Abstract
Salmonella enterica serovar Typhimurium, a common enteric pathogen, possesses three NiFe uptake-type hydrogenases. The results from mouse infection studies suggest that the H2 oxidation capacity provided by these hydrogenases is important for virulence. Since the three enzymes are similar in structure and function, it may be expected that they are utilized under different locations and times during an infection. A recombination-based method to examine promoter activity in vivo (RIVET) was used to determine hydrogenase gene expression in macrophages, polymorphonuclear leukocyte (PMN)-like cells, and a mouse model of salmonellosis. The hyd and hya promoters showed increased expression in both murine macrophages and human PMN-like cells compared to that in the medium-only controls. Quantitative reverse transcription-PCR results suggested that hyb is also expressed in phagocytes. A nonpolar hya mutant was compromised for survival in macrophages compared to the wild type. This may be due to lower tolerance to acid stress, since the hya mutant was much more acid sensitive than the wild type. In addition, hya mutant cells were internalized by macrophages the same as wild-type cells. Mouse studies (RIVET) indicate that hyd is highly expressed in the liver and spleen early during infection but is expressed poorly in the ileum in infected animals. Late in the infection, the hyd genes were expressed at high levels in the ileum as well as in the liver and spleen. The hya genes were expressed at low levels in all locations tested. These results suggest that the hydrogenases are used to oxidize hydrogen in different stages of an infection.
There are an estimated 13.8 million food-borne illnesses per year in the United States, resulting in about 5,020 deaths annually (17). Salmonella species are some of the most common pathogens associated with food-borne illness in adults living in developed countries (5) and are responsible for about 30% of food-related deaths in the United States (17). Salmonella enterica serovar Typhimurium causes gastroenteritis in humans and a typhoid fever-like disease in mice (25).
Salmonella serovar Typhimurium possesses three uptake-type NiFe hydrogenases that are important for virulence (16). H2 produced by colonic bacterial fermentation can be split by these membrane-bound hydrogenases. This process yields energy in the form of protons that contribute to the proton gradient and electrons that can be passed down the electron transport chain (27). H2 use may be common among bacterial pathogens, since the ability to use H2 is also important for Helicobacter pylori to efficiently colonize the mouse stomach (21).
It was recently shown that the three uptake-type hydrogenases in Salmonella serovar Typhimurium are expressed under different conditions in vitro. The hyb promoter had the most expression under anaerobic respiration conditions with fumarate as the terminal electron acceptor. The hyd promoter was expressed the most under aerobic conditions, while the hya promoter had the most expression during fermentation (28). In addition, Park et al. reported that hya expression may be acid induced (23). Since the three hydrogenases are differentially expressed in vitro, it may be predicted that they are used at different locations and times during an infection.
Here we examine hydrogenase expression in vivo. Hydrogenase promoter fusions were made using resolvase-in vivo expression technology (RIVET). This system was originally developed to identify Vibrio genes induced during infection (3) and was later adapted for use with Salmonella serovar Typhimurium (18). In this study, expression of hydrogenase promoter fusions in murine and human phagocytes was assayed. Expression of the hya and hyb promoters was then assessed in the ileums, livers, and spleens of mice. Hydrogenase mutant survival was determined in murine macrophages. In order to examine the role of hydrogenases in macrophage survival in more depth, hydrogenase mutant survival was determined under acid stress conditions. Internalization assays were also performed in order to determine if there are differences in internalization of wild-type and mutant strains during macrophage infection.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and reagents.
The Salmonella serovar Typhimurium and Escherichia coli strains, plasmids, and phage used in this study are listed in Table 1. Strains were maintained in Luria-Bertani (LB) broth or on plates with appropriate antibiotics at the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 34 μg/ml; kanamycin, 25 μg/ml; and tetracycline, 12.5 μg/ml. CR-Hyd medium was used where indicated (26). The medium was supplemented with glucose (0.4%), glycerol (0.4%), or sodium fumarate (20 mM) where indicated. Anaerobic culture conditions were established by sparging sealed 165-ml bottles with N2 for 15 min and then with anaerobic mix (10% H2, 5% CO2, and 85% N2) for 20 min. Bismuth-sulfite agar, a selective and differential medium for Salmonella serovar Typhimurium, was used in mouse experiments. M9 minimal medium with 20% mannose was used where indicated. o-Nitrophenyl-β-d-galactopyranoside (ONPG) was purchased from Pierce (Rockford, IL). Primers were purchased from Integrated DNA Technologies (Coralville, IA).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli strains | ||
| SM10(λpir) | thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu (Kanr) λ::pir | John Gunn |
| DH5α(λpir) | F−lacU169 (ϕ80lacZΔM15) recA1 endA1 hsdR17 supE44 thi-1 gyrA96 (Nalr) relA1 λ::pir | John Gunn |
| S. enterica serovar Typhimurium strains | ||
| JSG210 | 14028s (wild type) | ATCC |
| ALZ7 | JSG210 with Δhyb::FRT | 28 |
| ALZ8 | JSG210 with Δhyd::FRT | 28 |
| ALZ9 | SG210 with Δhya::FRT | 28 |
| JS246 | 14028 yjeP8103::res1-tetAR-res1 | 18 |
| ALZ15 | JS246 hyb+::Φ(hyb′-tnpR-lacZY) | This study |
| ALZ16 | JS246 hyd+::Φ(hyd′-tnpR+-lacZY+) | This study |
| ALZ17 | JS246 hya+::Φ(hya′-tnpR+-lacZY+) | This study |
| ALZ18 | JS246 hyb+::Φ(hyb′-tnpRmut135-lacZY+) | This study |
| ALZ19 | JS246 hyd+::Φ(hyd′-tnpRmut135-lacZY+) | This study |
| ALZ20 | JS246 hya+::Φ(hya′-tnpRmut135-lacZY+) | This study |
| ALZ21 | JS246 hyb+::Φ(hyb′-tnpRmut168-lacZY+) | This study |
| ALZ22 | JS246 hyd+::Φ(hyd′-tnpRmut168-lacZY+) | This study |
| ALZ23 | JS246 hya+::Φ(hya′-tnpRmut168-lacZY+) | This study |
| Plasmids | ||
| pGOA1193 | pIVET5n with promoterless tnpR+-lacZY+ (Apr) | 18 |
| pGOA1194 | pIVET5n with promoterless tnpRmut135-lacZY+ (Apr) | 18 |
| pGOA1195 | pIVET5n with promoterless tnpRmut168-lacZY+ (Apr) | 18 |
| pZBL6 | pGOA1193 with Phyb | This study |
| pZBL7 | pGOA1193 with Phyd | This study |
| pZBL8 | pGOA1193 with Phya | This study |
| pZBL9 | pGOA1194 with Phyb | This study |
| pZBL10 | pGOA1194 with Phyd | This study |
| pZBL11 | pGOA1194 with Phya | This study |
| pZBL12 | pGOA1195 with Phyb | This study |
| pZBL13 | pGOA1195 with Phyd | This study |
| pZBL14 | pGOA1195 with Phya | This study |
FRT, FLP recognition target.
Construction of reporter gene fusions.
RIVET fusions were made as described previously (3, 18). In brief, three genes arranged in tandem, namely, a transposon resolvase gene (tnpR), lacZ, and lacY, were put under the control of each hydrogenase promoter. When the promoter was active, TnpR was made and catalyzed the excision of a chromosomal tetracycline resistance cassette (tetRA) located at the yjeP locus (yjeP8103::res1-tetRA-res1), resulting in the heritable loss of tetracycline resistance.
Primers Hyb1BamHI (5′-CGGGATCCTGGCTTTTATTTTGCAG-3′) and REVHyb1BamHI (5′-CGGGATCCAACAGGGGTATTATC-3′) were used to generate a 200-bp fragment containing the putative hyb promoter with BamHI sites engineered into both ends of the PCR product. Primers Hyb2BamHI (5′-CGGGATCCTAGTTAGTGTGGGCATC-3′) and REVHyb2BamHI (5′-CGGGATCCGCTCTCCTTGTTTGTA-3′) were used to generate a 500-bp PCR product containing the putative hyd promoter, while primers Hyb3BamHI (5′-CGGGATCCTTGCACCACGCTCCGC-3′) and REVHyb3BamHI (5′-CGGGATCCGCCCTCTTTCAAACAGTC-3′) were used to generate a 550-bp product containing the putative hya promoter. Each PCR product was digested with BamHI and ligated into three vectors containing different ribosome binding sites that allow varied levels of TnpR translation (pGOA1193, pGOA1194, and pGOA1195). The resulting nine plasmids are listed in Table 1. Plasmids were transferred into E. coli DH5α(λpir) and then into E. coli SM10(λpir). Finally, each of the nine fusion constructs was then transferred into Salmonella serovar Typhimurium JS246 by conjugation, yielding nine strains (Table 1). The fusion constructs integrated into the JS246 chromosome by homologous recombination, since the plasmids cannot replicate in JS246. The three strains constructed for each hydrogenase promoter confer different levels of expression of the transposon resolvase genes. This occurs because each strain contains a unique ribosome binding site mutation or no ribosome binding site mutation in front of the transposon resolvase genes.
RT-PCR.
In order to confirm the nonpolar nature of the hya mutation, reverse transcription-PCR (RT-PCR) was performed on wild-type and hya mutant strains. Bacteria were grown overnight in LB medium at 37°C. RNAs were extracted using an Aurum Total RNA Mini kit (Bio-Rad) and were digested with RQ1 DNase (Promega) to remove contaminating genomic DNA. The digested RNAs were the template to generate cDNA, using an iScript select cDNA synthesis kit (Bio-Rad) and primers UPST1788 (5′-ATGTTGGTACTGATGGTAAC-3′) and REVST1788 (5′-AATAGCGGTTGCCGACACAG-3′), which amplified 178 bp from the gene directly downstream (STM 1788) of the deleted hya genes. The same primers were used to amplify the fragment by PCR.
In vitro RIVET transcription assays.
RIVET fusion cells were grown overnight at 37°C under conditions previously shown to induce hydrogenase promoter activity or under noninducing conditions (28). After overnight growth under these conditions, β-galactosidase activity was assayed according to the method of Miller (19). Cells were diluted and plated on M9 minimal medium agar plates supplemented with ampicillin. After overnight growth, between 50 and 100 colonies were patched on LB plates containing tetracycline. Percent resolution, a measurement of promoter activity, was determined by dividing the number of tetracycline-sensitive cells by the total number of colonies patched. One strain for each promoter (ALZ18, ALZ16, and ALZ17 for hyb, hyd, and hya, respectively) was chosen for further study based on detectable and reliable gene expression.
Tissue culture conditions and cell invasion assays.
RAW 264.7 murine macrophages (ATCC) or HL-60 human polymorphonuclear leukocyte (PMN)-like cells (D. Evans, University of Georgia) were grown at 37°C and 5% CO2 in Dulbecco's modified Eagle medium (DMEM) with 10% heat-inactivated fetal bovine serum. Cells were passaged every 3 to 5 days as required. HL-60 cells were incubated with 1 μM retinoic acid (Sigma, St. Louis, MO) for 4 days immediately prior to cell invasion assay to stimulate differentiation and maturation of the PMNs. Tissue culture cells were added to 24-well plates at a concentration of 1 × 105 cells per well 1 day prior to infection with Salmonella serovar Typhimurium. Cells were infected with RIVET fusion strains or hydrogenase deletion mutants at a multiplicity of infection (MOI) of 10 (1 × 106 bacteria per well). This was considered time zero for the assay in order to determine the survival of each strain at early time points, before gentamicin was added. The plate was then centrifuged at 100 × g and incubated at 4°C for 30 min and then at 37°C for 1 h. Extracellular bacteria were killed by being incubated with gentamicin (100 μg/ml) at 37°C for 2 h and then for an additional 16 h with 10 μg/ml gentamicin. Tissue culture cells were lysed with 1% Triton X-100, and the lysate was diluted and plated on LB. Expression of each of the three hydrogenase promoters was monitored by determining the ratio of tetracycline-sensitive colonies to ampicillin-resistant colonies, as described above. Bacterial survival of wild-type or hydrogenase deletion mutant strains was determined by diluting and plating lysates from RAW 264.7 macrophages on LB plates at different time points during the assay. The resulting colonies were then counted, and the number of CFU per ml was determined.
Real-time PCR.
RAW 264.7 murine macrophages (5 × 105) were grown overnight in 12-well plates and then infected with 5 × 107 wild-type Salmonella serovar Typhimurium cells. Total RNAs from bacteria and macrophages were isolated using TRIzol (Invitrogen) or an Aurum Total RNA Mini kit (Bio-Rad) immediately after infection and at 2 h, 4 h, 12 h, and 24 h postinfection (p.i.). RNAs were digested with RQ1 DNase (Promega) to remove contaminating genomic DNA. cDNA was then synthesized using 0.5 to 1 μg RNA and an iScript Select cDNA synthesis kit (Bio-Rad). Quantitative PCR was performed with Platinum SYBR Green qPCR SuperMix UDG (Invitrogen) and an iCycler iQ PCR detection system (Bio-Rad). Primers were designed to amplify a 100- to 200-bp product and were homologous to the small subunit of the Salmonella serovar Typhimurium hyb (STM 3150), hyd (STM 1539), or hya (STM 1786) structural gene or to the rpoA gene, encoding the α subunit of the RNA polymerase (STM 3415), used for sample normalization. Transcript abundance for each gene in DMEM only was standardized to 1, and transcript levels in macrophages are given relative to levels in DMEM only. Relative quantification was used to calculate differences between samples, using the ΔΔCT method. Standard deviations and the range of ΔΔCT values were calculated for each sample, as described in Applied Biosystems user bulletin 2.
Internalization assays.
The ability of the hya mutant and wild-type cells to enter RAW 264.7 murine macrophages was assessed by using a fluorescence quenching assay, as described by Fallman et al. (8). Wild-type and hya mutant Salmonella serovar Typhimurium cells were grown overnight in 2 ml LB broth upright, without shaking. Bacterial cells (1 × 109 cells/ml) were washed in phosphate-buffered saline (PBS) and labeled with 0.1 mg/ml 5-(6)-carboxyfluorescein-succinylester or fluorescein isothiocyanate (FITC; Molecular Probes) in PBS for 30 min at 4°C. Cells were washed in sterile PBS and resuspended in DMEM plus 10% fetal bovine serum, and 1 × 108 cells were added to 1 × 105 RAW 264.7 cells that had been grown overnight in 24-well tissue culture plates. FITC-conjugated 0.5-μm-diameter polystyrene beads (1 × 108) or FITC-labeled E. coli (5 × 106) was added to the macrophages as a control. Plates were centrifuged at 100 × g for 10 min at 4°C and then were incubated at 37°C for 1.5 h to allow for phagocytosis. The number of macrophages in one-fourth of a random frame of view was counted by using a Leica TCS inverted epifluorescence microscope. Trypan blue (0.1 ml; 1 mg/ml in citrate buffer, pH 4.4) was then added to quench the fluorescence of extracellular bacteria or beads. The number of macrophages containing intracellular bacteria or beads was then counted.
Acid survival assays.
Hydrogenase deletion mutants and wild-type cells were grown anaerobically overnight in sealed 165-ml bottles containing 20 ml LB broth. Cells were harvested by centrifugation and resuspended in McIlvaine's citric acid-phosphate buffer (7). Sealed anaerobic 165-ml bottles containing 5 ml McIlvaine's buffer at pH 4 or pH 7 were inoculated with 1 × 108 cells. Samples were taken at various time points up to 24 h postinoculation, diluted in PBS, and plated on LB agar. The resulting colonies were counted, and the number of CFU per ml was determined.
Animal studies.
Female 4.5-week-old BALB/c mice (National Cancer Institute, Frederick, MD) were orally inoculated with 1 × 106 RIVET fusion cells that had been grown overnight in LB medium with 100 μg/ml ampicillin and 12.5 μg/ml tetracycline, washed in PBS, and then resuspended in PBS. Mice were sacrificed at various time points as indicated in Fig. 8, and ileums, livers, and spleens were mechanically homogenized in PBS with 10-ml Dounce hand homogenizers. The homogenate was plated on LB or bismuth-sulfite agar containing 100 μg/ml ampicillin, and resulting colonies were patched onto LB plates containing 12.5 μg/ml tetracycline to determine percent resolution.
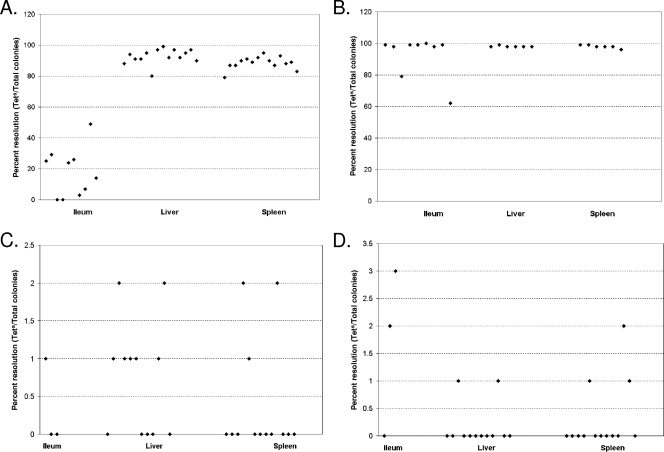
FIG. 8.
Hydrogenase gene expression in mouse tissues. Female BALB/c mice were orally inoculated with 1 × 106 ALZ16 (hyd fusion) or ALZ17 (hya fusion) cells. Mice were sacrificed at 1 day (A) and 2.5 days (B) p.i. for hyd or at 2 days (C) and 4 days (D) p.i. for hya. Ileums, livers, and spleens were taken. The organs were mechanically homogenized in PBS and plated on bismuth-sulfite or LB agar containing ampicillin. The resulting colonies were patched on LB containing tetracycline, and the percentage of Tets colonies/total colonies patched per mouse organ was calculated. Cells isolated from the liver and spleen had significantly more hyd expression at day 1 (A) than did cells isolated from the ileum on the same day, according to the Mann-Whitney-Wilcoxon test (α = 0.01).
RESULTS
Hydrogenase expression in vitro.
In order to study the expression of hydrogenase genes, transcriptional reporter fusions were made using the RIVET technique. The resulting constructs (Table 1) have a lacZ gene and a transposon resolvase gene under the control of the hydrogenase promoter. Three strains were made for every promoter, with each containing a different ribosome binding site mutation that would result in differential translation of the TnpR resolvase.
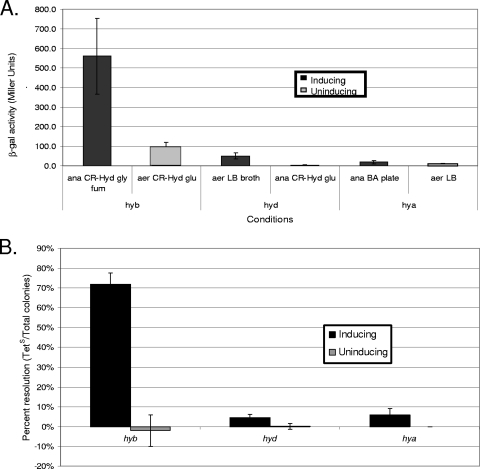
In order to determine which fusions expressed detectable levels of TnpR, strains were grown under conditions known to induce or repress promoter activity (28). The hyb promoter fusion strains were grown in CR-Hyd medium with glycerol and fumarate (anaerobic respiration to fumarate) for inducing conditions and in CR-Hyd with added glucose (fermentation) for noninducing conditions. The hyd fusion strains were grown aerobically (under atmospheric conditions) in LB broth (inducing) or anaerobically in CR-Hyd with glucose (noninducing). The hya fusion strains were grown on blood agar (BA) plates in anaerobic jars (inducing) or aerobically on LB plates (noninducing). β-Galactosidase assays were used to determine lacZ expression. Transposon resolvase expression was determined by finding the percentage of tetracycline-sensitive colonies amongst the total colonies (percent resolution). The fusions that showed higher lacZ and tnpR expression under inducing conditions than under noninducing conditions were chosen for further study (ALZ18, ALZ16, and ALZ17 for hyb, hyd, and hya, respectively). The in vitro expression of these three fusions is shown in Fig. 1.
FIG. 1.
Expression of RIVET fusions in vitro. RIVET fusion strains ALZ18, ALZ16, and ALZ17, for hyb, hyd, and hya, respectively, were grown overnight under conditions previously shown to induce the promoter or under noninducing conditions (aerobically [aer] or anaerobically [ana] on CR-Hyd, LB, or BA medium). β-Galactosidase activities were measured (A), and tetracycline sensitivity was determined (B) (n = 3 to 7). According to the Mann-Whitney-Wilcoxon test, β-galactosidase activities were significantly higher for ALZ17 cells grown anaerobically on BA than for cells grown aerobically on LB agar (α = 0.01).
Hydrogenase expression in phagocytes.
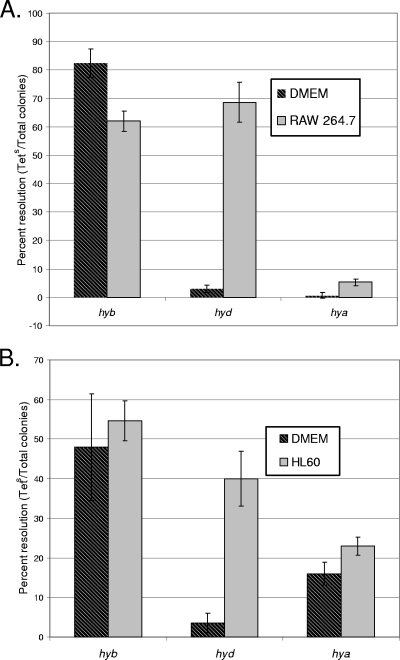
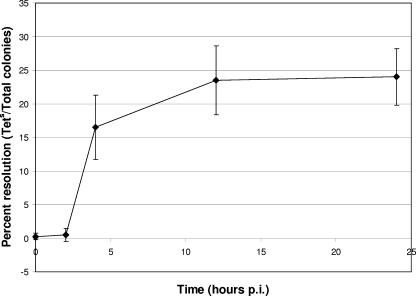
It was recently shown that uptake-type hydrogenases are important for Salmonella serovar Typhimurium virulence in mice (16). We wanted to examine whether hydrogenase gene expression is upregulated intracellularly or, in contrast, expressed only outside phagocytes in order to predict where each gene might be expressed during a mouse infection. RAW 264.7 murine macrophages were infected with each of the three hydrogenase RIVET fusion strains, and gene expression was determined. The hyd genes were upregulated inside the macrophages compared to the medium-only control (Fig. 2). Expression of hyd was then determined at various time points during a tissue culture survival assay. The results suggest that hyd has the highest level of expression at around 12 h p.i. (Fig. 3). Quantitative RT-PCR (qRT-PCR) results also indicate that the hyd genes are expressed in macrophages starting at the 12-h time point (approximately 3-fold more [range, 1.8- to 4.3-fold] expression than that of the DMEM-only control).
FIG. 2.
Expression of RIVET fusions in murine macrophages and human PMN-like cells. A total of 1 × 105 RAW 264.7 murine macrophages (A) or HL-60 human PMN-like cells (B) were infected with 1 × 106 CFU of RIVET fusion strain ALZ18 (hyb), ALZ16 (hyd), or ALZ17 (hya) at an MOI of 10 in 24-well plates. Hydrogenase expression was monitored by determining the ratio of tetracycline-sensitive colonies to ampicillin-resistant colonies, as described in Materials and Methods. Results are averages for three replicates. Hya gene expression was significantly higher in bacterial cells isolated from RAW 264.7 or HL-60 tissue culture cells than in medium-only controls, based on Student's t test (P < 0.005).
FIG. 3.
Expression of hyd genes in RAW 264.7 murine macrophages. RAW 264.7 murine macrophages (1 × 105) were infected with 1 × 106 CFU of RIVET fusion strain ALZ16 (hyd) at an MOI of 10 in 24-well plates. Tissue culture cells were lysed with Triton X-100 at various time points, as indicated, and bacterial cells were diluted in PBS and plated. Hydrogenase expression was monitored by determining the ratio of tetracycline-sensitive colonies to ampicillin-resistant colonies, as described in Materials and Methods. Results are averages for four replicates.
According to Student's t test, hya expression was significantly upregulated in cells isolated from RAW 264.7 macrophages compared to the medium-only controls (P < 0.005). Overall expression for hya seems to be low in macrophages, and relative hya transcript abundance was too low to make significant conclusions from qRT-PCR experiments.
The results from RIVET experiments indicated that the hyb genes are not upregulated in RAW 264.7 murine macrophages, since expression was similar in this cell line and in controls (Fig. 2); however, background expression was high in these experiments. qRT-PCR results suggested that hyb was indeed expressed in RAW 264.7 macrophages, starting at 4 h p.i. Expression in one sample was about 3-fold higher (range, 2.3- to 3.4-fold among three replicates) at 4 h p.i. and 43-fold higher (range, 28- to 66-fold among six replicates) at 12 h p.i. than that in DMEM-only controls.
PMNs are among the first cells recruited to the intestine to combat a Salmonella serovar Typhimurium infection in mice and humans (9). In order to determine whether hydrogenases would be expressed in the human PMN-like cell line HL-60, cells were infected with hydrogenase fusion strains. The hyd fusion was upregulated in this cell line compared to medium-only controls, and the hyb fusion was not upregulated, just as in RAW 264.7 cells (Fig. 2). The hya fusion was slightly but significantly upregulated in cells isolated from HL-60 PMNs compared to medium-only controls, according to Student's t test (P < 0.005).
Hydrogenase mutant survival in macrophages and internalization assays.
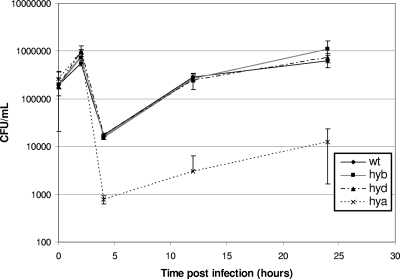
Since hydrogenase expression was upregulated for hyd and hya during intracellular growth and since hydrogenases are important for virulence, we wanted to determine whether hydrogenase expression was important for survival and growth in macrophages. RAW 264.7 macrophages were infected with hydrogenase deletion mutants, and bacterial numbers were counted at various time points p.i. Only the hya genes were necessary for survival inside macrophages, since there were about 22 times fewer hya mutant cells than wild-type cells as early as 4 hours p.i., and hya cell survival remained lower than wild-type levels at all time points tested. The hyb and hyd mutants were similar to the wild type at all time points (Fig. 4).
FIG. 4.
Hydrogenase mutant survival in RAW 264.7 murine macrophages. RAW 264.7 murine macrophages (1 × 105) were infected at an MOI of 10 (1 × 106) with wild-type (wt) or hyb, hyd, or hya mutant cells (strain ALZ7, ALZ8, or ALZ9, respectively). Extracellular bacteria were killed with 100 μg/ml gentamicin at 2 h p.i. Macrophages were lysed with 1% Triton X-100 at various time points, and bacteria were enumerated by dilution plating on LB. Results are averages for four replicates.
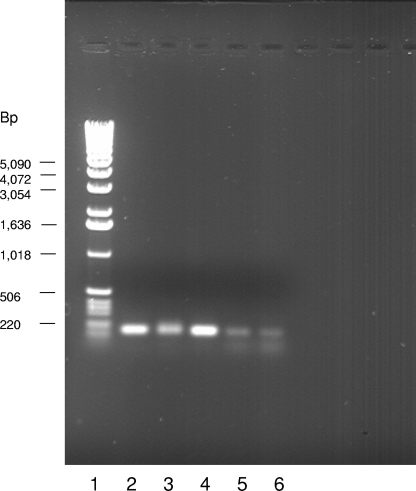
In order to confirm that the survival defect was due to the hya gene deletion and not some polar effect, RT-PCR was performed on wild-type and hya mutant cells. Cells were grown overnight in LB medium, RNAs were isolated from both strains, and cDNAs were synthesized. Primers specific to the gene directly downstream of the hya mutation, i.e., STM 1788, were used to generate a PCR product from the cDNA. Both the wild-type and hya strain cDNAs yielded PCR products in greater quantities than in the no-reverse-transcriptase control (Fig. 5), demonstrating that the gene directly downstream of the hya deletion is transcribed in the hya mutant and that the mutation is nonpolar.
FIG. 5.
STM 1788 expression in wild-type and hya mutant cells. RNAs were extracted from wild-type and hya mutant cell cultures grown aerobically overnight in LB broth, using an Aurum Total RNA Mini kit (Bio-Rad). RNAs were digested with RQ1 DNase (Promega) to remove contaminating genomic DNA. These were used as templates to generate cDNAs by use of an iScript Select cDNA synthesis kit (Bio-Rad) and primers homologous to the gene directly downstream of the hya mutation (STM 1788). PCR was performed with the same primers used to generate cDNA. Lane 1, Promega 1-kb DNA ladder; lane 2, positive control (PCR product from wild-type genomic DNA); lanes 3 and 4, PCR products from wild-type and hya cDNAs, respectively; lanes 5 and 6, negative controls (PCR products from wild-type and hya cDNA reactions, respectively, where reverse transcriptase was not added to the mix).
In order to determine whether the observed PCR products were within the linear range of the amplification reaction, PCR was performed with 20, 25, 27, and 30 amplification cycles. After 20 amplification cycles, no product was observed for the cDNA for either strain or for the no-reverse-transcriptase control, although product was observed for the positive control (genomic DNA). After 25 and 27 amplification cycles, faint bands of approximately the same intensity were observed for both wild-type and hya mutant strains, while no product was observed for the no-reverse-transcriptase control samples (results not shown). The PCR products shown in Fig. 5 are the results of 30 amplification cycles. Since faint bands were observed at 27 cycles, it can be assumed that the reactions were within the linear range after 25 or 27 cycles and were not saturating at 30 cycles.
Interestingly, in macrophage survival assays, the hya mutant had similar cell survival to that of the wild type as late as 2 hours p.i., suggesting that these cells were able to combat reactive oxygen and nitrogen species and enzymes released by the macrophages. However, cell numbers decreased dramatically after extracellular bacteria were killed with gentamicin compared to wild-type cell numbers. This may indicate that hya mutants have difficulty entering macrophages or surviving the early Salmonella-containing vacuole (SCV). The hya mutant was able to grow inside macrophages, since cell numbers increased at a logarithmic rate as early as 4 hours p.i. (Fig. 4).
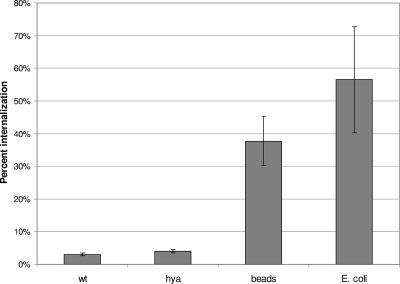
Internalization assays using the fluorescence quenching method were performed in order to determine whether the decreased survival of the hya mutant in macrophages was due to reduced survival once cells were inside macrophages (as hypothesized) or due to decreased entry into macrophages. The results (Fig. 6) demonstrated that the hya mutant entered RAW 264.7 macrophages just as well as wild-type cells did. The macrophages were also able to internalize inert polystyrene beads or killed E. coli, which demonstrates that they were capable of effective phagocytosis.
FIG. 6.
Phagocytosis of wild-type and hya mutant Salmonella serovar Typhimurium by RAW 264.7 murine macrophages. Macrophages (1 × 105) were infected with 1 × 108 FITC-conjugated live wild-type (wt) or hya cells or beads or with 5 × 106 FITC-conjugated killed E. coli cells. Macrophages were examined 1.5 h later for adherence or phagocytosis by fluorescence quenching, using a Leica TCS inverted epifluorescence microscope. The number of macrophages containing one or more bacterial cells or beads per 100 to 200 macrophages was counted and expressed as percent internalization. The results are averages for three trials.
The hya mutant is compromised for survival under acidic conditions.
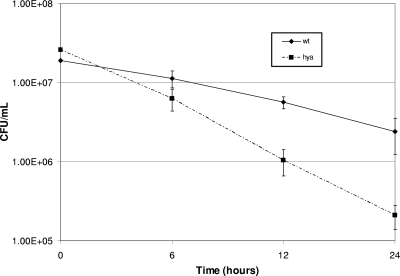
Salmonella must withstand hostile conditions in order to survive and replicate inside macrophages. The SCV acidifies soon after bacteria enter the cells and macrophages release reactive oxygen and nitrogen species (10, 13). If the hya mutant was more sensitive to acid stress than the wild type, it might explain why this mutant did not survive as well inside macrophages.
In order to test resistance to acid stress, the hya, hyb, and hyd mutant cells, as well as wild-type cells, were exposed to citric acid-phosphate buffer at pH 4 or 7, and cell numbers were determined at various time points. The hya mutant had a lower tolerance for acid stress, with about 90% fewer cells than wild-type cells after 24 h at pH 4 (Fig. 7). The hyb and hyd mutants had similar cell numbers compared to the wild type after exposure to pH 4 (results not shown). hya gene expression is also upregulated by acid. hya genes had about fourfold more expression after overnight growth in anaerobic bottles containing LB buffered with 2-(N-morpholino)ethanesulfonic acid (MES) at pH 5.8 than that in LB at pH 7 (results not shown), according to β-galactosidase assays using a lacZ fusion to the hya promoter. Park et al. also suggested that hya is acid induced (23). The results above suggest that the hya mutant is compromised for survival in macrophages, at least in part because it cannot survive acid stress as well as the wild type does.
FIG. 7.
Hya mutant survival under acid stress conditions. Anaerobic bottles containing citric acid-phosphate buffer at pH 4 were inoculated with 108 wild-type (wt) or hya mutant cells that were grown anaerobically in LB overnight. Bottles were incubated for various times, as indicated, and samples were taken. Cells were enumerated by dilution plating on LB agar. Results are averages for at least four replicates.
Hydrogenase expression in BALB/c mice.
In order to determine hydrogenase expression during infection, female BALB/c mice were orally inoculated with 1 × 106 CFU of a hyb, hyd, or hya RIVET fusion strain. The hyb RIVET fusion strain ALZ18 had similar levels of resolution in the mouse to those in the medium-only background (around 80% resolution) and therefore was not studied further. The hyb RIVET fusion strains ALZ15 and ALZ21 were not used to determine hyb expression in the mouse because they did not produce significant TnpR in vitro. Mice were inoculated with hyd and hya strains at a young age (30 to 34 days old) and began to achieve morbidity at 4 days p.i. Therefore, at day 1 p.i., the infection was considered to be in the early stages, while days 3 to 4 were considered late in the infection. Mice were euthanized at various time points, and bacteria were recovered from the ileum, liver, and spleen. Bacteria were plated on bismuth-sulfite agar or LB agar containing ampicillin to select for Salmonella serovar Typhimurium colonies that contained RIVET fusions. The percent resolution of each promoter was determined by subtracting the level of resolution in the inoculum from the percent resolution in cells isolated from the mouse. There was significant contamination in ileum samples when cells were plated on LB plates containing ampicillin. Further investigation revealed that the contaminant was naturally ampicillin-resistant Enterobacter cloacae. The experiments were then repeated, with plating of ileum samples on bismuth-sulfite agar, which is a selective and differential medium for Salmonella serovar Typhimurium. Liver and spleen samples were not significantly contaminated, and percentages of resolution for colonies plated on LB and bismuth-sulfite agar were similar.
At day 1 p.i., the hyd promoter was expressed poorly in the ileum but highly in the liver and spleen (Fig. 8). During mouse infection, Salmonella serovar Typhimurium resides primarily within macrophages and other phagocytic cells, especially in the liver and spleen (24, 25). Since hyd was highly expressed in macrophages, it is reasonable that it would be expressed in the liver and spleen early in the infection, since Salmonella serovar Typhimurium resides within phagocytes in those organs. At 2.5 days postinoculation, hyd was highly expressed in all locations (Fig. 8). This increase of expression in the ileum was probably due to the accumulation of low levels of resolution over time, not to bacterial reinfection of the ileum from the bile duct. A recent study examined the expression of several Salmonella serovar Typhimurium genes in mice by use of the RIVET system and showed that bacterial reinfection of the ileum can occur, but at such a low level that it does not affect the apparent gene expression of bacteria isolated from the ileum (18).
The hya promoter was expressed to some extent in all locations tested in the mouse (Fig. 8), albeit with considerable variability among all tissues. hya gene expression was low at both initial (1 day [data not shown] or 2 days [Fig. 8C]) and late (4 days) (Fig. 8D) stages in the infection, including the point at which mice began to succumb to the infection. These levels of resolution are in agreement with the relatively low levels of hya expression in vitro and in macrophages. However, because hya is important for survival in both macrophages and mice, it appears that it is expressed at high enough levels to be important during infection.
DISCUSSION
In this study, we used the RIVET reporter system to demonstrate that hyd and hya are expressed in phagocytic cells and in the mouse during Salmonella infection. Prior to this work, it was shown that the ability to use H2 was necessary for Salmonella serovar Typhimurium to cause morbidity in the mouse model (16). The results from the RIVET expression studies in this report suggest where and when two of the hydrogenases are expressed inside the animal and, therefore, at which point each hydrogenase may be used during infection.
The hyd genes were initially expressed in the livers and spleens of mice, probably within phagocytes. Later in the infection, hyd was highly expressed in the ileum as well. The hyd genes were previously shown to be upregulated under aerobic conditions (28). After activation, phagocytes typically undergo a respiratory burst in order to generate toxic oxidants; therefore, we can assume that the environment inside phagocytes is somewhat oxic. A recent study reported that an activated RAW 264.7 murine macrophage had a respiratory increase of 1.5 to 2 nmol O2/106 cells-min after activation with E. coli lipopolysaccharide (22). Another study used a microelectrode to measure the amount of superoxide (O2−) present within the PMN-like cell line HL-60 (12). It was reported that there was about 15 ± 5 fmol O2− present per cell. The oxygen present in these cell lines could cause hyd genes to be upregulated. It is possible that Hyd provides a reductant used for oxidative stress-combating enzymes.
The same hydrogenase (Hyd) may be used to oxidize hydrogen with oxygen as the terminal electron acceptor in order to conserve energy while the bacterium resides in the host. Comparisons between the hyd large subunit and the gene database on the NCBI website revealed that Salmonella serovar Typhimurium hyd is more similar to the oxygen-tolerant hydrogen-oxidizing enzymes from Methylococcus capsulatus (hup) and Azotobacter vinelandii (hox) than to the anaerobic hydrogen-oxidizing hydrogenases from other enteric bacteria (www.ncbi.nlm.nih.gov). In addition, it has been shown that H2 oxidation can be coupled to O2 reduction in Salmonella serovar Typhimurium (16). Hyd may oxidize hydrogen with oxygen as the terminal electron acceptor while cells are residing in macrophages. This could either conserve energy in the form of ATP production and/or decrease the amount of O2 present in macrophages, which would reduce the toxic oxidation of molecules inside the bacterium.
The hya genes were expressed at low levels both in macrophages and in all locations tested within the mouse. Although it was expressed at low levels, hya was important for survival within macrophages. This may be due to reduced acid resistance in the SCV, since the hya mutant strain entered macrophages just as well as the wild type did yet did not survive acidic conditions as well as the wild type in acid survival assays. It has been shown that the macrophage SCV pH decreases to between 4.5 and 6 at 1 hour p.i. with Salmonella serovar Typhimurium (4, 6). The Hyc proteins in E. coli are known to produce H2 when the growth medium is maintained at a low pH during mixed-acid fermentation (1, 20). Hya may be used to recycle the H2 produced by Hyc at a low pH, thereby conserving energy, as suggested by Sawers et al. (26). Hydrogenase activity has been shown to affect acid resistance in E. coli, since a recent study demonstrated that a complete loss of hydrogenase activity (by a hyp mutation) reduced acid resistance to 3% of the wild-type levels (11). Alternatively, previous Salmonella serovar Typhimurium mutant strain analysis supports the hypothesis that Hya can operate in an H2 uptake manner coupled to respiration as well (16).
We have shown that the Salmonella serovar Typhimurium uptake-type hydrogenases are differentially expressed in the mouse and in murine macrophages. Although hyb was important for survival in the mouse, we were unable to determine where this gene was expressed in vivo. qRT-PCR results suggested that hyb is expressed in macrophages, which supports the likelihood that it is expressed in the animal as well. Further studies with promoter fusions to other reporter proteins, such as green fluorescent protein, would be useful to examine hyb expression in mice. Several studies have used green fluorescent protein fusions to determine bacterial or host promoter activity in animal models (2, 14). This report shows that hya plays a role in both acid tolerance and survival in macrophages. Additional studies that examine the mechanism behind hya acid resistance will be useful for exploring this relationship further.
Acknowledgments
We thank John Gunn for supplying bacterial strains JS246 and SM10(λpir), phage, and plasmids pGOA1193, pGOA1194, and pGOA1195. We thank Donald Evans for supplying tissue culture strain HL-60 and Michael Strand for helpful discussions and assistance.
This work was supported by the University of Georgia Foundation.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 14 July 2008.
REFERENCES
- 1.Bagramyan, K., N. Mnatsakanyan, A. Poladian, A. Vassilian, and A. Trchounian. 2002. The roles of hydrogenases 3 and 4, and the F0F1-ATPase, in H2 production by Escherichia coli at alkaline and acidic pH. FEBS Lett. 516172-178. [DOI] [PubMed] [Google Scholar]
- 2.Bumann, D., and R. H. Valdivia. 2007. Identification of host-induced pathogen genes by differential fluorescence induction reporter systems. Nat. Protocols 2770-777. [DOI] [PubMed] [Google Scholar]
- 3.Camilli, A., and J. J. Mekalanos. 1995. Use of recombinase gene fusions to identify Vibrio cholerae genes induced during infection. Mol. Microbiol. 18671-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuellar-Mata, P., N. Jabado, J. Liu, W. Furuya, B. B. Finlay, P. Gros, and S. Grinstein. 2002. Nramp1 modifies the fusion of Salmonella typhimurium-containing vacuoles with cellular endomembranes in macrophages. J. Biol. Chem. 2772258-2265. [DOI] [PubMed] [Google Scholar]
- 5.de Bruyn, G. 2000. Infectious disease: diarrhea. West. J. Med. 172409-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drecktrah, D., L. A. Knodler, R. Ireland, and O. Steele-Mortimer. 2006. The mechanism of Salmonella entry determines the vacuolar environment and intracellular gene expression. Traffic 739-51. [DOI] [PubMed] [Google Scholar]
- 7.Elving, P. J., J. M. Markowitz, and I. Rosenthal. 1956. Preparation of buffer systems of constant ionic strength. Anal. Chem. 281179-1180. [Google Scholar]
- 8.Fallman, M., K. Andersson, S. Hakansson, K. Magnusson, O. Stendahl, and H. Wolf-Watz. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 633117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fierer, J. 2001. Polymorphonuclear leukocytes and innate immunity to Salmonella infections in mice. Microbes Infect. 31233-1237. [DOI] [PubMed] [Google Scholar]
- 10.Gorvel, J.-P., and S. Meresse. 2001. Maturation steps of the Salmonella-containing vacuole. Microbes Infect. 31299-1303. [DOI] [PubMed] [Google Scholar]
- 11.Hayes, E. T., J. C. Wilks, P. Sanfilippo, E. Yohannes, D. P. Tate, B. D. Jones, M. D. Radmacher, S. S. BonDurant, and J. L. Slonczewski. 2006. Oxygen limitation modulates pH regulation of catabolism and hydrogenases, multidrug transporters, and envelope composition in Escherichia coli K-12. BMC Microbiol. 689-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isogai, Y., T. Tsuyama, H. Osada, T. Iizuka, and K. Tanaka. 1996. Direct measurement of oscillatory generation of superoxide anions by single phagocytes. FEBS Lett. 380263-266. [DOI] [PubMed] [Google Scholar]
- 13.John, G. S., N. Brot, J. Ruan, H. Erdjument-Bromage, P. Tempst, H. Weissbach, and C. Nathan. 2001. Peptide methionine sulfoxide reductase from Escherichia coli and Mycobacterium tuberculosis protects bacteria against oxidative damage from reactive nitrogen intermediates. Proc. Natl. Acad. Sci. USA 989901-9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josserand, V., I. Texier-Nogues, P. Huber, M.-C. Favrot, and J.-L. Coll. 2007. Non-invasive in vivo optical imaging of the lacZ and luc gene expression in mice. Gene Ther. 141587-1593. [DOI] [PubMed] [Google Scholar]
- 15.Luo, Y., E. Cook, B. C. Fries, and A. Casadevall. 2006. Phagocytic efficacy of macrophage-like cells as a function of cell cycle and Fcγ; receptors (FcγR) and complement receptor (CR)3 expression. Clin. Exp. Immunol. 145380-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier, R. J., A. Olczak, S. Maier, S. Soni, and J. Gunn. 2004. Respiratory hydrogen use by Salmonella enterica serovar Typhimurium is essential for virulence. Infect. Immun. 726294-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merighi, M., C. D. Ellermeier, J. M. Slauch, and J. S. Gunn. 2005. Resolvase-in vivo expression technology analysis of the Salmonella enterica serovar Typhimurium PhoP and PmrA regulons in BALB/c mice. J. Bacteriol. 1877407-7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, J. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 20.Mnatsakanyan, N., K. Bagramyan, and A. Trchounian. 2004. Hydrogenase 3 but not hydrogenase 4 is major in hydrogen gas production by Escherichia coli formate hydrogenlyase at acidic pH and in the presence of external formate. Cell Biochem. Biophys. 41357-365. [DOI] [PubMed] [Google Scholar]
- 21.Olson, J. W., and R. J. Maier. 2002. Molecular hydrogen as an energy source for Helicobacter pylori. Science 2981788-1790. [DOI] [PubMed] [Google Scholar]
- 22.Palazzolo-Ballance, A. M., C. Suquet, and J. K. Hurst. 2007. Pathways for intracellular generation of oxidants and tyrosine nitration by a macrophage cell line. Biochemistry 467536-7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park, K. R., J. C. Giard, J. H. Eom, S. Bearson, and J. W. Foster. 1999. Cyclic AMP receptor protein and TyrR are required for acid pH and anaerobic induction of hyaB and aniC in Salmonella typhimurium. J. Bacteriol. 181689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter-Dahlfors, A., A. M. J. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos, R. L., S. Zhang, R. M. Tsolis, R. A. Kingsley, L. Garry Adams, and A. J. Baumler. 2001. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 31335-1344. [DOI] [PubMed] [Google Scholar]
- 26.Sawers, R. G., D. J. Jamieson, C. F. Higgins, and D. H. Boxer. 1986. Characterization and physiological roles of membrane-bound hydrogenase isoenzymes from Salmonella typhimurium. J. Bacteriol. 168398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vignais, P. M., B. Billoud, and J. Meyer. 2001. Classification and phylogeny of hydrogenases. FEMS Microbiol. Rev. 25455-501. [DOI] [PubMed] [Google Scholar]
- 28.Zbell, A. L., S. L. Benoit, and R. J. Maier. 2007. Differential expression of NiFe uptake-type hydrogenase genes in Salmonella enterica serovar Typhimurium. Microbiology 1533508-3516. [DOI] [PubMed] [Google Scholar]