS2 containing three MS2 affinity tags were prepared as described before.
In vitro Splicing and Spliceosome Assembly
Nuclear extracts from HeLa and WERI-1 cells were prepared as described previously28. In vitro splicing was carried out as described 33. Splicing complexes were assembled as described previously10. For the ATP dependent complexes, at the indicated time, standard splicing reactions were stopped by addition of heparin to a final concentration of 0.2 mg mL−1, and the incubation was continued for another 5 minutes. The splicing complexes were separated and visualized using native agarose gels. Of the 25 μL splicing reaction, 4 μL was loaded onto a 2% agarose (Seakem GTG agarose) gel. The native gels (10 cm × 14 cm) were cast and run in 25 mM Tris-glycine buffer at 100 V for 4 hrs at room temperature. The gels were fixed in of 10% (v/v) acetic acid, 10% (v/v) methanol for 30 min, dried under vacuum and visualized by Phosphorimager (Molecular Dynamics). For assembly of the ATP-independent complexes, the nuclear extracts were depleted of ATP by pre-incubating them at room temperature for 30 min. The assembly reactions lacked ATP and creatine phosphate. The complexes were resolved on a 1.5% native agarose gels without addition of heparin.
Trans-splicing was carried out as described38. Briefly, uniformly 32P-labeled 5′ RNA at 5 nM and varying amounts of the cold 3′ RNA (0, 0.1, 0.5, 1, 2, and 5 nM) were added to standard splicing reactions and incubated at 30°C for 90 mins. RNA was extracted, ethanol precipitated, separated on 8% urea-PAGE, and visualized by Phosphorimager. Reactions mixes for the pull down assay contained 5 nM MS2 tagged 3′ RNA, 50 nM of MS2-MBP protein, and 0 or 5 nM of 5′ RNA in HeLa and WERI extract in the presence or absence of ATP. The reactions were incubated for 90 min at 30°C. The MS2 hairpin tagged RNA was pulled down using amylose beads, extracted, visualized as described above.
Psoralen Crosslinking
The EDE and EDA complexes were assembled at 30°C for 0 min in HeLa and WERI extracts. AMT-psoralen was added to a final concentration of 20 μg ml−1 and reactions were UV irradiated as described previously10. The pre-mRNA and U1 and U2 snRNA crosslinked RNAs were detected by northern blot analysis using digoxigenin labeled probes55. The pre-mRNA probe was directed against residues 1–153 of the Ex4 RNA and the U1 and U2 snRNA probes were made as described55.
Isolation of Splicing Complexes
Purification of the pre-mRNP complexes was carried out according to the method described by Reed and coworkers56, with some modifications as described before10. MS2 binding site-containing pre-mRNAs (10 nM) were first pre-incubated with 10-fold excess of recombinant MS2-MBP fusion protein (a gift from J. Vilardell). The splicing complexes were then assembled on this pre-mRNA/MS2-MBP complex. For assembly of ATP dependent complexes, a typical reaction (500 μL), contained 10 nM pre-mRNA, 100 nM MS2-MBP, 20 mM ATP, 0.4 mM creatine phosphate, 2.2 mM MgCl2, 10U RNAguard, and 300 μL of nuclear extract. The reaction mixture was incubated for 90 min at 30 °C. The ATP independent complexes were assembled in extracts that had been depleted of ATP and the assembly reactions lacked ATP and creatine phosphate. The reaction mix was then layered on a 15–30% (v/v) glycerol gradient prepared in buffer DG. The gradients were centrifuged at 24,000 rpm for 16 hours, at 4°C in a SW41 rotor. The gradients were fractionated into 24 aliquots and the radioactivity determined by scintillation counting. The peak fractions were pooled and passed twice through a column of amylose beads (500 μL; New England Biolabs) pre-equilibrated in buffer DGM (buffer DG containing 2.2 mM MgCl2). The column was washed with 10 column volumes of buffer DGM. Bound complexes were eluted with 20 mM maltose in Buffer DGM. Concentrations of the purified complexes were determined from the specific activity of the labeled transcript. The yield of the purified complexes using this method varied between 20–30% of the input transcript. E. coli ribosomal subunits, 50S and 30S were used as markers in parallel glycerol gradients.
RNA from the purified complexes was extracted with PCA and ethanol precipitated. The RNA was labeled with 32P-pCp in a 10 μl reaction containing the RNA, RNAguard, 10 units RNA ligase (NEB), 1 μl of 10x ligase buffer and 2 μl of 32P-pCp (3000Ci mmol−1) incubated at 4°C overnight. The RNA was extracted with PCA, ethanol precipitated, and separated on 8% urea-PAGE gel.
PTB purification and immunodepletion
Recombinant His-PTB was expressed in E. coli and purified using Ni-NTA agarose (Invitrogen). HeLa nuclear extract was immunodepleted for PTB using the mouse monoclonal anti-PTB antibody, BB7 as described previously10. Western analysis was carried out using antibodies against PTB (BB7), Raver1, and U1A.
Mass Spectrometry
To obtain proteins, the complexes were treated with RNase A (0.05mg ml−1) at 37°C for one hour and TCA precipitated. Precipitated proteins were resuspended in 8M urea, subjected to carboxyamidomethylation of cysteines, and digested with trypsin. A nano LC column was packed in a 100 μm inner diameter glass capillary with an emitter tip. The column consisted of 10 cm of Polaris c18 5 μm packing material (Varian), followed by 4 cm of Partisphere 5 SCX (Whatman), then another 2 cm of Polaris C18 to form a column for 3 phase MudPIT analysis57. The column was loaded by use of a pressure bomb and washed extensively with buffer A (see below). The column was then directly coupled to an electrospray ionization source mounted on a Thermo-Finnigan Decca XP Plus mass spectrometer. An Agilent 1100 HPLC equipped with a split line so as to deliver a flow rate of 30 nl/min was used for chromatography. Peptides were eluted using a 14-step MudPIT procedure35. Buffer A was 5% (v/v) acetonitrile/0.02% (v/v) heptaflurobutyric acid (HBFA); buffer B was 80% (v/v) acetonitrile/0.02% (v/v) HBFA. Buffer C was 250 mM ammonium acetate/5% (v/v) acetonitrile/0.02% (v/v) HBFA; buffer D was same as buffer C, but with 500 mM ammonium acetate. The programs SEQUEST and DTASELECT were used to identify peptides and proteins from the complete human database58,59. Xcorr cutoffs of 1.8, 2.2 and 3.5 were used for inclusion of peptides with +1, +2 and +3 charge states, respectively. These cutoffs have been shown to yield peptide identifications with considerably less than a 1% false positive rate60. Two or more peptides meeting these statistical criteria were required to consider a protein as positively identified. The resulting false positive rate for protein identification is calculated to be less than 0.01%.
Supplementary Material
Acknowledgments
We thank Kristen Lynch, Tim Nilsen, and members of the Black lab for helpful discussion and comments. We thank Brigitte Jokusch (Technical University of Braunschweig, Braunschweig) for the gift of anti Raver-1 antibody and Carol Lutz (University of Medicine and Dentistry of New Jersey, Newark) for the anti-U1A antibody. This work was supported by NIH grants RO1:GM49662 to DLB, and RO1:GM61987 and 1S10RR017780-01 to DCR. DLB in an investigator of the Howard Hughes Medical Institute.
References
- 1.Smith CW, Valcarcel J. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem Sci. 2000;25:381–8. doi: 10.1016/s0968-0004(00)01604-2. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Lee JA, Black DL. Neuronal regulation of alternative pre-mRNA splicing. Nat Rev Neurosci. 2007 doi: 10.1038/nrn2237. [DOI] [PubMed] [Google Scholar]
- 3.Shin C, Manley JL. Cell signalling and the control of pre-mRNA splicing. Nat Rev Mol Cell Biol. 2004;5:727–38. doi: 10.1038/nrm1467. [DOI] [PubMed] [Google Scholar]
- 4.Matlin AJ, Clark F, Smith CW. Understanding alternative splicing: towards a cellular code. Nature. 2005;6:386–398. doi: 10.1038/nrm1645. [DOI] [PubMed] [Google Scholar]
- 5.Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- 6.House AE, Lynch KW. Regulation of alternative splicing:more than just the ABCs. J Biol Chem. 2007 doi: 10.1074/jbc.R700031200. in press. [DOI] [PubMed] [Google Scholar]
- 7.Nilsen TW. The spliceosome: the most complex macromolecular machine in the cell? Bioessays. 2003;25:1147–9. doi: 10.1002/bies.10394. [DOI] [PubMed] [Google Scholar]
- 8.Jurica MS, Moore MJ. Pre-mRNA splicing: awash in a sea of proteins. Mol Cell. 2003;12:5–14. doi: 10.1016/s1097-2765(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 9.Will CL, Luhrmann R. Spliceosome Structure and Function. In: Gesteland RF, Cech TR, Atkin JF, editors. RNA World. Cold Spring Harbor Laboratory Press; 2006. pp. 525–560. [Google Scholar]
- 10.Sharma S, Falick AM, Black DL. Polypyrimidine tract binding protein blocks the 5′ splice site-dependent assembly of U2AF and the prespliceosomal E complex. Mol Cell. 2005;19:485–96. doi: 10.1016/j.molcel.2005.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gozani O, Potashkin J, Reed R. A potential role for U2AF-SAP 155 interactions in recruiting U2 snRNP to the branch site. Mol Cell Biol. 1998;18:4752–60. doi: 10.1128/mcb.18.8.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donmez G, Hartmuth K, Kastner B, Will CL, Luhrmann R. The 5′ end of U2 snRNA is in close proximity to U1 and functional sites of the pre-mRNA in early spliceosomal complexes. Mol Cell. 2007;25:399–411. doi: 10.1016/j.molcel.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Kent OA, MacMillan AM. Early organization of pre-mRNA during spliceosome assembly. Nat Struct Biol. 2002;9:576–81. doi: 10.1038/nsb822. [DOI] [PubMed] [Google Scholar]
- 14.Kent OA, Ritchie DB, Macmillan AM. Characterization of a U2AF-independent commitment complex (E′) in the mammalian spliceosome assembly pathway. Mol Cell Biol. 2005;25:233–40. doi: 10.1128/MCB.25.1.233-240.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brow DA. Allosteric cascade of spliceosome activation. Annu Rev Genet. 2002;36:333–60. doi: 10.1146/annurev.genet.36.043002.091635. [DOI] [PubMed] [Google Scholar]
- 16.Staley JP, Guthrie C. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell. 1998;92:315–26. doi: 10.1016/s0092-8674(00)80925-3. [DOI] [PubMed] [Google Scholar]
- 17.Berget SM. Exon recognition in vertebrate splicing. J Biol Chem. 1995;270:2411–4. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- 18.Black DL. Finding splice sites within a wilderness of RNA. Rna. 1995;1:763–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Fox-Walsh KL, et al. The architecture of pre-mRNAs affects mechanisms of splice-site pairing. Proc Natl Acad Sci U S A. 2005;102:16176–81. doi: 10.1073/pnas.0508489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterner DA, Carlo T, Berget SM. Architectural limits on split genes. Proc Natl Acad Sci U S A. 1996;93:15081–5. doi: 10.1073/pnas.93.26.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner EJ, Garcia-Blanco MA. Polypyrimidine tract binding protein antagonizes exon definition. Mol Cell Biol. 2001;21:3281–8. doi: 10.1128/MCB.21.10.3281-3288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spellman R, Smith CW. Novel modes of splicing repression by PTB. Trends Biochem Sci. 2006;31:73–6. doi: 10.1016/j.tibs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Oberstrass FC, et al. Structure of PTB bound to RNA: specific binding and implications for splicing regulation. Science. 2005;309:2054–7. doi: 10.1126/science.1114066. [DOI] [PubMed] [Google Scholar]
- 24.Lin CH, Patton JG. Regulation of alternative 3′ splice site selection by constitutive splicing factors. Rna. 1995;1:234–45. [PMC free article] [PubMed] [Google Scholar]
- 25.Singh R, Valcarcel J, Green MR. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995;268:1173–6. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- 26.Sauliere J, Sureau A, Expert-Bezancon A, Marie J. The polypyrimidine tract binding protein (PTB) represses splicing of exon 6B from the beta-tropomyosin pre-mRNA by directly interfering with the binding of the U2AF65 subunit. Mol Cell Biol. 2006;26:8755–69. doi: 10.1128/MCB.00893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlet BN, Logan P, Singh G, Cooper TA. Dynamic antagonism between ETR-3 and PTB regulates cell type-specific alternative splicing. Mol Cell. 2002;9:649–58. doi: 10.1016/s1097-2765(02)00479-3. [DOI] [PubMed] [Google Scholar]
- 28.Chan RC, Black DL. The polypyrimidine tract binding protein binds upstream of neural cell-specific c-src exon N1 to repress the splicing of the intron downstream. Mol Cell Biol. 1997;17:4667–76. doi: 10.1128/mcb.17.8.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southby J, Gooding C, Smith CW. Polypyrimidine tract binding protein functions as a repressor to regulate alternative splicing of alpha-actinin mutally exclusive exons. Mol Cell Biol. 1999;19:2699–711. doi: 10.1128/mcb.19.4.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amir-Ahmady B, Boutz PL, Markovtsov V, Phillips ML, Black DL. Exon repression by polypyrimidine tract binding protein. Rna. 2005;11:699–716. doi: 10.1261/rna.2250405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou MY, Underwood JG, Nikolic J, Luu MH, Black DL. Multisite RNA binding and release of polypyrimidine tract binding protein during the regulation of c-src neural-specific splicing. Mol Cell. 2000;5:949–57. doi: 10.1016/s1097-2765(00)80260-9. [DOI] [PubMed] [Google Scholar]
- 32.Markovtsov V, et al. Cooperative assembly of an hnRNP complex induced by a tissue-specific homolog of polypyrimidine tract binding protein. Mol Cell Biol. 2000;20:7463–79. doi: 10.1128/mcb.20.20.7463-7479.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black DL. Activation of c-src neuron-specific splicing by an unusual RNA element in vivo and in vitro. Cell. 1992;69:795–807. doi: 10.1016/0092-8674(92)90291-j. [DOI] [PubMed] [Google Scholar]
- 34.Das R, Reed R. Resolution of the mammalian E complex and the ATP-dependent spliceosomal complexes on native agarose mini-gels. Rna. 1999;5:1504–8. doi: 10.1017/s1355838299991501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacCoss MJ, et al. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci U S A. 2002;99:7900–5. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rideau AP, et al. A peptide motif in Raver1 mediates splicing repression by interaction with the PTB RRM2 domain. Nat Struct Mol Biol. 2006;13:839–48. doi: 10.1038/nsmb1137. [DOI] [PubMed] [Google Scholar]
- 37.Behzadnia N, et al. Composition and three-dimensional EM structure of double affinity-purified, human prespliceosomal A complexes. Embo J. 2007;26:1737–48. doi: 10.1038/sj.emboj.7601631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiara MD, Reed R. A two-step mechanism for 5′ and 3′ splice-site pairing. Nature. 1995;375:510–3. doi: 10.1038/375510a0. [DOI] [PubMed] [Google Scholar]
- 39.Bruzik JP, Maniatis T. Enhancer-dependent interaction between 5′ and 3′ splice sites in trans. Proc Natl Acad Sci U S A. 1995;92:7056–9. doi: 10.1073/pnas.92.15.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konforti BB, Konarska MM. A short 5′ splice site RNA oligo can participate in both steps of splicing in mammalian extracts. Rna. 1995;1:815–27. [PMC free article] [PubMed] [Google Scholar]
- 41.Makarova OV, et al. A subset of human 35S U5 proteins, including Prp19, function prior to catalytic step 1 of splicing. Embo J. 2004;23:2381–91. doi: 10.1038/sj.emboj.7600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blencowe BJ, et al. The SRm160/300 splicing coactivator subunits. Rna. 2000;6:111–20. doi: 10.1017/s1355838200991982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eldridge AG, Li Y, Sharp PA, Blencowe BJ. The SRm160/300 splicing coactivator is required for exon-enhancer function. Proc Natl Acad Sci U S A. 1999;96:6125–30. doi: 10.1073/pnas.96.11.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soares LM, Zanier K, Mackereth C, Sattler M, Valcarcel J. Intron removal requires proofreading of U2AF/3′ splice site recognition by DEK. Science. 2006;312:1961–5. doi: 10.1126/science.1128659. [DOI] [PubMed] [Google Scholar]
- 45.Will CL, et al. Characterization of novel SF3b and 17S U2 snRNP proteins, including a human Prp5p homologue and an SF3b DEAD-box protein. Embo J. 2002;21:4978–88. doi: 10.1093/emboj/cdf480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu YZ, et al. Prp5 bridges U1 and U2 snRNPs and enables stable U2 snRNP association with intron RNA. Embo J. 2004;23:376–85. doi: 10.1038/sj.emboj.7600050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perriman R, Barta I, Voeltz GK, Abelson J, Ares M., Jr ATP requirement for Prp5p function is determined by Cus2p and the structure of U2 small nuclear RNA. Proc Natl Acad Sci U S A. 2003;100:13857–62. doi: 10.1073/pnas.2036312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin KT, Lu RM, Tarn WY. The WW domain-containing proteins interact with the early spliceosome and participate in pre-mRNA splicing in vivo. Mol Cell Biol. 2004;24:9176–85. doi: 10.1128/MCB.24.20.9176-9185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abovich N, Rosbash M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell. 1997;89:403–12. doi: 10.1016/s0092-8674(00)80221-4. [DOI] [PubMed] [Google Scholar]
- 50.Gromak N, Matlin AJ, Cooper TA, Smith CW. Antagonistic regulation of alpha-actinin alternative splicing by CELF proteins and polypyrimidine tract binding protein. Rna. 2003;9:443–56. doi: 10.1261/rna.2191903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho TH, et al. Muscleblind proteins regulate alternative splicing. Embo J. 2004;23:3103–12. doi: 10.1038/sj.emboj.7600300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu J, Mayeda A, Krainer AR. Exon identity established through differential antagonism between exonic splicing silencer-bound hnRNP A1 and enhancer-bound SR proteins. Mol Cell. 2001;8:1351–61. doi: 10.1016/s1097-2765(01)00409-9. [DOI] [PubMed] [Google Scholar]
- 53.Izquierdo JM, et al. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol Cell. 2005;19:475–84. doi: 10.1016/j.molcel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 54.House AE, Lynch KW. An exonic splicing silencer represses spliceosome assembly after ATP-dependent exon recognition. Nat Struct Mol Biol. 2006;13:937–44. doi: 10.1038/nsmb1149. [DOI] [PubMed] [Google Scholar]
- 55.Damianov A, Schreiner S, Bindereif A. Recycling of the U12-type spliceosome requires p110, a component of the U6atac snRNP. Mol Cell Biol. 2004;24:1700–8. doi: 10.1128/MCB.24.4.1700-1708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou Z, Licklider LJ, Gygi SP, Reed R. Comprehensive proteomic analysis of the human spliceosome. Nature. 2002;419:182–5. doi: 10.1038/nature01031. [DOI] [PubMed] [Google Scholar]
- 57.McDonald WH, Ryoma O, Miyamoto DT, Mitchison TJ, Yates JR., 3rd Comparison of three directly coupled HPLC MS/MS strategies for identification of proteins from complex mixtures: single-dimension LC-MS/MS, 2-phase MudPIT, and 3-phase MudPIT. Int J Mass Spectrom. 2002;219:245–251. [Google Scholar]
- 58.Eng JK, MacCormak AL, Yates JR., 3rd An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Specrtom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 59.Tabb DL, McDonald WH, Yates JR., 3rd DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res. 2002;1:21–6. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elias JE, Haas W, Faherty BK, Gygi SP. Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat Methods. 2005;2:667–75. doi: 10.1038/nmeth785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.