Abstract
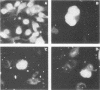
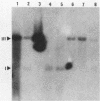
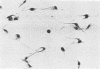
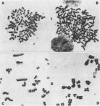
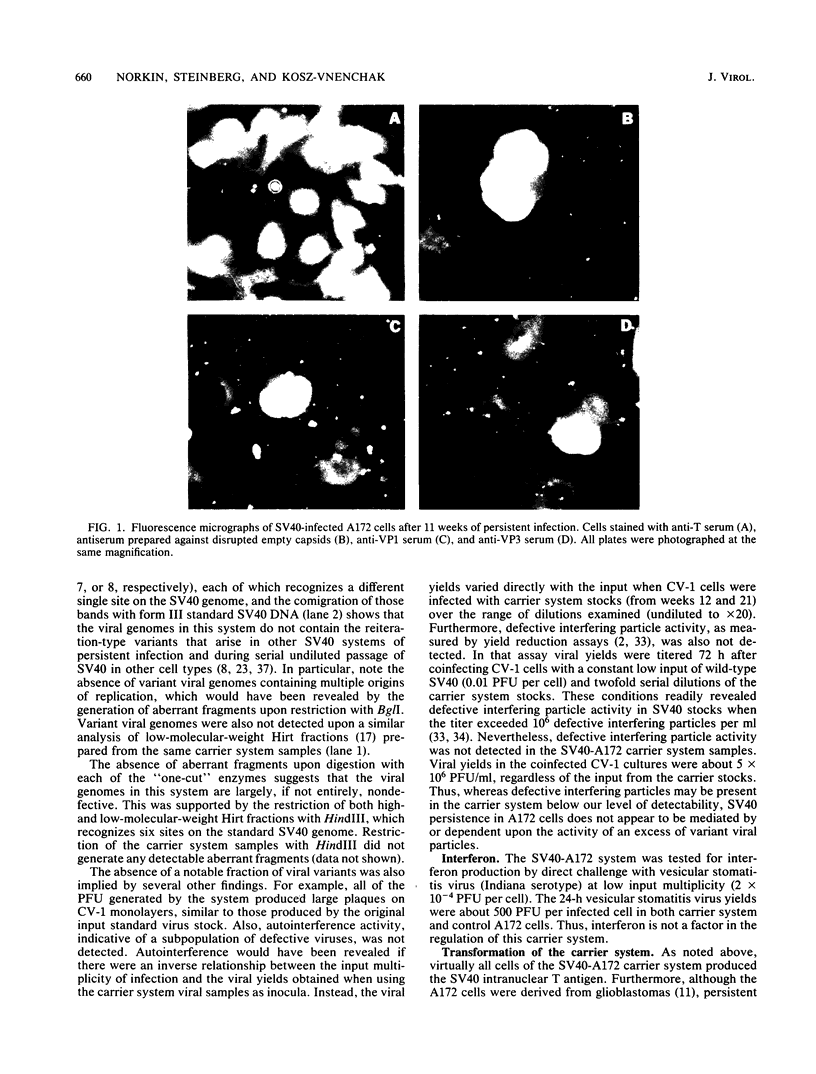
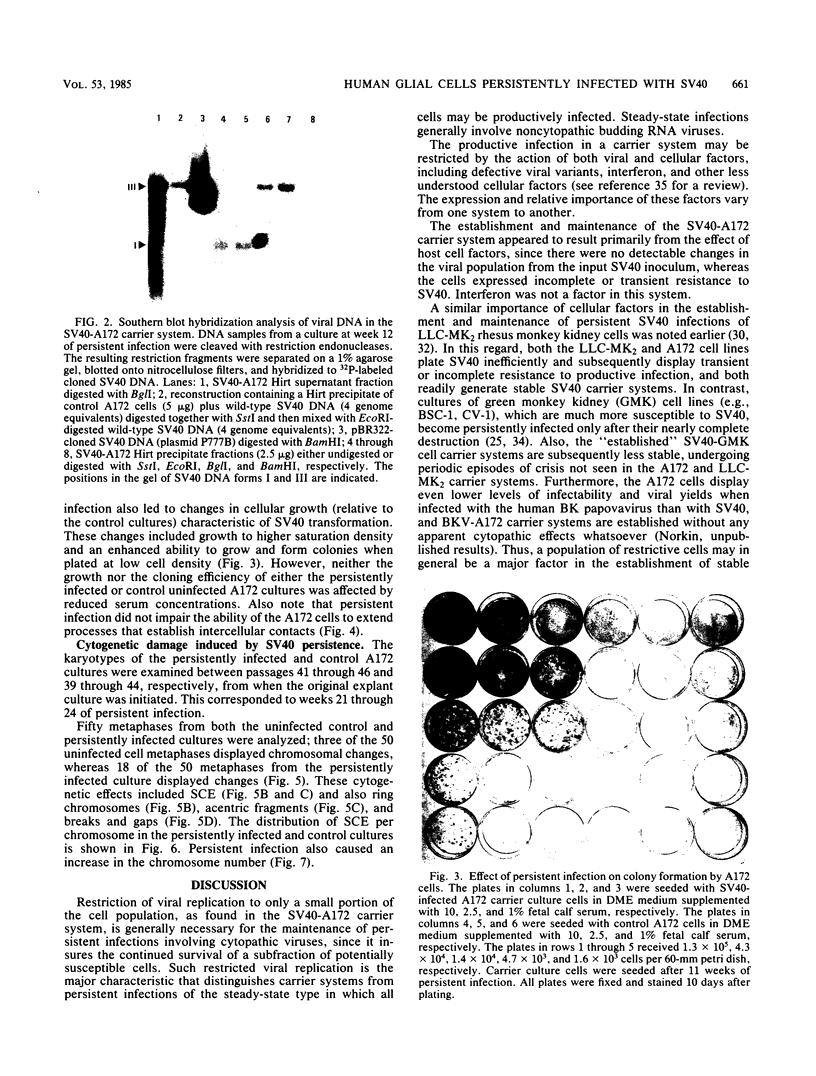
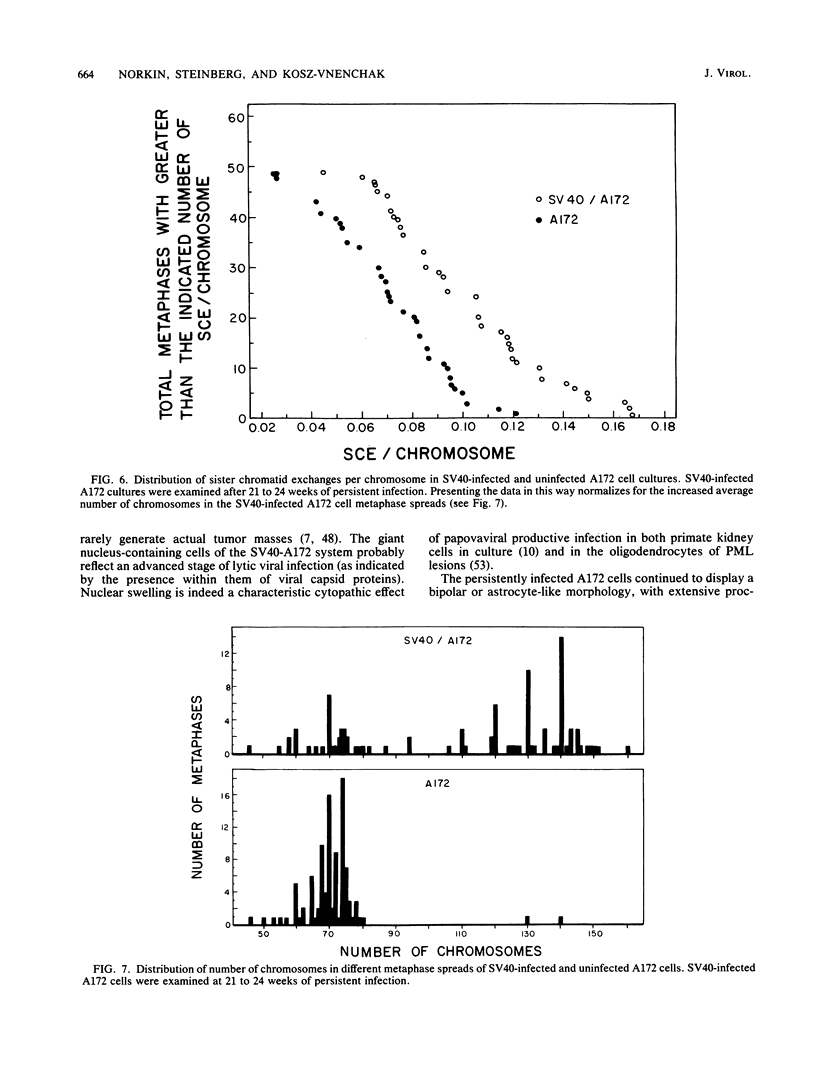
A stable, persistent infection of A172 human glioblastoma cells with simian virus 40 (SV40) was readily established after infection at an input of 450 PFU per cell. Only 11% of the cells were initially susceptible to SV40, as shown by indirect immunofluorescent staining for the SV40 T antigen at 48 h. However, all cells produced T antigen by week 11. In contrast, viral capsid proteins were made in only about 1% of the cells in the established carrier system. Weekly viral yields ranged between 10(4) and 10(6) PFU/ml. Most of the capsid protein-producing cells contained enormous aberrant (lobulated or multiple) nuclei. Persistent viral DNA appeared in an episomal or "free" state exclusively in Southern blots and was indistinguishable from standard SV40 DNA by restriction analysis. Viral autointerference activity was not detected, and yield reduction assays did not indicate defective interfering particle activity, further implying that variant viruses were not a factor in this carrier system. Interferon was also not a factor in the system, as shown by direct challenge with vesicular stomatitis virus. Persistent infection resulted in cellular growth changes (enhanced saturation density and plating efficiency) characteristic of SV40 transformation. Persistent infection also led to an increased frequency of cytogenetic effects. These included sister chromatid exchanges, a variety of chromosomal abnormalities (ring chromosomes, acentric fragments, breaks, and gaps), and an increase in the chromosome number. Nevertheless, the persistently infected cells continued to display a bipolar glial cell-like morphology with extensive process extension and intercellular contacts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTROM K. E., MANCALL E. L., RICHARDSON E. P., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958 Mar;81(1):93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- BELLETT A. J., COOPER P. D. Some properties of the transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:498–509. doi: 10.1099/00221287-21-3-498. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Brown E. H., Basilico C. Induction of sister chromatid exchange by polyoma large viral tumor antigen in transformed rat fibroblasts. Cancer Res. 1982 May;42(5):1909–1912. [PubMed] [Google Scholar]
- Carrano A. V., Thompson L. H., Lindl P. A., Minkler J. L. Sister chromatid exchange as an indicator of mutagenesis. Nature. 1978 Feb 9;271(5645):551–553. doi: 10.1038/271551a0. [DOI] [PubMed] [Google Scholar]
- Carroll D., Hansen J. L., Maryon E. B., O'Neill F. J. SV40 defectives selected during low multiplicity passage on A172 human glioblastoma cells. Virology. 1981 Jul 30;112(2):461–471. doi: 10.1016/0042-6822(81)90293-2. [DOI] [PubMed] [Google Scholar]
- Castaigne P., Rondot P., Escourolle R., Ribadeau dumas J. L., Cathala F., Hauw J. J. Leucoencéphalopathie multifocale progressive et "gliomes" multiples. Rev Neurol (Paris) 1974 Sep-Oct;130(9-10):379–392. [PubMed] [Google Scholar]
- Davoli D., Ganem D., Nussbaum A. L., Fareed G., Howley P. M., Khoury G., Martin M. A. Genome Structures of reiteration mutants of simian virus 40. Virology. 1977 Mar;77(1):110–124. doi: 10.1016/0042-6822(77)90411-1. [DOI] [PubMed] [Google Scholar]
- Dörries K., Johnson R. T., ter Meulen V. Detection of polyoma virus DNA in PML-brain tissue by (in situ) hybridization. J Gen Virol. 1979 Jan;42(1):49–57. doi: 10.1099/0022-1317-42-1-49. [DOI] [PubMed] [Google Scholar]
- Einck K. H., Norkin L. C. Lysosome stability during lytic infection by simian virus 40. Intervirology. 1979;12(1):47–56. doi: 10.1159/000149068. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Wertheim-van Dillen P., van Strien A., van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10(2):91–99. doi: 10.1002/jmv.1890100203. [DOI] [PubMed] [Google Scholar]
- Gribble D. H., Haden C. C., Schwartz L. W., Henrickson R. V. Spontaneous progressive multifocal leukoencephalopathy (PML) in macaques. Nature. 1975 Apr 17;254(5501):602–604. doi: 10.1038/254602a0. [DOI] [PubMed] [Google Scholar]
- Grinnell B. W., Martin J. D., Padgett B. L., Walker D. L. Is progressive multifocal leukoencephalopathy a chronic disease because of defective interfering particles or temperature-sensitive mutants of JC virus? J Virol. 1982 Sep;43(3):1143–1150. doi: 10.1128/jvi.43.3.1143-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Padgett B. L., Walker D. L. Distribution of nonintegrated DNA from JC papovavirus in organs of patients with progressive multifocal leukoencephalopathy. J Infect Dis. 1983 Apr;147(4):669–675. doi: 10.1093/infdis/147.4.669. [DOI] [PubMed] [Google Scholar]
- Heritage J., Chesters P. M., McCance D. J. The persistence of papovavirus BK DNA sequences in normal human renal tissue. J Med Virol. 1981;8(2):143–150. doi: 10.1002/jmv.1890080208. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Welsh R. M., Oldstone M. B., Kohne D., Lazzarini R., Scolnick E. Long-term persistent vesicular stomatitis virus and rabies virus infection of cells in vitro. J Gen Virol. 1976 Nov;33(2):193–211. doi: 10.1099/0022-1317-33-2-193. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Rentier-Delrue F., Heilman C. A., Law M. F., Chowdhury K., Israel M. A., Takemoto K. K. Cloned human polyomavirus JC DNA can transform human amnion cells. J Virol. 1980 Dec;36(3):878–882. doi: 10.1128/jvi.36.3.878-882.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. N., Brockman W. W., Nathans D. Evolutionary variants of simian virus 40: cloned substituted variants containing multiple initiation sites for DNA replication. Virology. 1975 Jul;66(1):53–69. doi: 10.1016/0042-6822(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Lombardi P. S., Balduzzi P., Hare J. D., Morgan H. R. Mechanism of the development of a stable carrier system of L cells with polyoma virus. J Natl Cancer Inst. 1970 Jul;45(1):171–178. [PubMed] [Google Scholar]
- McKeever P. E., Chronwall B. M., Houff S. A., Sever J. L., Kornblith P. L., Padgett B. L., Walker D. L., London W. T. Glial and divergent cells in primate central nervous system tumors induced by JC virus isolated from human progressive multifocal leukoencephalopathy (PML). Prog Clin Biol Res. 1983;105:239–251. [PubMed] [Google Scholar]
- Nichols W. W., Bradt C. I., Toji L. H., Godley M., Segawa M. Induction of sister chromatid exchanges by transformation with simian virus 40. Cancer Res. 1978 Apr;38(4):960–964. [PubMed] [Google Scholar]
- Norkin L. C. Cell killing by simian virus 40: impairment of membrane formation and function. J Virol. 1977 Mar;21(3):872–879. doi: 10.1128/jvi.21.3.872-879.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Effect of input multiplicity on the establishment of simian virus 40 persistent infections in rhesus monkey kidney cells. Infect Immun. 1977 Dec;18(3):868–871. doi: 10.1128/iai.18.3.868-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C., Ouellette J. Cell killing by simian virus 40: variation in the pattern of lysosomal enzyme release, cellular enzyme release, and cell death during productive infection of normal and simian virus 40-transformed simian cell lines. J Virol. 1976 Apr;18(1):48–57. doi: 10.1128/jvi.18.1.48-57.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Papovaviral persistent infections. Microbiol Rev. 1982 Dec;46(4):384–425. doi: 10.1128/mr.46.4.384-425.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Persistent infections of green monkey kidney cells initiated with temperature-sensitive mutants of simian virus 40. Virology. 1980 Dec;107(2):375–388. doi: 10.1016/0042-6822(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Norkin L. C. Rhesus monkeys kidney cells persistently infected with Simian Virus 40: production of defective interfering virus and acquisition of the transformed phenotype. Infect Immun. 1976 Sep;14(3):783–792. doi: 10.1128/iai.14.3.783-792.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. The emergence of simian virus 40 variants in a persistent infection of rhesus monkey kidney cells and their interaction with standard simian virus 40. Virology. 1979 Jun;95(2):598–603. doi: 10.1016/0042-6822(79)90515-4. [DOI] [PubMed] [Google Scholar]
- Norkin L. C., Wojcik J. B., Goguen C. A. Effect of the host cell on the generation of defective Simian Virus 40 during undiluted serial passages and persistent infection. Virology. 1981 Jan 30;108(2):525–530. doi: 10.1016/0042-6822(81)90462-1. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Rogers C. M., Walker D. L. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977 Feb;15(2):656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Varakis J. N. Differential neurooncogenicity of strains of JC virus, a human polyoma virus, in newborn Syrian hamsters. Cancer Res. 1977 Mar;37(3):718–720. [PubMed] [Google Scholar]
- Palitti F., Matarese G. P., Diana G., Sorrentino V., Rossi G. B. In vivo studies of increased incidence of sister chromatid exchanges in target cells of replication-component Friend murine leukemia virus. Cancer Res. 1982 Nov;42(11):4753–4757. [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974 Sep 13;251(5471):156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rima R. K., Martin S. J. Persistent infection of tissue culture cells by RNA viruses. Med Microbiol Immunol. 1976 Jun 1;162(2):89–119. doi: 10.1007/BF02121320. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Finkelstein S. D., McLachlan D. R. Multiple malignant astrocytomas in a patient with spontaneous progressive multifocal leukoencephalopathy. Ann Neurol. 1983 Aug;14(2):183–188. doi: 10.1002/ana.410140205. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Mullarkey M. F. Human papovavirus, BK strain: biological studies including antigenic relationship to simian virus 40. J Virol. 1973 Sep;12(3):625–631. doi: 10.1128/jvi.12.3.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner L. P., Herndon R. M., Narayan O., Johnson R. T., Shah K., Rubinstein L. J., Preziosi T. J., Conley F. K. Isolation of virus related to SV40 from patients with progressive multifocal leukoencephalopathy. N Engl J Med. 1972 Feb 24;286(8):385–390. doi: 10.1056/NEJM197202242860801. [DOI] [PubMed] [Google Scholar]
- ZURHEIN G., CHOU S. M. PARTICLES RESEMBLING PAPOVA VIRUSES IN HUMAN CEREBRAL DEMYELINATING DISEASE. Science. 1965 Jun 11;148(3676):1477–1479. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
- Zu Rhein G. M. Association of papova-virions with a human demyelinating disease (progressive multifocal leukoencephalopathy). Prog Med Virol. 1969;11:185–247. [PubMed] [Google Scholar]