Abstract
Yersinia type III machines secrete protein substrates across the bacterial envelope and, following assembly of their secretion needles, transport effector Yops into host cells. According to their destination during type III secretion, early, middle, and late secretion substrates can be distinguished; however, the signals and mechanisms whereby these proteins are recognized and transported by the secretion machine are not understood. Here, we examine several hybrids between secretion substrates and the impassable reporter protein glutathione S-transferase (GST). YscP-GST and YopR-GST blocked type III secretion; however, YscF-, YopD-, YopN-, and LcrV-GST did not. Unlike YopR-GST, which can block type III machines only during their assembly, expression of YscP-GST led to an immediate and complete block of all secretion. The secretion signal of YscP was mapped to its first 10 codons or amino acids; however, YscPΔ2-15-GST, lacking this secretion signal, imposed a partial blockade. YscP-GST copurified with the type III ATPase complex (YscN, YscL, and YscQ) and with YscO, suggesting that the association of specific machine components with the impassable substrate may cause the block in type III secretion.
Pathogenic yersiniae, including Yersinia enterocolitica, use the type III secretion system (T3SS) to combat host immune defenses (50, 71). Yersinia type III secretion machines are constructed from more than 25 proteins, resulting in the production of a needlelike structure on the bacterial cell surface known as the type III secretion apparatus (3, 7, 21, 30, 36). The needle is inserted into host cell membranes and creates a conduit through which effector Yop proteins are injected, hindering phagocytosis through the inhibition of actin polymerization and causing cell death (23, 36, 49, 70, 83). In yersiniae, the genes for the type III secretion machine (Yersinia secretion genes; ysc) and its substrates (Yersinia outer proteins; yop) are found on a 70-kb virulence plasmid clustered into several operons (3, 21, 53). Spanning the bacterial membrane, the proximal part of the type III secretion apparatus assumes rotational symmetry and shares significant structural homology with the basal body of flagella (10, 30, 41, 51). Assembly of the needle portion of the apparatus requires the secretion of an early substrate, namely, YscF, which polymerizes into a hollow tube on the bacterial surface (36, 49, 59). YopR and YscP are additional early secretion substrates (3, 45, 63). YscP is known to regulate the length of the needle, whereas YopR has no known function (2, 40, 76). Once needle construction is complete, middle substrates, known as translocators (LcrV, YopB, and YopD), are secreted (33, 47). LcrV is deposited at the tips of needles, and YopB and YopD are thought to be inserted into host cell membranes, forming a translocation pore (13, 60, 61, 73). Upon insertion into the cytosol of host cells, needles are thought to encounter a drop in perceived calcium levels, thereby triggering type III machines to transport late substrates, Yop effectors (YopE, YopH, YopM, YopN, YopO, YopP, YopQ, and YopT), into host cells (6, 11, 18, 26, 31, 34, 37, 44, 54, 70).
Each T3SS apparatus is thought to undergo a defined set of developmental steps as it matures into a functional needle complex (19, 24, 75). How bacteria coordinate the assembly of this apparatus with the ordered secretion of different classes of substrates remains a mystery. Do the secretion signals of individual substrates define the developmental stage at which they are recognized and secreted by the machine? In order to study the secretion signals of proteins that travel the type III pathway, translational fusions of secreted substrates to impassable reporter proteins were constructed (77). These impassable reporters include glutathione S-transferase (GST) and dihydrofolate reductase (DHFR), as well as ubiquitin (Ub) (46, 76). The best-characterized impassable substrates of the Yersinia T3SS are YopE and YopR. YopE, a late effector protein, travels the pathway at its final stage and is destined for injection into host cells (20, 70, 80). Injection requires SycE, a specific chaperone that binds to residues 15 to 100 of YopE and dissociates from the substrate before it leaves the bacterial cell (17, 20, 43, 79, 84, 87). YopE-DHFR, when expressed in wild-type yersiniae, does not block the machine and is also rejected from the type III secretion (TTS) pathway (77, 78). YopE-DHFR also represses the synthesis of other secretion substrates (77). The type III rejection phenotype of YopE-DHFR holds true for hybrids with other effectors, including YopQ and YopH (77, 78). YopR, an early substrate, is secreted into extracellular media prior to completion of the type III conduit and presumably does not require a chaperone for its secretion (3, 45). In contrast to YopE, YopR hybrids with impassable substrates block type III machines; however, this occurs only during early stages of development, when the T3SS conduit has not yet been completed (76). From this and other information on the positions of secreted products along the T3SS, one can infer that the selection of substrates by the type III machine must be ordered.
Here, we sought to use translational fusions of substrates with both passable and impassable reporter proteins to investigate the mechanisms whereby type III machines select their substrates. A unique curiosity of type III secretion is that hybrids formed between middle or late substrates and an impassable hybrid are rejected and cannot block the secretion machine. Nevertheless, fusions with two early substrates, YopR and YscP, cause a block of T3SS. Understanding the molecular attributes of these events may provide insight into the mechanisms of substrate recognition.
MATERIALS AND METHODS
Bacterial strains and media.
Y. enterocolitica strains W22703 (wild type) (22) and EC2 Δ(yscM1 yscM2) (16) were propagated in tryptic soy broth (TSB) or agar, brain heart infusion (BHI) broth, or M9-Casamino Acids minimal medium as indicated. Escherichia coli was grown in Luria broth or agar at 37°C. Antibiotics were added to a final concentration of 35 μg/ml of chloramphenicol or 50 μg/ml of kanamycin where appropriate for plasmid retention. Yersinia pestis KIM D27 (14), a KIM5 (nonpigmented) variant of Y. pestis biovar medievalis KIM lacking the 102-kb pgm locus (29), was grown in heart infusion broth or agar (HIA) at 26°C. The media were supplemented with 20 mM MgCl2 and 20 mM oxalic acid (HIA/MgOx) for low-calcium conditions and 35 μg/ml chloramphenicol for plasmid maintenance. Standard methods for transformation of plasmids into E. coli, Y. pestis, and Y. enterocolitica were employed.
Plasmid construction.
Construction of yopR-gst (pJS116) and pgst have been previously reported (69, 76). To construct the remaining glutathione-S transferase fusion proteins, we followed a common strategy. yscF and yscP were amplified from the Y. enterocolitica pYVe227 virulence plasmid via PCR using Go-Taq DNA polymerase. The following primers were used: 5′YscF-NdeI (5′-AACATATGATGAGTAATTTCTCTGGGTTTACAAAAGG-3′) and 3′YscF-BglII (5′-AAAGATCTTGGGAACTTCTGTAGGATGCCTTGC-3′), and 5′YscP-NdeI (5′-AACATATGAATAAAATCACCACTCGTTCCCCATTAG-3′) and 3′YscP-BglII (5′-AAAGATCTTTCTTCAGCCTCCCACTCCTCATAG-3′). The PCR products were cloned into pCR2.1 (Invitrogen), digested with NdeI and BglII, and ligated into pJS116 (a moderate-copy-number pBBR1MCS-2 plasmid derivative containing a C-terminal GST fusion protein) (76) cut with NdeI and BglII to yield pJS169 and pJS170, respectively.
The GST gene (gst) was amplified from pGEX-2TK with Go-Taq DNA polymerase using primers KpnI GST F (5′-AAGGTACCTCCCCTATACTAGGTTATTGGAA-3′) and BglII GST R (5′-AAAGATCTTCAGTCACGATGAATTCCCG-3′). gst was cloned into pCR2.1 and digested with KpnI and BglII. pDA41 (a low-copy-number pHSG576/pSC101 derivative modified to contain the Ptac promoter driving expression of yopE-npt) was digested with KpnI and BamHI (4, 81). gst was ligated into digested pDA41 to yield pKER127 (yopE-gst). yopD, lcrV, and yopN were PCR amplified from the Y. enterocolitica pYV227 virulence plasmid using Go-Taq DNA polymerase. The following primers were used: NdeI-YopD F (5′-AACATATGACAATAAATATCAAGACAGACAGC-3′), KpnI YopD R (5′-AAGGTACCGACAACACCAAAAGCGGCTTTCAT-3′), NdeI-LcrV F (5′-AACATATGATTAGAGCCTACGAACAAAACCCA-3′), KpnI LcrV R (5′-AAGGTACCCCTCGTGTCATCTAGCAGACGTTGCAT-3′), NdeI-YopN F (5′AAAACATATGACGACGCTTCATAACCTATC-3′), and KpnI YopN FL R (5′-AAGGTACCGAAAGGTCGTAAGCCATTAGTTAT-3′). The PCR products were cloned into pCR2.1 (Invitrogen), digested with NdeI and KpnI, and ligated into pKER127 previously digested with NdeI and KpnI to yield pKER131 (yopD-gst), pKER132 (yopN-gst), and pKER133 (lcrV-gst), respectively.
For the construction of pyscP, YscP was amplified from the Y. enterocolitica pYVe227 virulence plasmid via PCR using Go-Taq DNA polymerase. The following primers were used: 5′YscPext-NdeI (5′-AACATATGTTTCTACAGCATCACAGGAACG-3′) and 3′YscPext-BamHI (5′-AAGGATCCGTTCCAACAGCGCAAGTTGCA-3′). The PCR products were cloned into pCR2.1 (Invitrogen), digested with NdeI and BamHI, and ligated into pDA37 previously digested with NdeI and BamHI to yield pKER76 (pyscP).
For the construction of Ub fusion proteins, the Ub gene and its variant Ub3,13 gene (carrying two mutations that replace Ub isoleucine codons 3 and 13 with glycine) were amplified using the following primers: 5′Ub-BglII (5′-GGAAGATCTATGCAGATTTTCGTCAAGACTTTG-3′), 3′Ub-XbaI (5′-CTAGTCTAGATTAACCACCTCTTAGCCTTAGCACAAG-3′), and 5′Ub3,13-BglII (5′-GGAAGATCTATGCAGGGTTTCGTCAAGACTTTGACCGGTAAAACCGG AACATTG-3′). The PCR products were cloned into pCR2.1, digested with BglII and XbaI, and ligated into pJS170 previously digested with BglII and XbaI to generate pJS200 and pJS201 expressing yscP-Ub gene and yscP-Ub3,13 gene, respectively.
For construction of the C-terminal yscP truncations (1-300, 1-200, 1-100, and 1-25) fused to gst, yscP fragments were PCR amplified with Pfu polymerase and cloned into pCR2.1. The recombinant plasmid was digested with NdeI and BglII and ligated into pJS116 digested with the same enzymes to yield pJS182 (yscP1-300-gst), pJS183 (yscP1-200-gst), pJS184 (yscP1-100-gst), and pJS186 (yscP1-25-gst). The low-copy-number plasmid pDA44 (81) was digested with NdeI and KpnI. The minimal secretion signals of yscP and its variants were generated by annealed oligonucleotide cloning and ligated into the NdeI/KpnI site to generate neomycin phosphotransferase (npt) fusions.
For construction of the N-terminal yscP truncations (Δ2-15, Δ2-50, Δ2-100, and Δ2-150), yscP fragments were PCR amplified with Pfu polymerase and cloned into pCR2.1. The recombinant plasmid was digested with NdeI and KpnI and ligated into pKER127 digested with the same enzymes to yield pKER147 (yscPΔ2-15-gst), pKER148 (yscPΔ2-50-gst), pKER152 (yscPΔ2-100-gst), and pKER151 (yscPΔ2-150-gst).
For the construction of 3′ npt fusions, full-length, 1-300, 1-200, 1-100, and Δ2-15 yscP fragments were PCR amplified with Pfu polymerase and cloned into pCR2.1. The recombinant plasmids were digested with NdeI and KpnI and ligated into pDA41 previously digested with the same enzymes to yield plasmids pKER156 (yscP-npt), pKER161 (yscP1-300-npt), pKER162 (yscP1-200-npt), pKER163 (yscP1-100-npt), and pKER155 (yscPΔ2-15-npt). All sequences were verified by the University of Chicago Cancer Research Center DNA-sequencing facility. Table 1 lists additional primers used in this study.
TABLE 1.
Primers used in this study
| No. | Name | Sequencea |
|---|---|---|
| 1 | YscP1-15 Nde-Kpn 1 | TATGAATAAAATCACCACTCGTTCCCCATTAGAACCTGAGTATCAAGGTAC |
| 2 | YscP1-15 Nde-Kpn 2 | CTTGATACTCAGGTTCTAATGGGGAACGAGTGGTGATTTTATTCA |
| 3 | YscP1-14 Nde-Kpn 1 | TATGAATAAAATCACCACTCGTTCCCCATTAGAACCTGAGTATGGTAC |
| 4 | YscP1-14 Nde-Kpn 2 | CATACTCAGGTTCTAATGGGGAACGAGTGGTGATTTTATTCA |
| 5 | YscP1-13 Nde-Kpn 1 | TATGAATAAAATCACCACTCGTTCCCCATTAGAACCTGAGGGTAC |
| 6 | YscP1-13 Nde-Kpn 2 | CCTCAGGTTCTAATGGGGAACGAGTGGTGATTTTATTCA |
| 7 | YscP1-12 Nde-Kpn 1 | TATGAATAAAATCACCACTCGTTCCCCATTAGAACCTGGTAC |
| 8 | YscP1-12 Nde-Kpn 2 | CAGGTTCTAATGGGGAACGAGTGGTGATTTTATTCA |
| 9 | YscP1-11 Nde-Kpn 1 | TATGAATAAAATCACCACTCGTTCCCCATTAGAAGGTAC |
| 10 | YscP1-11 Nde-Kpn 2 | CTTCTAATGGGGAACGAGTGGTGATTTTATTCA |
| 11 | YscP1-10 Nde-Kpn 1 | TATGAATAAAATCACCACTCGTTCCCCATTAGGTAC |
| 12 | YscP1-10 Nde-Kpn 2 | CTAATGGGGAACGAGTGGTGATTTTATTCA |
| 13 | YscP1-9 Nde-Kpn 1 | TATGAATAAAATCACCACTCGTTCCCCAGGTAC |
| 14 | YscP1-9 Nde-Kpn 2 | CTGGGGAACGAGTGGTGATTTTATTCA |
| 15 | YscP1-8 Nde-Kpn 1 | TATGAATAAAATCACCACTCGTTCCGGTAC |
| 16 | YscP1-8 Nde-Kpn 2 | CGGAACGAGTGGTGATTTTATTCA |
| 17 | YscP1-7 Nde-Kpn 1 | TATGAATAAAATCACCACTCGTGGTAC |
| 18 | YscP1-7 Nde-Kpn 2 | CACGAGTGGTGATTTTATTCA |
| 19 | YscP1-6 Nde-Kpn 1 | TATGAATAAAATCACCACTGGTAC |
| 20 | YscP1-6 Nde-Kpn 2 | CAGTGGTGATTTTATTCA |
| 21 | YscP15 mut NdeI-KpnI F | TATGAACAAGATAACAACGCGGAGCCCCCTGGAGCCGGAATACCAGGGTAC |
| 22 | YscP15 mut NdeI-KpnI R | CCTGGTATTCCGGCTCCAGGGGGCTCCGCGTTGTTATCTTGTTCA |
| 23 | YscP 15 + 1 NdeI-KpnI F | TATGAAATATAATCACCACTCGTTCCCCATTAGAACCTGTGTATCAGGTAC |
| 24 | YscP 15 + 1 NdeI-KpnI R | CTGATACACAGGTTCTAATGGGGAACGAGTGGTGATTATATTTCA |
| 25 | 3′YscP300 BglII | AAAGATCTGGTATCTTTGCTGCTATCAACGCCAT |
| 26 | 3′YscP200 BglII | AAAGATCTGACAGACCACACAATAACCTCTGCG |
| 27 | 3′YscP100 BglII | AAAGATCTGTTTAAATCATGATTATTCTGATGGT |
| 28 | 3′YscP25 BglII | AAAGATCTTTGCAAATCATGATGCAGCTTCCCC |
| 29 | 5′YscP-NdeI | AACAACAACATATGAATAAAATCACCACTCGTTCCCCATTAG |
| 30 | NdeI YscP Δ2-15 F | GGGAATTCCATATGCCTCTGGGGAAGCTGCATCATGATTTGCAA |
| 31 | NdeI YscP Δ2-50 F | GGGAATTCCATATGAGACCTGTACGTCCGCATGACCTTGGC |
| 32 | NdeI YscP Δ2-100 F | GGGAATTCCATATGAACTTATCTCCGCTTGCCGAAGGTGTTACC |
| 33 | NdeI YscP Δ2-150 F | GGGAATTCCATATGAGTGAATCCCCCTGCGAGCCGTCTGGACAT |
| 34 | KpnI YscP R (no stop) | CGGGGTACCTTCTTCAGCCTCCCACTCCTCATAGACGTGGCG |
Boldface indicates restriction sites.
TTS needle purification and electron microscopy.
Needles were purified as described previously (59). Bacteria were grown overnight in 300 ml BHI supplemented with 20 mM MgCl2 and 20 mM sodium oxalate. The bacteria were sedimented by centrifugation for 15 min at 2,700 × g and suspended with 10 ml 1 M Tris-HCl, pH 7.5. The bacteria were again sedimented at 8,000 × g, and the supernatant was passed through a 0.45-μm cellulose acetate membrane filter (Whatman) and centrifuged for 30 min at 40,000 × g. The resulting pellet was suspended in 100 μl 1 M Tris-HCl, separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotted with antisera specific for YscF and LcrV. Samples were placed on a carbon-coated copper grid, rinsed with H2O, and stained with 2% uranyl acetate. Samples were viewed on a Tecnai F30 electron microscope.
Hydroxylamine mutagenesis of yscP-gst and LCR phenotypes.
Plasmid pJS170 containing the yscP gene under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Ptac promoter was subjected to hydroxylamine mutagenesis. A fresh 1 M NH2OH solution of hydroxylamine at pH 6.0 was mixed in a 1:1 (vol/vol) ratio with newly isolated pJS170. The solution was incubated at 68°C for 2 h, after which DNA was isolated using QIAquick PCR purification (Qiagen). Ten microliters of hydroxylamine-treated pJS170 was electroporated into Y. pestis KIM D27 that had been diluted 1:20 into 20 ml of heart infusion broth and grown for 3 h at 26°C. Transformants were recovered at 26°C for 2 h and plated on HIA containing 35 μg/ml kanamycin for plasmid selection. Colony material was patched on HIA/MgOx plates containing 35 μg/ml of kanamycin and 1 mM IPTG and grown at 26°C and 37°C. Colonies that no longer grew at 37°C (phenotype, low calcium response positive [LCR+]) were selected. The yscP gene was amplified using primers 5′YscP F NdeI (5′-AAACATATGAATAAAATCACCACTCGTTCCCCA-3′) and 3′ YscP fl R KpnI (5′-AAAGGTACCTTCTTCAGCCTCCCACTCCTCATAGAC-3′) and sequenced with the above-mentioned primers and internal primers YscP screen 1 (5′-TTTGTACGGAAAACGAGCAGATTTTTCTGCTAC-3′) and YscP screen 2 (5′-GCTCTTCATCAAAAAGCATTGCCAGAGATATGT-3′). The PCR products were sequenced at the University of Chicago Cancer Research Center DNA-sequencing facility, and mutations were confirmed by comparison with the native yscP sequence.
Type III secretion.
Yersinia strains were grown overnight in TSB containing 35 μg/ml of either kanamycin or chloramphenicol. The cultures were diluted 50-fold into fresh BHI supplemented with antibiotic, 20 mM oxalic acid, and 20 mM MgCl2 or M9 minimal medium and grown for 2 h at 26°C. The cultures were shifted to 37°C for 3 h to induce type III secretion. Where indicated, IPTG was added to induce the expression of hybrid fusion proteins. Samples were fractionated and analyzed for type III secretion.
Digitonin fractionation of Yersinia-infected HeLa cells.
HeLa cells were grown in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and 2 mM glutamine at 37°C in 5% CO2. Two hours prior to infection, overnight Yersinia cultures were diluted 1:50 into fresh TSB containing 35 μg/ml kanamycin. Ten minutes prior to infection, 1 mM IPTG was added to induce expression of the indicated genes. One hour prior to infection, 90% confluent HeLa cells were washed two times with phosphate-buffered saline (PBS), and 10 ml of Optimem medium and 1 mM IPTG were added. HeLa cells were infected with the indicated Yersinia strains at a multiplicity of infection (MOI) of 10. Three hours after infection, the tissue culture medium was decanted and centrifuged at 15,000 rpm for 15 min. Proteins in 7 ml of supernatant were precipitated with methanol/chloroform, and the rest of the supernatant was discarded. The pellets were suspended in 10 ml of 1% SDS in PBS, and 7 ml of the pellet fraction was precipitated with methanol/chloroform. Digitonin (1%) in PBS was added to the tissue cultures cells and their attached bacteria for 20 min at room temperature to disrupt HeLa cell plasma membranes. Following HeLa cell disruption, the cell remnants were detached from the flasks using a cell scraper, the digitonin lysates were centrifuged, and proteins in the supernatant and pellet were processed as described above. Samples were separated on 15% SDS-PAGE gels, transferred to polyvinylidene difluoride (PVDF) membranes, and immunoblotted using specific polyclonal antisera to YopR, YopE, YopH, YopN, YopB, YopD, LcrV, IκB, and RpoA.
Cytotoxicity assay.
HeLa cell tissue cultures (3 × 105 cells) were grown in 12-well tissue culture plates and infected as described above. After 3 hours of infection, the medium was removed and the cells were fixed for 20 min in a solution of 3.7% formaldehyde in PBS. Fixation was quenched with 0.1 M glycine in PBS. The cells were permeabilized with 0.1% Triton X-100 in PBS for 30 min at 4°C. The cells were washed and blocked for 15 min with PBS containing 0.05% Tween 20 and 5% skim milk. Actin filaments were labeled with 99 nM (3 U) of rhodamine-phalloidin (Flexa) for 20 min at room temperature. The labeling solution was removed, and each well was washed with PBS. The cells were visualized with a Nikon TE2000-U inverted microscope. Rhodamine visualization was achieved through excitation at 591 nm and emission at 608 nm. Images were captured with a Cascade 1K charge-coupled device camera.
Protein electrophoresis and immunodetection.
Proteins were separated by electrophoresis on 15% SDS-PAGE where indicated. For immunoblots, proteins were transferred to PVDF membranes (Millipore) and probed with antisera as described previously (17).
Protein purification.
For purification of YscP-GST, YscPΔ2-15-GST, and YscP1-100-GST, Y. enterocolitica W22703 containing pJS170, pKER147, and pJS184 were grown overnight in TSB medium containing 35 μg/ml kanamycin and 35 μg/ml chloramphenicol. The overnight cultures were diluted 20-fold into 1 liter of M9 minimal medium containing 35 μg/ml kanamycin/chloramphenicol and grown at 26°C for 3 h (optical density at 600 nm, 0.6). Upon shift to 37°C for 3 h, 1 mM IPTG was added to induce the expression of hybrid proteins. The cultures were harvested by centrifugation at 7,000 × g for 10 min, and the bacterial pellet was suspended in 20 ml of PBS. Samples were passed through a French pressure cell at 16,000 lb/in2 twice, and the lysate was clarified by ultracentrifugation at 100,000 × g for 30 min. The clarified lysate was loaded onto a 1-ml glutathione-Sepharose column (GE Healthcare) preequilibrated with PBS. The column was then washed with 10 column volumes of PBS, and the bound proteins were eluted with 20 mM glutathione, pH 8.0, in PBS.
RESULTS
Substrate fusions that block the Yersinia type III secretion pathway.
We constructed plasmid-encoded translational hybrids between the Yersinia type III substrate genes yscF, yscP, yopD, lcrV, and yopN and GST under the control of the IPTG-inducible Ptac promoter. Y. enterocolitica W22703 harboring various plasmids was induced for type III secretion by shifting broth cultures to 37°C and chelating calcium ions. The expression of translational hybrids was either induced with IPTG or left uninduced during bacterial growth over 3 hours. The cultures were then centrifuged, and the proteins secreted into the supernatant were separated on SDS-PAGE and stained with Coomassie brilliant blue (Fig. 1A). IPTG-induced expression of YscP-GST, YopD-GST, or YopN-GST blocked the secretion of YopB, YopD, YopE, YopH, YopN, YopR, and LcrV, whereas YscF-GST and LcrV-GST had no effect on Yersinia type III secretion (Fig. 1A). YopR-GST blocked the secretion of all substrates, whereas YopE-GST caused a reduction in the secretion of type III substrates (Fig. 1A) (76). The abilities of GST hybrids to block type III secretion were not correlated with the expression levels of fusion proteins, as the abundances of blocking substrates, e.g., YscP-GST, appeared to be reduced compared to those of nonblocking substrates (LcrV-GST) (Fig. 1A). The suppression of type III secretion implemented by YopE-GST is at least in part overcome by class II regulatory mutations, for example, Δ(yscM1 yscM2) in Y. enterocolitica strain EC2 (15, 77). IPTG-induced expression of substrate gene fusions with gst revealed that yscP-gst and yopR-gst, but not yopD-gst, yopN-gst, or yopE-gst, caused a blockade in type III secretion (Fig. 1B). Thus, GST hybrids with the needle shaft protein, YscF; the needle tip protein, LcrV; or the translocator, YopD, or with substrates that travel into the cytosol of host cells, YopE and YopN, can be rejected by the secretion machine. As a result, these polypeptides fail to jam the type III pathway (Fig. 1C). In contrast, YscP-GST, similarly to YopR-GST, causes a complete blockade that cannot be relieved even by class II mutations, preventing feedback inhibition of gene expression in the type III pathway (Fig. 1C).
FIG. 1.

YscP-GST and YopR-GST block the Yersinia T3SS. (A) Wild-type Y. enterocolitica W22703 expressing yscF-gst, yscP-gst, yopR-gst, yopD-gst, lcrV-gst, yopN-gst, and yopE-gst was induced for type III secretion by the chelation of calcium. The cultures were grown in the absence (−) and presence (+) of the inducer IPTG, and following centrifugation of culture aliquots, Yop proteins in the supernatant were separated by 12% SDS-PAGE and visualized by Coomassie blue staining. (Bottom) Proteins in the bacterial sediment (plus IPTG) were electrotransferred to PVDF membranes and immunoblotted with antisera to GST. (B) Y. enterocolitica strain EC2 [Δ(yscM1 yscM2)] expressing yscP-gst, yopR-gst, yopD-gst, yopN-gst, and yopE-gst was induced for type III secretion, and Yop proteins in the culture supernatant were detected by SDS-PAGE and Coomassie blue staining. Molecular mass markers (kilodaltons) are indicated to the left of the gel, while secreted Yop effectors are indicated to the right. (C) Illustration of a model in which YscP-GST and YopR-GST, but not YscF-, YopD-, LcrV-, YopN-, and YopE-GST, block the type III secretion machine.
YscP-GST blocks the formation of type III secretion needles.
Y. enterocolitica W22703 harboring gst, yscP-gst, and yopR-gst was grown overnight at 26°C in BHI containing 20 mM MgCl2 and 20 mM sodium oxalate in the absence or presence of the inducer, IPTG. Culture aliquots were centrifuged, and proteins in the supernatant and pellet were separated on SDS-PAGE and probed with antibodies directed against YscF, LcrV, YopE, and Cat or Npt (Fig. 2A). IPTG-induced expression of YscP-GST and YopR-GST, but not GST, blocked secretion of YscF, the needle shaft protein, and LcrV, the needle tip protein. Type III secretion needles were harvested from the same cultures, and purified needle preparations were separated by SDS-PAGE, probed with antisera to YscF and LcrV, and viewed by transmission electron microscopy (Fig. 2B and C). Even in the absence of IPTG, purified needles were not obtainable from Y. enterocolitica harboring yscP-gst, suggesting that only minor amounts of YscP-GST were required to prevent the production of polymerized TTS needles. YopR-GST prevented needle production only upon induction with IPTG, whereas expression of GST had no effect on needle production (Fig. 2B and C).
FIG. 2.
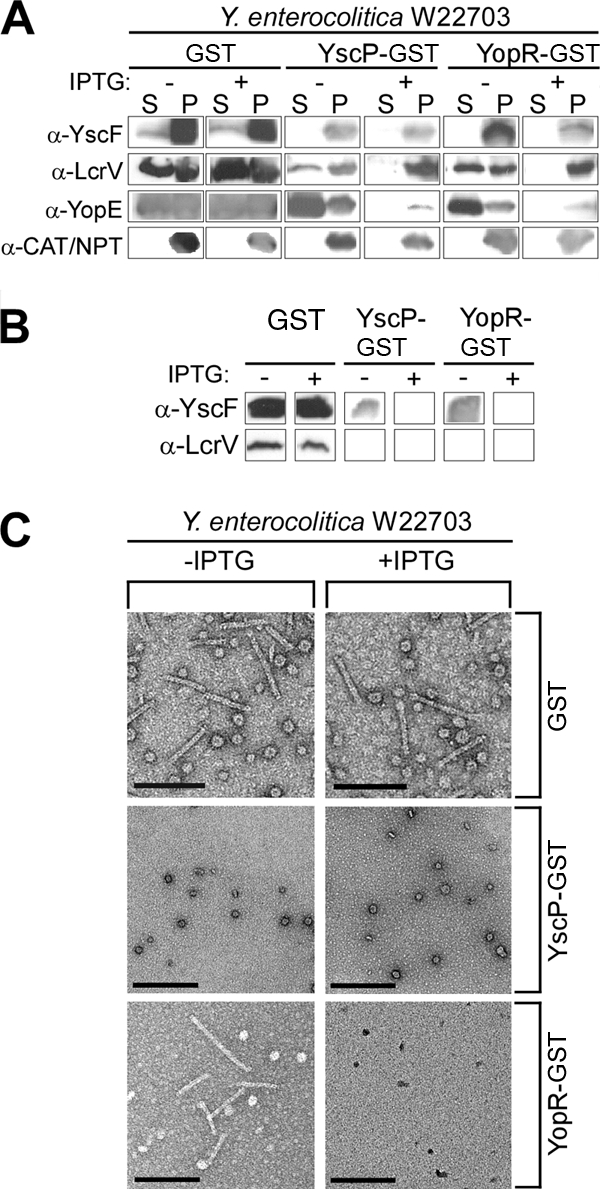
Expression of yscP-gst blocks needle formation in wild-type Y. enterocolitica. (A) Wild-type (WT) Y. enterocolitica expressing gst, yscP-gst, and yopR-gst was grown in the absence (−) and presence (+) of the inducer IPTG, and following centrifugation of culture aliquots, proteins in the supernatant (S) and pellet (P) were separated by SDS-PAGE, transferred to PVDF membranes, and probed with antisera to YscF, LcrV, YopE, and chloramphenicol acetyltransferase (CAT) or NPT. (B) Needles were purified from the cultures in panel A, and the purified needle preparations were separated by SDS-PAGE, transferred to PVDF membranes, and probed with antisera to YscF and LcrV. (C) Purified needle preparations from the strains in panel A were immobilized on carbon-coated copper grids. Samples were negatively stained with 2% uranyl acetate and viewed by transmission electron microscopy. The bars indicate a distance of 10 nm.
The type III blockade of translational yscP hybrids is due to protein folding of the reporter protein.
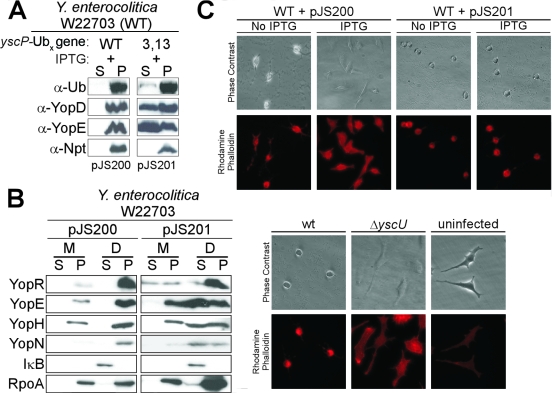
To test whether the tight folding of an impassable YscP hybrid is responsible for the type III secretion blockade, yscP was fused to the Ub gene. Ub is a highly conserved 76-residue polypeptide present in eukaryotic cells that folds rapidly into a tight structure and cannot pass through the type III secretion pathway (39, 64, 76). IPTG-inducible expression of yscP-Ub gene generated a protein product that not only failed to travel into the extracellular medium, but also blocked the Yersinia type III pathway, as immunoblotting experiments revealed the absence of YopD and YopE secretion (Fig. 3A). The Ub3,13 gene carries two mutations that replace Ub isoleucine codons 3 and 13 with glycine, thereby destabilizing the Ub3,13 mutant product and permitting its travel through the type III pathway (39). The product of a translational yscP-Ub3,13 gene hybrid was secreted by yersiniae (Fig. 3A). YscP-Ub3,13 did not block the secretion of YopD and YopE, suggesting that tight folding of the Ub domain prevented YscP-Ub from jamming the Yersinia type III pathway, consequently imposing a blockade (76).
FIG. 3.
Expression of yscP-Ub gene, but not yscP-Ub3,13 gene, blocks Yersinia type III secretion. (A) Y. enterocolitica expression of YscP-Ub and YscP-Ub3,13 was induced with 1 mM IPTG, and type III secretion was activated with sodium oxalate. Following centrifugation of culture aliquots, proteins in the supernatant (S) and pellet (P) were separated by SDS-PAGE, transferred to PVDF membranes, and probed with antisera to Ub, YopD, YopE, and Npt. WT, wild type. (B) Y. enterocolitica W22703 containing pJS200 or pJS201 was used to infect HeLa tissue culture cells at an MOI of 10. IPTG was added to the medium to induce expression of yscP-Ub gene and yscP-Ub3,13 gene. The infection medium (M) was removed and centrifuged, separating the supernatant (S) and pellet (P). Digitonin was added to tissue culture cells with adherent bacteria. Digitonin-extracted samples (D) were centrifuged, separating the supernatant and pellet. All samples were precipitated with methanol/chloroform. Proteins were separated by SDS-PAGE and immunoblotted with polyclonal antisera specific for YopR, YopE, YopH, YopN, IκB, and RpoA. (C) HeLa tissue culture cells were infected at an MOI of 10 with Y. enterocolitica W22703 containing pJS200 or pJS201. The cytotoxicity of Y. enterocolitica-infected HeLa cells was visualized by staining F-actin with rhodamine-conjugated phalloidin. Wild-type-infected, ΔyscU mutant-infected (TTS−), and uninfected HeLa cells are shown as controls.
YscP-Ub blocks Yersinia injection of effector Yops.
Arguably, in vitro secretion assays cannot measure the physiological role of Yersinia type III machines in transporting Yop effector proteins into host cells. To test whether the YscP-Ub in vitro blockade also occurs under physiological conditions, HeLa tissue culture cells were infected with Yersinia, and type III transport of Yop proteins was analyzed by digitonin fractionation (43) (Fig. 3B). The tissue culture medium was decanted and then centrifuged to separate extracellar medium from nonadherent bacteria in the sediment. Tissue culture cells were extracted with digitonin, a detergent that specifically disrupts cholesterol-containing plasma membranes of eukaryotic cells but not bacterial membranes, and centrifuged to separate proteins in the cytosol of HeLa cells from the sediment, containing bacteria and cellular organelles that are not disrupted by digitonin. Proteins in all fractions were precipitated with methanol/chloroform, separated by SDS-PAGE, and analyzed by immunoblotting (Fig. 3B). Y. enterocolitica W22703 expressing YscP-Ub failed to inject YopE, YopH, or YopN into the HeLa cell cytoplasm, whereas yersiniae expressing YscP-Ub3,13 transported effector Yops into host cells (Fig. 3B). As a second measure for Yersinia type III injection of host cells, actin filaments of infected tissue cultures were stained with rhodamine-phalloidin, and microscopy images were captured using phase-contrast and fluorescence technology (Fig. 3C). Y. enterocolitica W22703 expressing YscP-Ub3,13 induced HeLa cell rounding and actin cable rearrangements; however, yersiniae expressing YscP-Ub did not (Fig. 3C). Therefore, expression of YscP-Ub effectively blocks Yersinia type III injection of effector Yops into tissue culture cells. As controls, microscopy images of tissue cultures that were left uninfected or infected with wild-type, as well as yscU mutant (type III-defective), yersiniae revealed that cell rounding requires bacteria with functional type III machines (Fig. 3C).
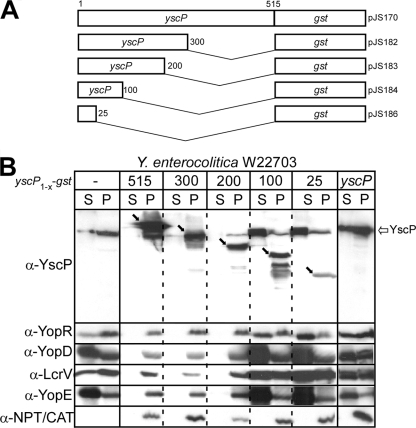
yscP sequences required for a type III secretion blockade.
To characterize the yscP sequences required for the YscP-GST blockade, Y. enterocolitica expressing stepwise 3′ truncations of yscP fused to gst (yscP1-515-gst, yscP1-300-gst, yscP1-200-gst, yscP1-100-gst, and yscP1-25-gst) (Fig. 4A) were induced with IPTG, and type III secretion was measured by immunoblotting. Strains expressing yscP1-515-gst (full-length yscP), yscP1-300-gst, or yscP1-200-gst blocked all type III secretion; however, 3′ yscP truncations beyond the first 200 codons (yscP1-100 and yscP1-25) did not (Fig. 4B). The products of yscP1-200-gst and yscP1-100-gst accumulated with similar abundances, suggesting that the defect in the type III blockade is not caused by differences in gene expression (Fig. 4B). The type III secretion blockade imposed by expression of yscP1-515-gst, yscP1-300-gst, and yscP1-200-gst also included YscP, whereas yscP1-100-gst and yscP1-25-gst did not block YscP secretion (Fig. 4B). As a control, overexpression of yscP alone did not block type III secretion (Fig. 4B). Thus, 3′ yscP sequences must harbor information that is necessary for the implementation of the YscP-GST-mediated type III blockade (Fig. 4B).
FIG. 4.
YscP sequences required for the type III secretion blockade. (A) Schematic diagram of the C-terminal truncations of YscP and subsequent fusion protein construction. (B) Wild-type Y. enterocolitica without plasmid (−) or expressing YscP1-515-GST, YscP1-300-GST, YscP1-200-GST, YscP1-100-GST, YscP1-25-GST, or YscP alone was induced for type III secretion. Following centrifugation of culture aliquots, proteins in the supernatant (S) and bacterial pellet (P) were separated by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with polyclonal antisera specific for YscP, YopR, YopD, LcrV, YopE, and Npt. YscP-GST fusions are denoted with black arrows, and native YscP is denoted by a white arrow.
YscP secretion signal.
The secretion signals for several Yersinia type III substrates have been mapped by generating translational hybrids between Yops and proteins that can be transported by the secretion machine, for example, neomycin phosphotransferase (Npt) (4, 17, 52, 74, 77-79). In all cases examined, the Yop secretion signal was encoded by sequences immediately adjacent to the 5′ end of the open reading frame, for example, the first 10 codons of yopR (76). Translational hybrids between the first 15 codons of yscP and the 5′ open reading frame of npt generated a hybrid product, YscP1-15-Npt, that was secreted by yersiniae (Fig. 5A). Stepwise 3′ truncations of yscP sequences in these hybrids revealed that the minimal secretion signal of yscP is positioned in its first 10 codons, as truncation to 9 codons or any further truncation abolished secretion of YscP-Npt hybrids (Fig. 5A). Secretion of YscP-Npt proteins was monitored by immunoblotting for the npt fusion partner and did not affect the secretion of another substrate, YopE (Fig. 5A). Previous work on the minimal secretion signal of Yop effectors, including yopE, yopQ, and yopN, suggested that features of the mRNA sequence may, at least in part, contribute to substrate recognition and/or transport (5, 32, 67, 68). For example, frameshift mutations, which disrupt the reading frame and are accompanied by suppressor mutations at the fusion site with the 3′ reporter gene, generate hybrids that continue to travel the type III pathway (4). We generated similar +1 frameshift and suppressor mutations in yscP1-15 and found that the YscP+1-Npt hybrid was secreted, albeit at a lower level than its wild-type parent. A second feature of substrate genes that led to the consideration of mRNA-encoded secretion signals is the identification of synonymous mutations that do not affect the protein sequence but alter the nucleic acid sequence (65, 66). Several of these synonymous mutations have been isolated and were shown to affect the secretion of translational hybrids (32, 78). We constructed yscP1-15,mut-npt with 16 synonymous mutations that changed the RNA sequence but not the encoded polypeptide; its protein product was also secreted by the Yersinia type III pathway (Fig. 5B and C). Thus, similar to the secretion signal of invJ, the Salmonella yscP homolog, the Yersinia yscP signal can tolerate drastic alterations of both its mRNA and protein sequences (12, 72, 76).
FIG. 5.
Mapping the minimal secretion signal of YscP. (A) Wild-type Y. enterocolitica expressing YscP1-x-Npt fusion proteins, where x represents codons 15, 14, 13, 12, 11, 10, 9, 8, 7, and 6, were induced for type III secretion in M9 minimal medium containing 35 μg/ml of chloramphenicol. Following centrifugation of culture aliquots, proteins in the supernatant (S) and pellet (P) were separated by 15% SDS-PAGE, transferred to PVDF membranes, and probed with antisera to NPT, YopE, and chloramphenicol acetyltransferase (CAT). (B) The +1 frameshift secretion signal maintains 47 of 50 nucleotides while altering 12 of the first 15 amino acids of YscP. The “mut” frameshift alters 16 of a possible 42 nucleotides of the mRNA sequence of the first 15 codons of YscP while maintaining the native protein sequence. Red, altered nucleotides; blue, altered amino acids. wt, wild type. (C) Wild-type Y. enterocolitica containing pKER144 and pKER145 (+1 and mut minimal secretion signals) were induced for type III secretion, and proteins in the supernatant (S) and pellet (P) were probed with antisera to NPT, YopE, and CAT.
The YscP secretion signal is required for the type III blockade.
We wondered whether the secretion signal of yscP is required for the type III blockade imposed by translational hybrids with gst and constructed sequential 5′ deletions in yscP-gst (Fig. 6A). Surprisingly, Y. enterocolitica expressing yscPΔ2-15-gst caused a nearly complete block in type III secretion when analyzed with Coomassie brilliant blue (Fig. 6A and B). Immunoblotting revealed that the type III block of YscPΔ2-15-GST was, however, incomplete, as YopR, YopD, LcrV, and YopE continued to be secreted. Further 5′ truncations, tested with yscPΔ2-50-gst, yscPΔ2-100-gst, and yscPΔ2-150-gst, were unable to block type III secretion of yersiniae for early (YscP and YopR), middle (YopD and LcrV), and late (YopE) substrates (Fig. 6B and C). From these observations, we surmise that initiation of YscP-GST into the type III secretion pathway is a prerequisite for the establishment of a complete secretion blockade. However, the data cannot exclude the possibility that YscPΔ2-15-GST retains another secretion signal element that imposes at least a partial engagement with the secretion machine, consistent with the report of Agrain and coworkers that YscP may harbor two secretion signals, one at its N terminus (5′ end) and one positioned at residues 97 to 137 (2).
FIG. 6.
The N-terminal secretion signal is required for the YscP-GST-mediated type III secretion blockade. (A) Schematic diagram of the N-terminal truncations of YscP and subsequent fusion protein construction. (B) Wild-type Y. enterocolitica expressing YscPΔ2-15-GST, YscPΔ2-50-GST, YscPΔ2-100-GST, and YscPΔ2-150-GST were induced for type III secretion and grown in the absence (−) and presence (+) of IPTG. Following centrifugation of culture aliquots, proteins in the supernatant were separated by SDS-PAGE and visualized by Coomassie blue staining. Molecular mass markers (in kilodaltons) are indicated to the left of the gel, while secreted Yop effectors are indicated to the right. (C) Wild-type Y. enterocolitica expressing the hybrids in panel B was induced for type III secretion, and the cultures, following centrifugation, were separated into supernatant (S) and pellet (P) fractions. Proteins were separated on SDS-PAGE, transferred to PVDF membranes, and immunoblotted with polyclonal antisera specific for YscP, YopR, YopD, LcrV, YopE, and chloramphenicol acetyltransferase (CAT). YscPΔ2-x-GST fusion proteins are denoted with black arrows; the faster-migrating immunoreactive species represents wild-type YscP.
Screening for mutations that abrogate the yscP-gst-imposed blockade.
We wondered whether yscP harbored discrete signals for initiation of YscP-GST into the type III pathway and the inability of the secretion machine to reject the impassable substrate. Assuming that single amino acid or nucleotide changes abolished the type III blockade, we should be able to isolate such mutations following hydroxylamine mutagenesis of pJS170 (yscP-gst) and by screening Y. pestis transformants for variants that failed to abolish the LCR, which prevents bacterial growth at 37°C on agar medium without calcium (8). Y. pestis biovar medievalis KIM D27 (pCD1+ pMT1+ pgm) was unable to grow at 37°C in the absence of calcium unless the plague bacteria had been transformed with pJS170. Following hydroxylamine mutagenesis and the isolation of mutants, the plasmids were purified and subjected to DNA sequencing (Fig. 7A). Approximately 1,200 colonies were screened for the LCR phenotype, and 20 mutants were isolated. Of the 20 potential mutants, only 14 yielded changes in the sequence of yscP, nine of which were unique. All of the isolated mutants harbored nonsense mutations that replaced glutamine (Q) or tryptophan (W) with stop codons (Q63 [two], Q93 [four], Q117, Q173, W198, Q277, Q411, Q413 [two], and Q472), causing premature termination of the impassable polypeptide chain. Surprisingly, missense mutations in yscP-gst were not isolated, suggesting that functional redundancy of multiple signals or the molecular complexity of these secretion signals prevented the isolation of simple substitutions (Fig. 7B).
FIG. 7.
Hydroxylamine-generated mutations in yscP-gst that abrogate the type III secretion blockade. (A) Experimental flowchart for the generation of yscP-gst mutants that no longer block the Yersinia type III pathway. (B) Amino acid and corresponding DNA sequences of Y. enterocolitica W22703 yscP. All nonsense mutations obtained by hydroxylamine mutagenesis and screening for LCR phenotypes are shaded in red. Notably, missense mutations were not obtained. YscPFL, full-length YscP.
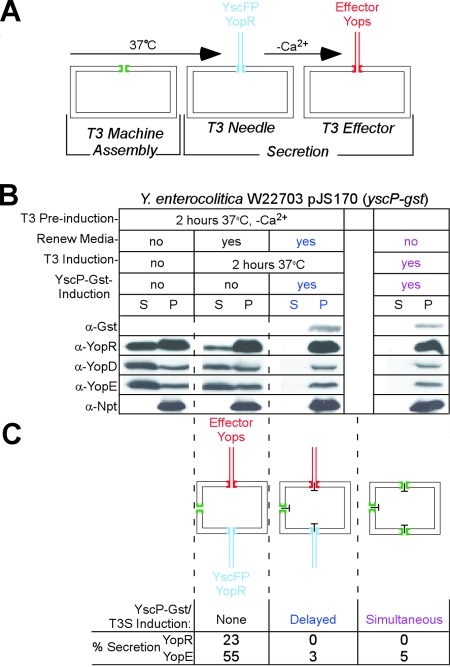
Impassable YscP hybrids block type III machines at any developmental stage.
When cultured in vitro, temperature shift to 37°C and calcium chelation triggers yersiniae to synthesize and assemble their type III secretion machines (Fig. 8A). Because their synthesis is activated by laboratory-controlled environmental signals, type III machines of all yersiniae in a culture are assembled synchronously. As yersiniae grow and generate new needle complexes, synchrony is lost, and bacterial cultures that were induced (37°C without calcium) for 2 hours are expected to harbor fully assembled secretion systems engaged in secretion of middle and late substrates (YopD and YopE), as well as machines undergoing assembly and secretion of early substrates (YscP) (Fig. 8B). What happens when blocking substrates are expressed in bacterial cells harboring type III machines at all stages of assembly and function? In previous work, our laboratory showed that YopR-DHFR could block the assembly of newly formed type III machines but did not inhibit secretion by machines that were already engaged in the secretion of effector Yops (76). To address this for YscP, we compared the effects of YscP-GST expression in cultures with synchronized type III assembly (simultaneous induction via IPTG and 37°C without calcium) and nonsynchronized secretion (2-h-delayed induction) (Fig. 8B). As expected, simultaneous induction of type III assembly and YscP-GST blocked the secretion of early (YopR), middle (YopD), and late (YopE) substrates (Fig. 7B and C). Upon delayed induction, YscP-GST also blocked the secretion of all substrates (Fig. 7B and C). Thus, unlike YopR-DHFR, the impassable hybrid YscP-GST jams type III machines without consideration for their developmental stage.
FIG. 8.
YscP-GST blocks all stages of the type III pathway. (A) Diagram displaying type III secretion machine assembly (green); secretion of the early substrates YscF, YopR, and YscP (blue); and secretion of late Yop effectors (red). (B) Wild-type Y. enterocolitica expressing YscP-GST was grown in BHI containing 20 mM MgCl2 and 20 mM sodium oxalate for 2 h at 26°C and shifted to 37°C for 2 h to induce type III secretion (T3 Pre-induction). After 2 hours, cultures in columns 2 and 3 (from left) were aliquoted, centrifuged, and suspended in fresh medium with or without IPTG (Renew Media) and incubated for an additional 2 h. Following centrifugation, all cultures were separated into supernatant (S) and pellet (P) fractions and separated on 15% SDS-PAGE. The proteins were transferred to a PVDF membrane and immunoblotted with polyclonal antisera to GST, YopR, YopD, YopE, and Npt. (C) Diagram displaying the effects of no induction of yscP-gst (no IPTG meant no blockade), delayed induction (IPTG added after 2 h at 37°C), and simultaneous induction (IPTG at the time of shift to 37°C). Percents secretion of YopR and YopE were calculated by the amount of signal in the supernatant (S) divided by the total signal in the supernatant and the pellet (S/S + P).
YscP-GST copurifies with YscN, YscL, YscQ, and YscO.
To explore whether the type III secretion blockade imposed by YscP-GST is caused by sequestering specific machine components, Y. enterocolitica expressing YscP-GST (pJS170), YscPΔ2-15-GST (pKER147), or YscP1-100-GST (pJS184) was grown at 26°C to an optical density at 600 nm of 0.8. The cultures were induced with IPTG for 4 h at 37°C. Fusion proteins were purified using affinity chromatography over glutathione-Sepharose. Eluate fractions were analyzed by Coomassie blue staining and immunoblotting for the presence of the fusion protein (Fig. 9A, B, and C). In all cases, the fusion protein was enriched in the elution fraction. In addition to YscP-GST, YscN, the ATPase of type III machines (9, 86); YscL, its negative regulator (9); YscQ, the C-ring protein (38, 82); and YscO, another mobile machine component (62), were also enriched during affinity chromatography. The association of YscN, YscL, YscQ, and YscO with the blocking substrate appears to be specific, as control purifications with Yersinia expressing GST alone did not yield type III machine components (data not shown). YscPΔ2-15-GST, which imposes a partial blockade, also captured the YscN ATPase complex, including YscL and YscQ, as well as YscO. In contrast, YscN did not copurify with YscP1-100-GST, a hybrid that cannot block the type III pathway, although small amounts of YscL, YscQ, and YscO did copurify with the hybrid.
FIG. 9.
YscP-GST interacts with the type III machine components YscN, YscL, YscQ, and YscO. Wild-type Y. enterocolitica expressing pJS170 (yscP-gst) (A), pKER147 (yscPΔ2-15-gst) (B), and pJS184 (yscP1-100-gst) (C) was grown under conditions that induced the type III secretion machine and YscP-GST in the absence of extracellular calcium. YscP-GST, YscPΔ2-15-GST, and YscP1-100-GST were purified from these strains, and samples corresponding to the lysate (L) and eluate (E) were analyzed by Coomassie blue-stained SDS-PAGE (left column) or immunoblotting (right column), probing with antisera to machinery components (YscP, YscK, YscL, YscN, YscO, YscQ, and YopR/YscH), as well as the secreted effector YopE. The relative intensities of immunoreactive signals in the lysate and eluate fractions (E/L) is expressed as a ratio to the right of each blot. The positions of the molecular mass markers are indicated to the left of the gels (in kilodaltons). In panel A, the black arrowhead at 83 kDa points to the full-length fusion protein and the white arrowheads to the cleaved species YscP (57 kDa) and GST (26 kDa). In panel B, the black arrowhead at 83 kDa points to the full-length fusion protein and the white arrowhead to GST (26 kDa). In panel C, the black arrowhead at 37 kDa points to the full-length fusion protein and the white arrowhead to GST (26 kDa).
DISCUSSION
Fusion of tightly folded proteins, for example, GST, DHFR, or Ub, to the C-terminal end of YopR (YscH), another early substrate, generates impassable substrates that block type III secretion (76). The attribute of YopR-GST in blocking secretion is associated with its ability to copurify with or bind to the type III secretion ATPase, YscN, thereby jamming the secretion pathway (76). Remarkably, the YopR-GST blockade occurs only for developing type III machines, but not for fully assembled secretion systems that are already advanced in their developmental programs toward transport of effector Yops (76). We wondered whether other type III substrates, when fused to an impassable reporter protein, display a similar phenotype. Thus far, our work has characterized fusions involving YopD, YopE, YopH, YopN, YopQ, YopR, YscF, YscP, and LcrV. Only YopR and YscP caused a complete blockade that could not be relieved via the introduction of class II mutations, e.g., yopD, lcrH, or Δ(yscM1 yscM2) (16). The residue length of YscP was found to be essential for the blockade, as truncations up to the first 200, but not less than 200, residues were tolerated (76). In order to block secretion, yopR and yscP clearly require additional sequence information tethered to the gene that encodes the impassable reporter than is minimally required when fusions occur with a reporter gene that specifies a passable product, such as Npt. From this, we conclude that substrate initiation into the secretion machine via its 5′ nucleotide sequence or N-terminal peptide can be revoked for all substrates examined; however, when additional sequences of yscP (or yopR) are added in cis, the engagement of impassable substrates with the type III machine is irrevocable (76). As in-frame deletions of 5′ sequences, which encompass only the minimal secretion signal, abrogate the blocking attributes of yopR-gst and yscP-gst, we infer that substrate initiation into the T3SS pathway is absolutely required for jamming to occur (76).
Immediate or delayed induction of yscP-gst, i.e., offering the impassable YscP-GST substrate to machines that develop synchronously or asynchronously, revealed that the nature of the YscP-GST blockade must differ from that of YopR-GST (76). YscP-GST appears to block machines at any stage of development, even in bacteria in which most type III needles are already engaged in the transport of late substrates, i.e., machines that flipped the switch toward secretion of later substrates. This unique phenotype suggests to us that YscP-GST may jam machine components that are required for the secretion of all substrates and at all developmental stages of the pathway. Affinity chromatography of YscP-GST hybrids that blocked machines led to copurification of YscN-YscL-YscO-YscQ, whereas a nonblocking substrate, YscP1-100-GST, captured only YscL-YscO-YscQ, but not YscN. Although this remains speculative, the collected data are consistent with a model in which an irrevocable engagement of impassable substrates with YscN AAA ATPase causes a blockade of the secretion pathway (9, 76).
The secretion signal of YscP has been extensively studied. Agrain and coworkers reported that, in the context of various YscP truncations fused to adenylate cyclase (Cya), the N-terminal 35 amino acids, as well as amino acids 97 to 137, functioned as two independent secretion signals of YscP (2). InvJ, the Salmonella homolog of YscP, also possesses an N-terminal secretion signal within the first seven codons that could tolerate changes in both amino acid and mRNA sequences (72). The possibility of a second secretion signal for InvJ has not yet been investigated. Here, we report that the first 10 codons of YscP function as a minimal secretion signal for passable reporter fusions and that the N-terminal secretion signal, codons 2 to 15, is also required for the type III blockade of YscP-GST. Significant changes in both the amino acid and mRNA sequences did not abolish the secretion signal function of the first 15 codons of yscP. Thus, similar to invJ and other type III secretion signals, an unequivocal distinction between protein- and mRNA-encoded signaling functions could not be achieved for the yscP signal (12, 72). The presence of a second secretion signal in YscP suggests the interesting possibility that substrates that block the pathway when fused to an impassable reporter require a second independent secretion signal to jam the type III machine. It is likely that such a signal may be complex (residues 97 to 137), as random-mutagenesis experiments failed to identify discrete missense mutations that abrogated the ability of yscP-gst to block the type III pathway.
YscP fulfills multiple roles during the maturation of the needle apparatus. Similarly to its flagellar homolog, FliK, YscP regulates the length of the assembled filament, i.e., a needle that emerges on the bacterial surface (2, 40, 56, 58, 59). Deletions of yscP result in needles of unregulated length, whereas deletions or loss-of-function mutations in fliK produce polyhooks, aberrant structures in which multiple hooks are joined with the flagellar basal body (40, 85). The residue length of YscP corresponds to the length of the type III needle, which prompted Journet and coworkers to propose that YscP functions as a molecular ruler (40). Length regulation could occur if YscP interacted with the polymerized needle protein YscF. If so, mechanisms that would allow YscP to perceive needle assembly progression could subsequently relay such information to the T3SS apparatus in the cytoplasm. Needle length regulation has been proposed to conclude with the release of YscP into the extracellular medium (59, 63). Nevertheless, N-terminally truncated FliK, which cannot enter the flagellar type III pathway, continues to regulate hook length from inside bacterial cells (35, 56). This finding has been interpreted to mean that FliK secretion cannot be an absolute requirement for hook length regulation. Instead of a ruler, FliK has been suggested to act as a molecular clock that times a switch within secretion machines to recognize different substrates during the assembly of hook and filament subunits (58). Another model envisioned that FliK may function as a measuring cup, collecting quanta of substrate molecules that subsequently generate the corresponding increments in structural assembly (35, 48).
Once hook length has been established, FliK, and presumably also YscP, perform a second task, flipping the substrate specificity switch of the needle apparatus (1, 25, 28, 55, 58). The C-terminal domain of FliK, designated T3S4, interacts with the switch protein FlhB, which is positioned in the inner membrane at the base of the type III machine (28, 55, 85). Flipping the switch is associated with FlhB autocleavage at its C-terminal cytoplasmic domain, thenceforth causing the type III machine to recognize later substrates (27, 57). YscU also undergoes autocleavage; however, its C-terminal domain does not seem to interact with YscP (42, 69, 75). Thus, there may be subtle differences between the flagellar assembly pathway and Yersinia type III secretion in the molecular mechanisms that couple hook and needle lengths to switch protein cleavage or FliK/YscP interactions with other machine and substrate components.
Acknowledgments
We thank Bill Blaylock for advice and members of our laboratory for critical comments on the manuscript.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases, Infectious Diseases Branch, AI42797 (to O.S.). K.E.R. is a trainee of the NIH Medical Scientist Training Program at The University of Chicago (GM07281). O.S. acknowledges membership in and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (GLRCE) (National Institute of Allergy and Infectious Diseases Award 1-U54-AI-057153).
Footnotes
Published ahead of print on 18 July 2008.
REFERENCES
- 1.Agrain, C., I. Callebaut, L. Journet, I. Sorg, C. Paroz, L. J. Mota, and G. R. Cornelis. 2005. Characterization of a type III secretion substrate specificity switch (T3S4) domain in YscP from Yersinia enterocolitica. Mol. Microbiol. 5654-67. [DOI] [PubMed] [Google Scholar]
- 2.Agrain, C., I. Sorg, C. Paroz, and G. R. Cornelis. 2005. Secretion of YscP from Yersinia enterocolitica is essential to control the length of the injectisome needle but not to change the type III secretion substrate specificity. Mol. Microbiol. 571415-1427. [DOI] [PubMed] [Google Scholar]
- 3.Allaoui, A., R. Schulte, and G. R. Cornelis. 1995. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol. Microbiol. 18343-355. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, D. M., and O. Schneewind. 1997. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 2781140-1143. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, D. M., and O. Schneewind. 1999. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol. Microbiol. 311139-1148. [DOI] [PubMed] [Google Scholar]
- 6.Andersson, K., N. Carballeira, K. Magnusson, C. Persson, O. Stendahl, H. Wolf-Watz, and M. Fallman. 1996. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol. Microbiol. 201057-1069. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann, T., K. Erickson, E. Galyov, C. Persson, and H. Wolf-Watz. 1994. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J. Bacteriol. 1762619-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann, T., S. Hakansson, A. Forsberg, L. Norlander, A. Macellaro, A. Backman, I. Bolin, and H. Wolf-Watz. 1991. Analysis of V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of lcrH and lcrV. J. Bacteriol. 1731607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaylock, B., K. E. Riordan, D. M. Missiakas, and O. Schneewind. 2006. Characterization of the Yersinia enterocolitica type III secretion ATPase YscN and its regulator, YscL. J. Bacteriol. 1883525-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri ‘needle complex’, a part of its type III secreton. Mol. Microbiol. 39652-663. [DOI] [PubMed] [Google Scholar]
- 11.Boland, A., M.-P. Sory, M. Iriarte, C. Kerbourch, P. Wattiau, and G. R. Cornelis. 1996. Status of YopM and YopN in the Yersinia yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 155191-5201. [PMC free article] [PubMed] [Google Scholar]
- 12.Broms, J. E., M. S. Francis, and A. Forsberg. 2007. Diminished LcrV secretion attenuates Yersinia pseudotuberculosis virulence. J. Bacteriol. 1898417-8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broz, P., C. A. Mueller, S. A. Muller, A. Philippsen, I. Sorg, A. Engel, and G. R. Cornelis. 2007. Function and molecular architecture of the Yersinia injectisome tip complex. Mol. Microbiol. 651311-1320. [DOI] [PubMed] [Google Scholar]
- 14.Brubaker, R. R. 1969. Mutation rate to non-pigmentation in Pasteurella pestis. J. Bacteriol. 981404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cambronne, E. D., L. W. Cheng, and O. Schneewind. 2000. LcrQ/YscM1, regulators of the Yersinia yop virulon, are injected into host cells by a chaperone dependent mechanism. Mol. Microbiol. 37263-273. [DOI] [PubMed] [Google Scholar]
- 16.Cambronne, E. D., and O. Schneewind. 2002. Yersinia enterocolitica type III secretion: yscM1 and yscM2 regulate yop gene expression by a post-transcriptional mechanism that targets the 5′-untranslated region of yop mRNA. J. Bacteriol. 1845880-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng, L. W., D. M. Anderson, and O. Schneewind. 1997. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol. Microbiol. 24757-765. [DOI] [PubMed] [Google Scholar]
- 18.Cheng, L. W., O. Kay, and O. Schneewind. 2001. Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J. Bacteriol. 1835293-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng, L. W., and O. Schneewind. 2000. Yersinia enterocolitica TyeA, an intracellular regulator of the type III machinery, is required for the specific targeting of YopE, YopH, YopM and YopN into the cytosol of eukaryotic cells. J. Bacteriol. 1823183-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng, L. W., and O. Schneewind. 1999. Yersinia enterocolitica type III secretion: on the role of SycE in targeting YopE into HeLa cells. J. Biol. Chem. 27422102-22108. [DOI] [PubMed] [Google Scholar]
- 21.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M.-P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 621315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornelis, G. R., and C. Colson. 1975. Restriction of DNA in Yersinia enterocolitica detected by the recipient ability for a derepressed R factor from Escherichia coli. J. Gen. Microbiol. 87285-291. [DOI] [PubMed] [Google Scholar]
- 23.Davis, A. J., and J. Mecsas. 2007. Mutations in the Yersinia pseudotuberculosis type III secretion system needle protein, YscF, that specifically abrogate effector translocation into host cells. J. Bacteriol. 18983-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day, J. B., F. Ferracci, and G. V. Plano. 2003. Translocation of YopE and YopN into eukaryotic cells by Yersinia pestis yopN, tyeA, sycN, yscB and lcrG deletion mutants measured using a phosphorylatable peptide tag and phosphospecific antibodies. Mol. Microbiol. 47807-823. [DOI] [PubMed] [Google Scholar]
- 25.Edqvist, P. J., J. Olsson, M. Lavander, L. Sundberg, A. Forsberg, H. Wolf-Watz, and S. A. Lloyd. 2003. YscP and YscU regulate substrate specificity of the Yersinia type III secretion system. J. Bacteriol. 1852259-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferracci, F., F. D. Schubot, D. S. Waugh, and G. V. Plano. 2005. Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol. Microbiol. 57970-987. [DOI] [PubMed] [Google Scholar]
- 27.Ferris, H. U., Y. Furukawa, T. Minamino, M. B. Kroetz, M. Kihara, K. Namba, and R. M. Macnab. 2005. FlhB regulates ordered export of flagellar components via autocleavage mechanism. J. Biol. Chem. 28041236-41242. [DOI] [PubMed] [Google Scholar]
- 28.Ferris, H. U., and T. Minamino. 2006. Flipping the switch: bringing order to flagellar assembly. Trends Microbiol. 14519-526. [DOI] [PubMed] [Google Scholar]
- 29.Fetherston, J. D., P. Schuetze, and R. D. Perry. 1992. Loss of the pigmentation phenotype in Yersinia pestis is due to the spontaneous deletion of 102 kb of chromosomal DNA which is flanked by a repetitive element. Mol. Microbiol. 62693-2704. [DOI] [PubMed] [Google Scholar]
- 30.Galan, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444567-573. [DOI] [PubMed] [Google Scholar]
- 31.Garcia, J. T., F. Ferracci, M. W. Jackson, S. S. Joseph, I. Pattis, L. R. Plano, W. Fischer, and G. V. Plano. 2006. Measurement of effector protein injection by type III and type IV secretion systems by using a 13-residue phosphorylatable glycogen synthase kinase tag. Infect. Immun. 745645-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goss, J. W., J. A. Sorg, K. S. Ramamurthi, H. Ton-That, and O. Schneewind. 2004. The secretion signal of YopN, a regulatory protein of the Yersinia enterocolitica type III secretion pathway. J. Bacteriol. 1866320-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakansson, S., T. Bergman, J.-C. Vanooteghem, G. Cornelis, and H. Wolf-Watz. 1993. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect. Immun. 6171-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hakansson, S., E. Gaylov, R. Rosqvist, and H. Wolf-Watz. 1996. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol. Microbiol. 20593-603. [DOI] [PubMed] [Google Scholar]
- 35.Hirano, T., S. Shibata, K. Ohnishi, T. Tani, and S.-I. Aizawa. 2005. N-terminal signal region of FliK is dispensable for length control of the flagellar hook. Mol. Microbiol. 56346-360. [DOI] [PubMed] [Google Scholar]
- 36.Hoiczyk, E., and G. Blobel. 2001. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl. Acad. Sci. USA 984669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iriarte, M., and G. R. Cornelis. 1998. YopT, a new Yersinia effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29915-929. [DOI] [PubMed] [Google Scholar]
- 38.Jackson, M. W., and G. V. Plano. 2000. Interactions between type III secretion apparatus components from Yersinia pestis detected using the yeast two-hybrid system. FEMS Microbiol. Lett. 18685-90. [DOI] [PubMed] [Google Scholar]
- 39.Johnsson, N., and A. Varshavsky. 1994. Ubiquitin-assisted dissection of protein transport across membranes. EMBO 132686-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Journet, L., C. Agrain, P. Broz, and G. R. Cornelis. 2003. The needle length of bacterial injectisomes is determined by a molecular ruler. Science 3021757-1760. [DOI] [PubMed] [Google Scholar]
- 41.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S.-I. Aizawa. 1998. Supermolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280602-605. [DOI] [PubMed] [Google Scholar]
- 42.Lavander, M., L. Sundberg, P. J. Edqvist, S. A. Lloyd, H. Wolf-Watz, and A. Forsberg. 2002. Proteolytic cleavage of the FlhB homologue YscU of Yersinia pseudotuberculosis is essential for bacterial survival but not for type III secretion. J. Bacteriol. 1844500-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, V. T., D. M. Anderson, and O. Schneewind. 1998. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol. Microbiol. 28593-601. [DOI] [PubMed] [Google Scholar]
- 44.Lee, V. T., S. K. Mazmanian, and O. Schneewind. 2001. A program of Yersinia enterocolitica type III secretion reactions is triggered by specific host signals. J. Bacteriol. 1834970-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee, V. T., and O. Schneewind. 1999. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol. Microbiol. 311619-1629. [DOI] [PubMed] [Google Scholar]
- 46.Lee, V. T., and O. Schneewind. 2002. Yop fusions to tightly folded protein domains and their effects on Yersinia enterocolitica type III secretion. J. Bacteriol. 1843740-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee, V. T., C. Tam, and O. Schneewind. 2000. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J. Biol. Chem. 27536869-36875. [DOI] [PubMed] [Google Scholar]
- 48.Makishima, S., K. Komoriya, S. Yamaguchi, and S. I. Aizawa. 2001. Length of the flagellar hook and the capacity of the type III export apparatus. Science 2912411-2413. [DOI] [PubMed] [Google Scholar]
- 49.Marenne, M. N., L. Journet, L. J. Mota, and G. R. Cornelis. 2003. Genetic analysis of the formation of the Ysc-Yop translocation pore in macrophages by Yersinia enterocolitica: role of LcrV, YscF and YopN. Microb. Pathog. 35243-258. [DOI] [PubMed] [Google Scholar]
- 50.Marketon, M. M., R. W. DePaolo, K. L. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 3091739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marlovits, T. C., T. Kubori, A. Sukhan, D. R. Thomas, J. E. Galan, and V. M. Unger. 2004. Structural insights into the assembly of the type III secretion needle complex. Science 3061040-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michiels, T., and G. R. Cornelis. 1991. Secretion of hybrid proteins by the Yersinia Yop export system. J. Bacteriol. 1731677-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michiels, T., J.-C. Vanooteghem, C. Lambert de Rouvroit, B. China, A. Gustin, P. Boudry, and G. R. Cornelis. 1991. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J. Bacteriol. 1734994-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mills, S. D., A. Boland, M.-P. Sory, P. van der Smissen, C. Kerbouch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 9412638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minamino, T., H. U. Ferris, N. Moriya, M. Kihara, and K. Namba. 2006. Two parts of the T3S4 domain of the hook-length control protein FliK are essential for the substrate specificity switching of the flagellar type III export apparatus. J. Mol. Biol. 3621148-1158. [DOI] [PubMed] [Google Scholar]
- 56.Minamino, T., B. Gonzalez-Pedrajo, K. Yamaguchi, S. I. Aizawa, and R. M. Macnab. 1999. FliK, the protein responsible for flagellar hook length control in Salmonella, is exported during hook assembly. Mol. Microbiol. 34295-304. [DOI] [PubMed] [Google Scholar]
- 57.Minamino, T., and R. M. Macnab. 2000. Domain structure of Salmonella FlhB, a flagellar export component responsible for substrate specificity switching. J. Bacteriol. 1824906-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moriya, N., T. Minamino, K. T. Hughes, R. M. Macnab, and K. Namba. 2006. The type III flagellar export specificity switch is dependent on FliK ruler and a molecular clock. J. Mol. Biol. 359466-477. [DOI] [PubMed] [Google Scholar]
- 59.Mota, L. J., L. Journet, I. Sorg, C. Agrain, and G. R. Cornelis. 2005. Bacterial injectisomes: needle length does matter. Science 3071278. [DOI] [PubMed] [Google Scholar]
- 60.Mueller, C. A., P. Broz, S. A. Muller, P. Ringler, F. Erne-Brand, I. Sorg, M. Kuhn, A. Engel, and G. R. Cornelis. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310674-676. [DOI] [PubMed] [Google Scholar]
- 61.Neyt, C., and G. R. Cornelis. 1999. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33971-981. [DOI] [PubMed] [Google Scholar]
- 62.Payne, P. L., and S. C. Straley. 1998. YscO of Yersinia pestis is a mobile component of the Yop secretion system. J. Bacteriol. 1803882-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Payne, P. L., and S. C. Straley. 1999. YscP of Yersinia pestis is a secreted component of the Yop secretion system. J. Bacteriol. 1812852-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quenee, L. E., and O. Schneewind. 2007. Ubiquitin-Yop hybrids as probes for post-translational transport by the Yersinia type III secretion pathway. Mol. Microbiol. 65386-400. [DOI] [PubMed] [Google Scholar]
- 65.Ramamurthi, K. S., and O. Schneewind. 2003. Substrate recognition by the Yersinia type III protein secretion machinery. Mol. Microbiol. 501095-1102. [DOI] [PubMed] [Google Scholar]
- 66.Ramamurthi, K. S., and O. Schneewind. 2005. A synonymous mutation in Yersinia enterocolitica yopE affects the function of the YopE type III secretion signal. J. Bacteriol. 187707-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramamurthi, K. S., and O. Schneewind. 2002. Yersinia enterocolitica type III secretion: mutational analysis of the yopQ secretion signal. J. Bacteriol. 1843321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramamurthi, K. S., and O. Schneewind. 2003. Yersinia yopQ mRNA encodes a bipartite type III secretion signal in the first fifteen codons. Mol. Microbiol. 501189-1198. [DOI] [PubMed] [Google Scholar]
- 69.Riordan, K. E., and O. Schneewind. 2008. YscU cleavage and the assembly of Yersinia type III secretion machine complexes. Mol. Microbiol. 681485-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosqvist, R., A. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 594562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosqvist, R., K.-E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russmann, H., T. Kubori, J. Sauer, and J. E. Galan. 2002. Molecular and functional analysis of the type III secretion signal of the Salmonella enterica InvJ protein. Mol. Microbiol. 46769-779. [DOI] [PubMed] [Google Scholar]
- 73.Sarker, M. R., C. Neyt, I. Stainier, and G. R. Cornelis. 1998. The Yersinia yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 1801207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schesser, K., E. Fritzh-Lindsten, and H. Wolf-Watz. 1996. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J. Bacteriol. 1787227-7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorg, I., S. Wagner, M. Amstutz, S. A. Muller, P. Broz, Y. Lussi, A. Engel, and G. R. Cornelis. 2007. YscU recognizes translocators as export substrates of the Yersinia injectisome. EMBO J. 263015-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sorg, J. A., B. Blaylock, and O. Schneewind. 2006. Secretion signal recognition by YscN, the Yersinia type III secretion ATPase. Proc. Natl. Acad. Sci. USA 10316490-16495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sorg, J. A., N. C. Miller, M. M. Marketon, and O. Schneewind. 2005. Rejection of impassable substrates by Yersinia type III secretion machines. J. Bacteriol. 1877090-7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorg, J. A., N. C. Miller, and O. Schneewind. 2005. Substrate recognition of type III secretion machines—testing the RNA signal hypothesis. Cell. Microbiol. 71217-1225. [DOI] [PubMed] [Google Scholar]
- 79.Sory, M.-P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. USA 9211998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sory, M.-P., and G. R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14583-594. [DOI] [PubMed] [Google Scholar]
- 81.Takeshita, S., M. Sato, M. Toba, W. Masahashi, and T. Hashimoto-Gotoh. 1987. High-copy-number and low-copy-number plasmid vectors for LacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 6163-74. [DOI] [PubMed] [Google Scholar]
- 82.Thomas, D. R., D. G. Morgan, and D. J. DeRosier. 1999. Rotational symmetry of the C ring and a mechanism for the flagellar rotary motor. Proc. Natl. Acad. Sci. USA 9610134-10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Torruellas, J., M. W. Jackson, J. W. Pennock, and G. V. Plano. 2005. The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol. Microbiol. 571719-1733. [DOI] [PubMed] [Google Scholar]
- 84.Wattiau, P., and G. R. Cornelis. 1993. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol. Microbiol. 8123-131. [DOI] [PubMed] [Google Scholar]
- 85.Williams, A. W., S. Yamaguchi, F. Togashi, S. I. Aizawa, I. Kawagishi, and R. M. Macnab. 1996. Mutations in fliK and flhB affecting flagellar hook and filament assembly in Salmonella typhimurium. J. Bacteriol. 1782960-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woestyn, S., A. Allaoui, P. Wattiau, and G. Cornelis. 1994. YscN, the putative energizer of the Yersinia Yop secretion machinery. J. Bacteriol. 1761561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woestyn, S., M.-P. Sory, A. Boland, O. Lequenne, and G. R. Cornelis. 1996. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol. Microbiol. 201261-1271. [DOI] [PubMed] [Google Scholar]