Abstract
The gram-negative bacterium Vibrio cholerae releases outer membrane vesicles (OMVs) during growth. In this study, we immunized female mice by the intranasal, intragastric, or intraperitoneal route with purified OMVs derived from V. cholerae. Independent of the route of immunization, mice induced specific, high-titer immune responses of similar levels against a variety of antigens present in the OMVs. After the last immunization, the half-maximum total immunoglobulin titer was stable over a 3-month period, indicating that the immune response was long lasting. The induction of specific isotypes, however, was dependent on the immunization route. Immunoglobulin A, for example, was induced to a significant level only by mucosal immunization, with the intranasal route generating the highest titers. We challenged the offspring of immunized female mice with V. cholerae via the oral route in two consecutive periods, approximately 30 and 95 days after the last immunization. Regardless of the route of immunization, the offspring was protected against colonization with V. cholerae in both challenge periods. Our results show that mucosal immunizations via both routes with OMVs derived from V. cholerae induce long-term protective immune responses against this gastrointestinal pathogen. These findings may contribute to the development of “nonliving,” OMV-based vaccines against V. cholerae and other enteric pathogens, using the oral or intranasal route of immunization.
The devastating diarrheal disease cholera is caused by the gram-negative, motile, curved-rod bacterium Vibrio cholerae (56). Over 200 serogroups of V. cholerae have been identified; however, epidemics are caused only by strains of serogroup O1 and the recently emerged serogroup O139 (27). Cholera is transmitted via the fecal-oral route. The infectious dose can vary tremendously, from 103 to 1011 cells, depending on multiple factors, including the specific V. cholerae isolate as well as the diet, age, blood type, and health status of the patient (16, 61, 79). In addition, it has been recently reported that V. cholerae secreted by the host is more infectious than in vitro-cultured V. cholerae (1, 12, 63). The existence of such a hyperinfectious state is supported by a mathematical model based on epidemiological data (41) and could help to explain the explosive nature of cholera outbreaks.
Soon after oral ingestion, V. cholerae reaches the small bowel, its primary site of colonization, and induces virulence factors, such as toxin-coregulated pilus (TCP) and cholera toxin (CT) (17, 42, 87). A complex regulatory cascade including ToxT as well as the inner membrane proteins ToxR/S and TcpP/H controls the expression of virulence factors (54, 78). With the infant mouse and rabbit ileal loop animal models of infection, several factors of V. cholerae have been demonstrated to be important for colonization. Interestingly, a large number of these factors are associated with the outer membrane (OM), including lipopolysaccharide (LPS), OM porins (OMPs), TCP, and flagella (5, 19, 68, 73, 84, 88, 100). Acute secretory diarrhea, which is mediated primarily by CT, causes massive dehydration. If untreated, the fluid loss can result in hypotensive shock and death within hours of the onset of diarrhea (6). Without treatment, the case fatality rate for severe cholera can be as high as 50% (82). Even today, treatment of cholera consists essentially of a simple rehydration therapy, sometimes in combination with antimicrobial agents (49, 82). The treatment is effective; however, appropriate sterile intravenous or oral solutions and medical expertise are not always available during cholera outbreaks. The global morbidity and mortality of cholera are difficult to determine because of gross underreporting, but the incidence may reach as high as 2 million cases globally in a given year (82, 97).
Three oral cholera vaccines have been developed for commercial use (98). The first is produced in Sweden and consists of killed whole-cell V. cholerae O1 and the purified recombinant B subunit of CT (44-46). The second is based on the live attenuated V. cholerae strain CVD-103-HgR. This vaccine was available in parts of Europe, but production was stopped in 2004 after it failed to demonstrate significant protection in a field trial (80, 92). The third, currently produced and used in Vietnam, is a variant of the first without the recombinant B subunit of CT (89). New live oral cholera vaccines are in testing, including the Peru-15 and the Cuban 638 strains (31, 74, 75). In addition, similar approaches are currently under way to develop live oral vaccines that protect against O139, including CVD112 and Bengal-15 (21, 86). However, licensing and large-scale use of live vaccines are uncertain because of the potential risk of reversion to a virulent state and dissemination of the vaccine in the population. In addition, efforts are being taken to develop cholera subunit vaccines and conjugate vaccines, focusing so far on V. cholerae antigens like LPS, TCP, and CT (3, 15, 35, 58). Despite their benefits, all commercially available vaccines have field use and logistical (“fieldability”) disadvantages. They require the use of a cold chain with large storage capacity, limited long-term protection, and complicated formulation as well as trained health care staff. Currently, no vaccine is licensed for children under the age of 2 years and none of the commercially available vaccines protect against cholera caused by O139 (81).
Therefore, there is need for the development of novel cholera vaccines that can meet the needs of fieldability as well as long-term protection. Recent studies revealed induction of a stable immune response and/or successful protection in mice after immunization with OM vesicles (OMVs) derived from Neisseria meningitidis, Porphyromonas gingivalis, Salmonella enterica serovar Typhimurium, or Helicobacter pylori (2, 22, 38, 51, 53, 83). The existence of OMVs, which are released from the envelopes of growing cells, has now been demonstrated to occur in a variety of gram-negative bacterial species, such as Escherichia coli (30, 43), Salmonella spp. (91, 93), Shigella spp. (26, 47), Neisseria spp. (24, 25), Bacteroides spp., H. pylori (28), and Pseudomonas aeruginosa (48). Most of the best-characterized OMVs are produced by pathogens, since they are implicated to play a role in virulence. For example, OMVs function in transport of virulence factors, adherence to and entry into host cells, or modulation of the host response (7, 59). In the case of V. cholerae, however, only the existence of OMVs has been described so far (18, 57). OMVs largely reflect the composition of the OM and periplasm of their donor gram-negative bacteria. Some proteins have been found to be enriched, whereas others are excluded in the OMVs compared to the OM, suggesting some specific sorting pathways (50, 72, 93, 96). Thus, the vesicles provide high concentrations of many of the most important surface antigens for immunization. The LPS, OMPs, and parts of flagella, fimbriae, or pili are known not only to be highly immunogenic and to have qualities as adjuvants but also to provide in most cases a strain-specific immune response. Hence, we investigated the use of OMVs derived from V. cholerae as a potential vaccine. In the following study, we demonstrate the immune response due to immunization with V. cholerae OMVs via intraperitoneal (i.p.), intragastric (i.g.), and intranasal (i.n.) routes by measuring antibody titers and by using a previously described protection model (34) whereby the offspring of immunized female mice are challenged orally.
MATERIALS AND METHODS
Strain constructions and growth conditions.
V. cholerae AC53, a spontaneous, streptomycin (Sm)-resistant mutant of V. cholerae E7946 (O1 El Tor Inaba, a clinical isolate from Bahrain, ATCC 55056, with hapR+), was used as a wild-type strain in all experiments (65, 90). For genetic manipulations, E. coli strain SM10λpir was used (66). Unless stated otherwise, bacteria were grown in Luria-Bertani broth (LB) at 37°C with aeration. Supplements were used at the following final concentrations: for Sm, 100 μg/ml; and for ampicillin (Ap), 50 μg/ml in combination with other antibiotics or 100 μg/ml alone. Deletion of ompU in AC53 was generated as described previously (85), using the suicide plasmid pΔompU (70). Briefly, the suicide plasmid was transferred from SM10λpir into V. cholerae by conjugation, which was achieved by cross-streaking colonies of recipient and donor cells on an LB plate and incubating them at 37°C for 6 h. Transfer into V. cholerae and integration on the chromosome were selected by isolating Smr Apr colonies. After one passage in LB in the absence of antibiotics, sucrose selection was used to obtain Aps colonies in which the plasmid had been excised. The correct chromosomal deletion was confirmed by PCR using an oligonucleotide pair consisting of ompU-F (5′-CAGCATGGTATTCCGCATTC-3′) and ompU-R (5′-GATCAGGTTTGTCGGACTCTTG-3′).
Preparation of OMVs.
OMVs were isolated from AC53 late-exponential-phase cultures by using a procedure adapted from the method of Balsalobre et al. (4). Briefly, 10 ml of an AC53 LB stationary-phase culture was used to inoculate 1 liter of LB and grown to late exponential phase for 8 h. Bacterial cells were removed by centrifugation (4,500 × g, 15 min, 4°C), and the supernatant was consecutively filtered through 0.45-μm- and 0.22-μm-pore-size filters. To confirm the absence of viable bacteria, 1 ml of the filtrate was plated on an LB agar plate, incubated overnight at 37°C, and examined for colonies. In all preparations, no colonies were observed. In order to prevent possible protein degradation, protease inhibitor (complete EDTA-free protease inhibitor cocktail, one tablet per liter of filtrate; Roche) was added to the filtrate and stored at 4°C. Within the same week, OMVs were subsequently purified from the filtrate by ultracentrifugation (140,000 × g, 4 h, 4°C) using a Beckmann SW32Ti or 50.2Ti rotor, washed once with phosphate-buffered saline (PBS), and finally resuspended in 625 μl of PBS. The protein concentration was determined by using a modified Lowry protein assay kit (Pierce) and adjusted to 2.5 μg/μl using PBS. Purified OMVs were stored at −80°C.
Preparation of OM proteins.
In order to isolate the proteins of the OM, 50 ml of the respective strain was grown to late exponential phase as described above. Cells were harvested (4,500 × g, 15 min, 4°C), washed once in 10 mM HEPES, pH 7.5, and resuspended in 10 mM HEPES, pH 7.5, with one tablet per liter of protease inhibitor. Cells were disrupted using a Mini-BeadBeater (Biospec) and 0.1-mm silica beads (Biospec). Unbroken cells were removed by centrifugation (13,000 × g, 1 min). The supernatant containing the OM proteins was transferred into a new tube and centrifuged again (13,000 × g, 30 min, 4°C). The pellet was resuspended in 0.8 μl 10 mM HEPES, pH 7.5, plus 1% sarcosyl and incubated for 30 min. After centrifugation (13,000 × g, 30 min, 4°C), the pellet was washed once with 1 ml of 10 mM HEPES, pH 7.5, and resuspended in 50 μl of 10 mM HEPES, pH 7.5. The protein concentration was determined as described above and adjusted to 2.5 μg/μl by using 10 mM HEPES, pH 7.5. Purified OM proteins were stored at −80°C.
Animals.
BALB/c mice (Charles River Laboratories or Jackson Laboratories) were used in all experiments. Mice were housed with food and water ad libitum and monitored under the care of full-time staff and in accordance with the rules of the Department of Laboratory Animal Medicine at Tufts Medical Center. All animals were acclimated for at least 1 week before use.
Immunization protocol.
Seven-week-old female mice were immunized at days 0, 14, and 28 with OMVs via the i.p., i.g., or i.n. route, using the following concentrations: for i.n. treatment, 25 μg in 10 μl PBS (5 μl per naris) for all immunizations; for i.g. treatment, 25 μg in 100 μl PBS for all immunizations; and for i.p. treatment, 1 μg in 50 μl PBS for the initial immunization at day 0 and 0.25 μg in 50 μl PBS at days 14 and 28. These immunization doses were based on recently published studies of immunization with OMVs derived from various gram-negative bacterial species (22, 29, 37, 51, 83). In these studies, the OMV doses ranged from 0.1 to 10 μg for i.p. and 10 to 250 μg for i.n. immunizations. Mice were anesthetized by inhalation of 2.5% isoflurane gas prior to all immunizations. Nonvaccinated control mice were housed in parallel with vaccinated mice for the duration of the experiment. A total of two independent rounds of immunizations, with two or three or with four or five animals per group, were conducted. At the end, each group of vaccinated mice and nonvaccinated mice had a total of at least six mice.
Collection of samples.
Blood was collected from the lateral tail vein at days 0, 14, 28, and 38, as well as 3 to 9 days after the first challenge, and by cardiac puncture 3 to 9 days after the second challenge. The collected blood was allowed to clot at room temperature (RT) for 30 min, after which serum was isolated by removing the blood clot with a sterile toothpick, followed by centrifugation (1,000 × g, 15 min). The supernatant was removed and diluted threefold in PBS. After addition of sodium azide to give a final concentration of 0.05%, the serum was stored at −80°C.
Determining the infectious dose of AC53.
In order to determine the infectivity of AC53 in neonatal mice from naïve dams, 10-week-old pregnant, naïve mice were monitored for birth. For all infections in this study, including this one using neonatal mice from naïve dams, 5- to 6-day-old mice were separated from their dams for 1 h, anesthetized by inhalation of 2.5% isoflurane gas, and then inoculated i.g. by oral gavage with 50 μl of the appropriate serial dilution of the inoculum. To prepare the inoculum, ∼60 colonies of AC53, grown overnight on an LB Sm plate, were resuspended in LB and adjusted to an optical density at 600 nm of 1, which is ∼2 × 109 CFU/ml. Dilutions of this suspension were prepared as inocula at the following ratios for infection: 1:10, 1:50, 1:100, 1:1,000, 1:10,000, and 1:100,000. The exact titer for the dilution series was determined by plating on LB-Sm plates in triplicate. The inoculated neonatal mice were kept either with or away from their dams for 24 h. At 24 h postinoculation, the mice were euthanized by humane measures consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medicine Association. The small bowel from each neonatal mouse was removed by dissection and mechanically homogenized in 1 ml LB with 20% glycerol. Appropriate dilutions of the homogenate, starting at 100 μl, were plated on LB-Sm plates in duplicate. Thus, our limit of detection was 10 CFU per small bowel. The dose at which 50% of mice were colonized (ID50) was determined graphically from the plotted data.
Challenge.
Control and vaccinated female mice were mated with age-matched males at a 2:1 ratio. After at least 14 days, the mice were separated, and the females were monitored for birth. Five- to six-day-old neonatal mice from control and vaccinated dams were challenged at two doses, which were ∼7- and 70-fold above the ID50 in the first challenge period, from day 55 to 67, and ∼70- and 700-fold above the ID50 in the second challenge period, from day 117 to 130. Neonatal mice from each litter were split equally for infection with the different doses, with the higher dose being favored in each case of uneven numbers of mice. The first-challenge experiments were conducted on neonatal mice obtained from the breeding period of days 41 to 55, and the second-challenge experiments were conducted on neonatal mice obtained from the breeding period of days 90 to 105 or 95 to 120. After i.g. inoculation, the neonatal mice were placed back in the presence of their respective dams. After 24 h, the neonatal mice were euthanized, and the small bowel of each neonatal mouse was harvested and analyzed as described above.
Quantitation of Igs.
Levels of immunoglobulin A (IgA), IgG1, IgG2, and IgM isotype antibodies to OMVs were determined by an enzyme-linked immunosorbent assay (ELISA) using 96-well ELISA microplates (BD Falcon). Plates were coated by incubation with OMVs (5 μg/ml in PBS) at 4°C for 2 days. To generate standard curves for each isotype, plates were coated in triplicate with twofold dilutions of the appropriate purified mouse Ig isotype standard (IgA, IgG1, IgG2a, or IgM; BD Biosciences), starting at 1 μg/ml, in PBS. After four washes with 0.05% Tween in PBS (PBS-T), nonspecific binding sites were blocked with 10% heat-inactivated fetal calf serum in PBS (PBS-F) for 1 h at RT. Starting at 1:400 in PBS-F, appropriate fivefold dilutions of the test samples were applied on the OMV-coated wells in triplicate, whereas PBS-F was used for the wells coated with the isotype standards. The plates were incubated for 1 h at RT and washed four times with PBS-T. The plates were then incubated for 1 h at RT with the appropriate alkaline phosphatase-conjugated, affinity-purified goat antibodies against mouse IgA (α-chain specific; Southern Biotech), IgG1 (γ1-chain specific; Southern Biotech), IgG2a (γ2a-chain specific; Southern Biotech), or IgM (μ-chain specific; Southern Biotech). After four washes with PBS-T, the plates were developed using the BluePhos Microwell phosphatase substrate system and stop solution (KPL) according to the manufacturer's instructions. Optical densities were read at 620 nm with a Synergy HT plate reader (Biotek Inc.). Titers were calculated using values from the appropriate dilutions of test samples, and a log-log regression was calculated from at least four dilutions of the isotype standards.
Half-maximum total Ig titers (IgA, IgG, and IgM) were determined by ELISA as described above, with the exceptions that alkaline phosphatase-conjugated, affinity-purified goat antibodies against mouse (IgM plus IgG plus IgA, heavy plus light chains; Southern Biotech) served as a secondary antibody, no Ig standard was used, and at least four fivefold dilutions, starting at 1:4 and increasing to 1:400, were used to calculate the titers. Half-maximum titers were calculated using the solution of the sigmoidal line of the plot of the log of the reciprocal dilutions of mouse sera and the resulting absorbances to determine the reciprocal that gave half of the maximum optical density.
SDS-PAGE.
The standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) procedure (60) in combination with 4 to 15% gradient Ready Gels (Tris-HCl; Bio-Rad) was used. Gels were stained with the Imperial protein stain (Pierce).
Immunoblot analysis.
Five micrograms of purified OMVs was separated by SDS-PAGE and transferred onto a nitrocellulose membrane (Invitrogen). The membrane was blocked by incubation in 10% skim milk in TBS (10 mM Tris-Cl, pH 7.5, 150 mM NaCl) for 2 h at RT. The membrane was washed twice in TBS-TT (20 mM Tris-Cl, pH 7.5, 500 mM NaCl, 0.05% Tween 20, 0.2% Triton X-100) and once in TBS for 10 min each wash. For use as the primary antibody, the mouse serum was diluted 1:500 in 10% skim milk in TBS. The diluted serum was added to a membrane and incubated with gentle rocking at 4°C overnight. The membrane was then washed as described above. After being washed, the membrane was incubated for 1 h in the secondary antibody solution by using horseradish peroxidase-conjugated anti-mouse IgG from sheep (GE Healthcare UK Limited) in 10% skim milk in TBS. The membrane was washed four times in TBS-TT for 10 min each wash. Chemiluminescence detection was performed by using the ECL plus Western blotting detection system (GE Healthcare UK Limited) and exposure to X-ray film (Kodak).
Statistical analysis.
Data were analyzed using the Mann-Whitney U test or a Kruskal-Wallis test and post hoc Dunn multiple comparisons. Differences were considered significant at P values of <0.05.
RESULTS
V. cholerae produces OMVs.
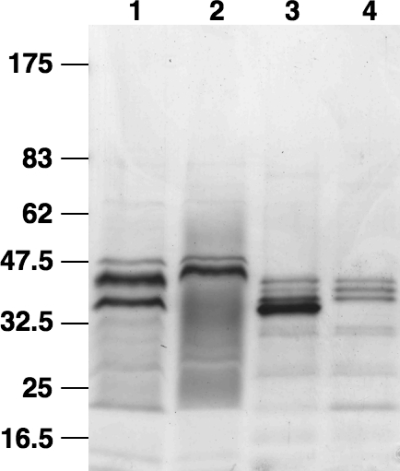
To analyze whether the V. cholerae serogroup O1 strain E7946 produces OMVs during growth in LB broth, we developed a purification method using filtration of culture supernatant and ultracentrifugation based on OMV isolation protocols previously described for E. coli (4, 94). The purified material, which was tested for the absence of culturable bacteria, was examined by negative-stain transmission electron microscopy and was found to contain numerous spherical vesicles, ranging in diameter from 50 to 300 nm, with about 80% of them being in the smaller size range (data not shown). A comparison at the protein level of the purified vesicles and the OM isolated from the wild type (Fig. 1, lanes 1 and 3) revealed similar patterns. Some protein bands are present or absent in the OMVs compared to the OM samples, but enrichment or exclusion of specific proteins in OMVs has also been reported for other organisms (50, 72, 93, 96). Furthermore, a deletion mutant for the most abundant OMP, OmpU, removes the corresponding protein band of 37 kDa in both the OM and the OMV samples (lanes 2 and 4), indicating that the proteins in the OMVs reflect the protein profile of the OM.
FIG. 1.
V. cholerae secretes OMVs. Shown are Coomassie blue-stained polyacrylamide gradient gels (4 to 15%) loaded with 5 μg protein from each sample: OM of wild-type AC53 (lane 1), OM of the ompU mutant (lane 2), OMVs of wild-type AC53 (lane 3), and OMVs of the ompU mutant (lane 4). Lines to the left indicate the molecular masses of the protein standards in kDa.
Immunization with OMVs induces strong antibody responses.
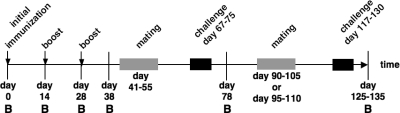
We immunized mice by i.n., i.g., and i.p. administration with OMVs as described in Materials and Methods. An overview of the experimental design is shown in Fig. 2. A nonvaccinated control group was housed in parallel for the duration of the experiment. Antibody titers in serum were monitored at six time points before, during, and after the immunization period. We challenged the respective offspring of the immunized female adult mice with V. cholerae via the oral route. Thus, we were able to monitor the immune response both biochemically and functionally up to 3 months after the last immunization. Two consecutive breeding cycles allowed two challenge periods at ∼30 and 90 days after the last immunization.
FIG. 2.
Schematic timetable of the immunization and challenge experiment. The timeline is given by a horizontal arrow from left to the right, starting with the initial immunization on day 0. Each vertical bar marks the day of a bleed, indicated by B. Mice were boosted on days 14 and 28. Immunizations are highlighted by vertical arrows. The breeding periods are indicated by gray rectangles, and the challenge periods for the infant mice are indicated by black rectangles.
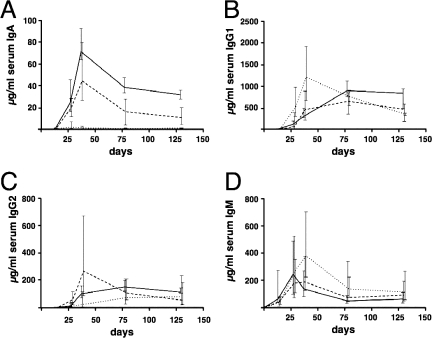
The temporal IgA, IgG1, IgG2, and IgM responses are shown in Fig. 3. The overall antibody titers for all three routes of immunization increased during the vaccination period, with a peak at day 38 or day 78. In general, this peak of the titers was followed by slight declines for IgG1 and IgG2 and moderate declines for IgA and IgM and then a leveling off. Except for the IgG1 titers in the i.p. immunized mice, the changes in antibody titers between day 78 and the end point were not significantly different (P < 0.05 and P > 0.3, respectively; Mann-Whitney U test). At day 0, the titers of isotype-specific antibodies against OMVs were below the limit of detection in all mice. Antibody titers in sera were also monitored for the nonvaccinated control mice, but the titers stayed below the limit of detection during the entire experiment for all isotypes tested (data not shown). IgM levels were already above the detection limit after the initial immunization at day 14 in all three immunization routes. For all other isotypes, the first point of detection was after one boost at day 28. The i.p. immunized mice exhibited the highest levels for IgG1, IgG2, and IgM on day 38, whereas IgA stayed almost undetectable in this group. Throughout the entire experiment, the highest IgA levels were observed in the i.n. immunized mice. Compared to the i.p. immunized group, mice immunized via the i.n. or i.g. route showed delayed but robust responses for IgG1 and IgG2, with a peak at day 78. The antibody responses in the i.n. immunized mice lasted longer than those in the other groups, because the end point median titers for both IgG isotypes were the highest in the i.n. group. The end point IgG2 and IgM titers were not significantly different for the three immunization routes, whereas the i.n. immunized group exhibited significantly higher IgA and IgG1 titers than mice immunized via the other routes (P < 0.05; Kruskal-Wallis test and post hoc Dunn multiple comparisons).
FIG. 3.
Ig titers in sera of mice immunized with OMVs. Shown are the median titers over time of IgA (A), IgG1 (B), IgG2 (C), and IgM (D) antibodies to OMVs in sera from i.n. (solid line), i.g. (dashed line), and i.p. (dotted line) immunized mice (n ≥ 6 for each group). The error bars indicate the interquartile range of each data set for each time point.
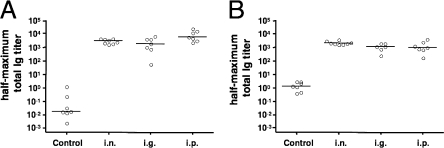
We determined the half-maximum total Ig titers on day 38 (Fig. 4A) and at the end point (Fig. 4B). All vaccinated mice had comparable half-maximum total Ig titers at both time points, which were significantly higher than those for the nonvaccinated control mice (P < 0.05; Kruskal-Wallis test and post hoc Dunn multiple comparisons). A comparison of the half-maximum total Ig titers for the immunized groups at both time points revealed that only the decrease in the i.p. group was significantly different (P < 0.05; Mann-Whitney U test).
FIG. 4.
Half-maximum total Ig titers in sera of mice immunized with OMVs derived from V. cholerae and the control group. Results are shown for sera collected at day 38 (A) and days 125 to 135 (B) from mice immunized via the i.n., i.g., and i.p. routes and the nonvaccinated control group. Each circle represents the half-maximum total Ig titer of one mouse. The horizontal bars indicate the median of each data set.
Specificity of the antibody response.
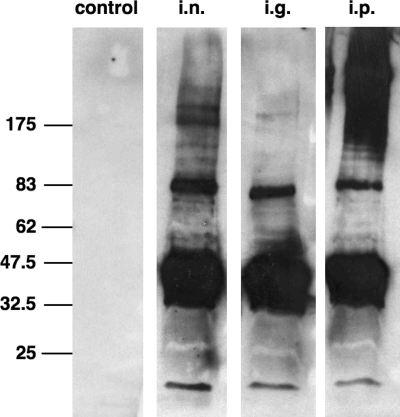
Immunoblots using OMVs as the antigen were used to test the specificity of the antibody response. Figure 5 shows representative immunoblots utilizing sera from one mouse of each immunization group and control group collected on day 78. No bands were detected on the immunoblot using the serum from a nonvaccinated control mouse, whereas multiple bands in the OMV protein profile were detected using the sera from vaccinated mice, demonstrating that the OMVs contained numerous proteins that can serve as antigens. The comparable intensities of the detected bands confirm that vaccinated mice from all routes of immunization have induced comparable levels of serum IgG response against OMV proteins at this time point. Furthermore, the comparable banding patterns indicated that, at least for the IgG isotype, there were no qualitative differences in antigen target between the routes of immunization. The most-reactive bands are located between 32.5 and 47.5 kDa, which correlates with the area of the most abundant proteins in the OMVs (Fig. 1). We also examined sera from convalescent cholera patients and healthy European volunteer controls in immunoblots using OMVs as the antigen. We detected multiple bands recognized by sera from the convalescent patients that were absent in immunoblots incubated with the control sera (data not shown).
FIG. 5.
Immunoblot analysis of IgG reactivity in sera from immunized mice. Shown are representative immunoblots loaded with OMVs and incubated with sera collected at day 78 from mice immunized via the i.n., i.g., and i.p. routes and the nonvaccinated control group. Lines to the left indicate the molecular masses of the protein standards in kDa.
Protection against colonization of V. cholerae.
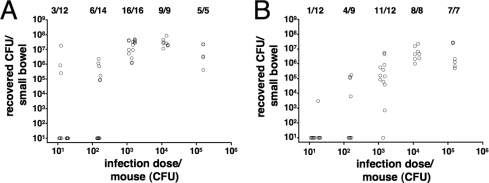
In order to determine whether the immunization with OMVs was protective against colonization with V. cholerae, we used a modified version of the infant mouse model wherein the offspring of immunized dams were challenged and the level of protection was measured by the degree of colonization in the small bowel. To allow for a continuous transfer of antibodies from the immunized dams to the offspring, the infected neonatal mice were given back to their respective dams for the challenge period of 24 h. Normally, in this model the infected neonatal mice are kept separate from their mothers, during the infection period to minimize variation in colonization due to the effects of milk. In order to investigate if the placement of the infant mice back with their mothers affected colonization, we infected neonatal mice from naïve dams with different doses of V. cholerae in an ID50 experiment (12, 13). The results of these colonization experiments are shown in Fig. 6. When the neonatal mice were given back to their dams, a maximal colonization level of around 107 CFU per small bowel within 24 h was achieved by infection with ∼1,500 or more CFU (Fig. 6A). In contrast, when mice were kept separated from their mothers, a maximal colonization level of around 106 CFU was achieved with ∼15,000 or more CFU (Fig. 6B). Thus, the overall colonization level was higher when neonatal mice were given back to their dams, although this increase was significantly different only for the group receiving a dose of ∼1,500 CFU (P < 0.05; Mann-Whitney U test). The ID50s were in both cases determined to be ∼200 CFU (data not shown). Thus, the milk from naïve BALB/c mice had no negative effect on colonization of V. cholerae and, if anything, appeared to enhance colonization at low inoculum doses.
FIG. 6.
Suckling from naïve dams does not interfere with colonization of V. cholerae in neonatal mice. Shown are the numbers of recovered CFU per small bowel on the y axis, correlated with the respective inoculum doses on the x axis. Each circle represents the number of CFU from one neonatal mouse. The infected neonatal mice were either given back to their naïve dams for the 24-h infection period (A) or kept separate for this period (B). Due to the nature of the experiment (explained in Materials and Methods), the exact numbers of CFU for the inoculum dose varied from day to day, ranging from 11 to 18, 137 to 150, 1,124 to 1,673, 11,235 to 15,000, and 150,000 to 156,000 CFU for the individual 10-fold dilutions of the inoculation mix. When no bacteria were recovered, the numbers of CFU were set to the limit of detection of 10 CFU/small bowel. The numbers of infected mice are given above the respective inoculum doses as follows: number of mice with detectable colonization/total number of challenged mice.
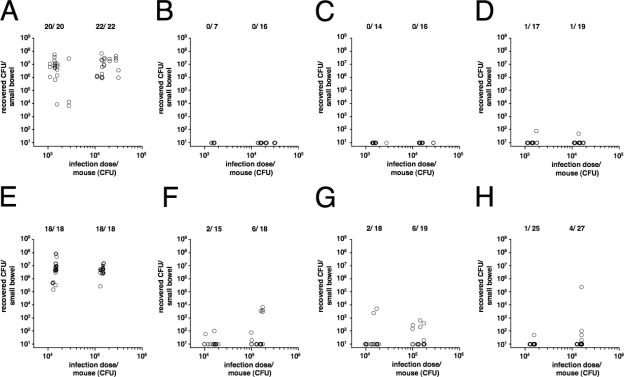
After it was determined that a stable colonization can be achieved with a dose of ∼1,500 or more CFU, neonatal mice from control and vaccinated dams were challenged with V. cholerae by using ∼1,500 or 15,000 CFU per mouse, which is about 7- or 70-fold above the ID50, respectively. The results for the first challenge period, from day 67 to 75, are presented in Fig. 7A to D. The neonatal mice for the first challenge period were born ∼27 days and challenged ∼33 days after the third and final immunization of the dams. Panel A shows the colonization results for neonatal mice from the control dams. With both doses, the median colonization level was around 7 × 106 CFU per small bowel and is therefore comparable to the results obtained with neonatal mice from naïve dams by using the same dose (Fig. 6A). In contrast, neonatal mice from i.g. and i.n. immunized dams exhibited full protection and no CFU were recovered (Fig. 7B and C). For the neonatal mice from i.p. immunized dams, colonization with V. cholerae was detectable in 2 out of 36 neonatal mice, but at least 10,000-fold-lower numbers of CFU were recovered than in the infection of naïve animals (Fig. 7D and 6). Therefore, neonatal mice from immunized dams, independent of the route of immunization, were protected from infection.
FIG. 7.
Neonatal mice from immunized dams show short- and long-term protection against colonization with V. cholerae. The numbers of recovered CFU per small bowel are shown on the y axis, versus the respective inoculum doses on the x axis. Results from the first challenge period, from day 67 to 75, are shown for neonatal mice born to the nonvaccinated control (A), i.n. immunized (B), i.g. immunized (C), or i.p. immunized (D) mice. Results from the second challenge period, from day 117 to 130, are shown for neonatal mice born to the nonvaccinated control (E), i.n. immunized (F), i.g. immunized (G), or i.p. immunized (H) mice. Each circle represents the number of CFU from one neonatal mouse. Due to the nature of the experiment (explained in Materials and Methods), the exact numbers of CFU for the inoculum doses varied from day to day, ranging from 1,100 to 2,740, 11,000 to 31,200, and 128,300 to 171,600 for the individual 10-fold dilutions of the inoculation mix. When no bacteria were recovered, the numbers of CFU were set to the limit of detection of 10 CFU/small bowel. The numbers of infected mice are given above the respective inoculum doses as follows: number of mice with detectable colonization/total number of challenged mice.
To measure long-term protection, dams were mated again after the first challenge period and neonatal mice from control and immunized dams from this round were challenged with even higher doses of V. cholerae, ∼70 and 700-fold above the ID50. These second challenge experiments were conducted on neonatal mice born ∼81 days and challenged ∼87 days after the third and final immunization of the dams. The results are shown in Fig. 7E to H. Similar to the results obtained from the experiments with naïve mice and the first challenge round, the neonatal mice from control dams were colonized with ∼107 CFU, whereas neonatal mice from i.n., i.g., and i.p. immunized dams showed in most of the cases no colonization. When the mice were challenged with a dose of ∼1,500 CFU, we found that 5 to 13% of the mice were colonized, which increased to 13 to 30% when a dose of ∼15,000 CFU was used. This result was independent of the immunization route, since colonized mice were found for each infectious dose in each group. However, it should be emphasized that the colonization level in mice from immunized dams was on average at least 1,000-fold lower than that in mice from naïve or control dams. In summary, neonatal mice from immunized dams demonstrated robust long-term protection independent of the immunization route.
DISCUSSION
The major goal of this study was to analyze the potential use of OMVs derived from V. cholerae as a new vaccine strategy. According to a previous report, V. cholerae produces OMVs when grown exponentially; however, purification of OMVs was successful only in the presence of glutaraldehyde (57). Adapting protocols established for OMV purification in other gram-negative bacterial species, we were able to isolate milligram quantities of vesicles from supernatants of V. cholerae cultures. Comparison of the OM and OMV protein profiles of the wild type and an ompU mutant, which lacks one of the most abundant OM proteins, showed that the isolated OMVs reflect the OM composition. Thus, we have established a simple protocol for isolation and purification of OMVs derived from V. cholerae that is amenable to scaling up.
We compared the immune responses of mice immunized via different routes with OMVs derived from V. cholerae and analyzed the protection resulting from this acquired immunity. Given that V. cholerae grows extracellularly in the small intestine, we focused on mucosal immunization via the i.g. and i.n. routes. The i.p. route of immunization, which is frequently used to obtain antibodies against specific antigens, was used as a positive control in this study. Based on recently published immunization protocols with OMVs derived from various gram-negative bacterial species (22, 29, 37, 51, 83), we decided to do an initial immunization, with two boosters at days 14 and 28. Immunization via all routes elicited specific systemic antibody responses. The half-maximum total Ig titers of immunized mice were at least 1,000-fold higher than those in the control group, indicating the substantial immunogenic capacity of OMVs even without the aid of an immunoadjuvant. Interestingly, the overall immune responses in the three differently immunized groups were very similar, as demonstrated by the comparable half-maximum total Ig titers. The temporal stability of the immune response is shown by the minimal declines in the half-maximum total Ig titers over a 3-month period without further immunization.
Although the titers for specific isotypes in the control group stayed below the limit of detection for the entire experiment, increases of the half-maximum total Ig titers of the control group were unexpectedly observed. However, this could be explained by the design of our challenge experiment, wherein the infected offspring were given back to the dams. Hence, during the 24-h infection period, the dams from the control group might have had contact with V. cholerae shed from the neonatal mice, and this could have triggered a slight immune response.
The specific isotype immune responses were different in the mice immunized via different routes. For example, i.p. immunization resulted in high IgG1 and IgG2 titers but failed to induce an IgA response. In contrast, i.n. immunized mice exhibited very high IgA titers and, additionally, high levels of IgG1 and IgG2. This is consistent with other OMV vaccination studies reporting high levels of IgG after i.p. or intravenous immunization and high levels of IgA after i.n. immunization (36, 83). In this study, we analyzed Ig titers in sera only. However, previous studies have demonstrated induction of secreted Ig, especially by i.n. immunization with OMVs (38, 51, 83). The importance of the specific isotypes for protection against cholera is unknown. However, high levels of mucosally secreted Igs in addition to systemic immunity might be advantageous in humans for prevention of gastrointestinal colonization by V. cholerae (10). It has been demonstrated that intestinal secretion of IgA protects against V. cholerae infection (99). It has also been shown that IgA in the milk of subcutaneously immunized dams contributes to passive protection of suckling mice from a lethal challenge of V. cholerae, although antibody titers and mucosal vaccination routes were not investigated (34). In the present study, i.n. immunized mice had the highest median titers in three of the four isotypes and had the highest half-maximum total Ig titers at the end of the experiment, ∼100 days after the last immunization. Thus, of the three routes tested, i.n. immunization induced the most stable, long-lasting immune response. The reason for this difference is unclear. It could be hypothesized that antigens delivered i.p. or i.g. are either rapidly diluted, degraded (11, 67), or excreted. Thus, antigens delivered i.p. or i.g. may result only in a stimulus of limited duration. In contrast, antigens delivered i.n. could stay immunogenic and remain on the nasopharyngeal mucosal surface for a longer period of time.
Challenge of immunized mice is necessary for demonstration of protection since Ig levels do not always correlate with reduced colonization, as recently demonstrated for H. pylori (52). The infant mouse model simulates most aspects of the human infection and is the most frequently used animal model for V. cholerae colonization studies (55). Colonization experiments using adult mice are complicated since the normal gastrointestinal flora must be reduced to allow for V. cholerae colonization. This either requires the use of germfree mice (14) or is achieved by oral treatment with antibiotics (64, 69). Thus, we decided to use the infant mouse model by mating vaccinated female mice and challenging their offspring as described by Guentzel and Berry (33). In both challenge rounds, the majority of neonatal mice from immunized dams were completely protected from V. cholerae. A minority of neonatal mice from immunized dams was colonized, but the level of colonization was significantly lower than that in mice from naïve or nonvaccinated control dams. Thus, all routes of immunization resulted in similar levels of short- and long-term protection against V. cholerae infection. Even at the highest inoculum dose, less than half of the neonatal mice in all immunized groups were colonized. Thus, we were not able to determine the ID50s for the neonatal mice from immunized dams. However, we can state that the immunization resulted in an increase in ID50 of at least 700-fold. The protection is clearly due to the induced immune response in the dams, but at this point the mechanism of protection can only be speculated upon. Immunity could be acquired prenatally via placental transfer of Igs or postnatally through suckling. Protection of breast-fed human infants against cholera has been shown in multiple studies (20, 77, 101). Furthermore, the level of this protection could be correlated with the level of the IgA antibodies in the mother's milk, and the majority of those antibodies are directed against V. cholerae surface structures and CT (32, 40). Hence, it is predicted to be advantageous for a cholera vaccine to induce an IgA response against OM structures, as exhibited by the i.n. and i.g. immunizations in this study.
Immunoblot analysis of the sera of immunized mice revealed diverse immune responses against many antigens present in the OMVs. No gross difference in specificity of immune response between the different routes of immunization was detected. However, some antigens are more immunogenic than others, as indicated by a massive response directed against proteins between 35 to 45 kDa. Similarly, analysis of convalescent patient sera revealed antibodies against multiple OMV antigens. Thus, antigens provided by the OMVs and/or the bacterial cells themselves are recognized by the human immune system during infection and are expressed by V. cholerae in vivo, as has been demonstrated previously (39, 62, 76). The human immune response against OMV antigens further supports the immunogenic potential of OMVs as a vaccine.
A cholera vaccine that is affordable for developing countries, does not require a cold chain, and can be easily administered is currently lacking. The available oral cholera vaccines require a buffer solution, and the hypothesized poor stability of antigens in killed vaccines delivered via the oral route may be a disadvantage (9, 67). In addition, oral vaccinations have been associated with complications like mild diarrhea and more-severe complications, such as intussusception, observed for example for a rotavirus vaccine (71, 95). Besides live attenuated oral vaccines, research currently focuses on subunit vaccines or conjugated vaccines. Expression and purification of single antigens and development of conjugated vaccines are time-consuming and costly. Most likely, the production of such vaccines will be very expensive. Besides a low production cost, administration of a vaccine intended for use in developing countries should be simple. OMVs are naturally released by gram-negative bacteria, can be purified relatively easily, and are ready for use without further treatment. OMVs naturally contain important features for a good vaccine, since they represent the composition of the OM and therefore contain some of the most immunogenic and important antigens. As indicated by a variety of clinical studies with meningococcal OMVs, they can be used safely and with high efficacy (8, 23, 102). As demonstrated by the present study, OMVs can be easily isolated from the supernatant of a V. cholerae culture. Our protocol does not demand any specialized equipment, with the exception of an ultracentrifuge. It can be speculated that purified OM proteins or whole-cell lysates might induce similar immune responses. However, both would require the production of cell lysates and, in the case of purified OM proteins, multiple extraction steps. The stability of such purified proteins or whole-cell lysates at ambient temperatures is questionable, as demonstrated by the requirement of a cold chain and buffer solutions for the killed whole-cell vaccine (44-46). According to the WHO, an ideal cholera vaccine should not require a cold chain or accessory buffer solutions (98). Qualitative analysis by SDS-PAGE indicates that V. cholerae OMVs purified by our method are very stable, since no significant protein degradation was observed after incubation at 37°C for a month (data not shown). OMVs were administered in all immunizations without any accessory buffer solutions. Thus, a cold chain and accessory buffer solutions are unlikely to be required for shipping and administration of a V. cholerae OMV vaccine.
To our knowledge, this is the first study characterizing OMVs as a candidate vaccine against cholera and also the first report to demonstrate protection against a gastrointestinal pathogen by i.n. immunization with OMVs. As such, this opens a new route of administration for vaccines against cholera and other gastrointestinal diseases. Future studies will be required to elucidate the mechanism(s) of protection against V. cholerae in this mouse immunization and challenge model.
Acknowledgments
This research was supported by NIH grant AI55058 to A.C. and the Center for Gastroenterology Research on Absorptive and Secretory Processes, New England Medical Center (P30 DK34928). A.C. is an investigator of the Howard Hughes Medical Institute.
We thank F. Qadri and S. B. Calderwood for kindly providing access to human samples from Bangladesh. We are grateful to M. Bergman and J. LeMieux for helpful assistance with the ELISAs and electron microscopy, respectively, as well as A. L. Bishop for helpful suggestions and discussions.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 4 August 2008.
REFERENCES
- 1.Alam, A., R. C. Larocque, J. B. Harris, C. Vanderspurt, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2005. Hyperinfectivity of human-passaged Vibrio cholerae can be modeled by growth in the infant mouse. Infect. Immun. 736674-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alaniz, R. C., B. L. Deatherage, J. C. Lara, and B. T. Cookson. 2007. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 1797692-7701. [DOI] [PubMed] [Google Scholar]
- 3.Asaduzzaman, M., E. T. Ryan, M. John, L. Hang, A. I. Khan, A. S. Faruque, R. K. Taylor, S. B. Calderwood, and F. Qadri. 2004. The major subunit of the toxin-coregulated pilus TcpA induces mucosal and systemic immunoglobulin A immune responses in patients with cholera caused by Vibrio cholerae O1 and O139. Infect. Immun. 724448-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balsalobre, C., J. M. Silvan, S. Berglund, Y. Mizunoe, B. E. Uhlin, and S. N. Wai. 2006. Release of the type I secreted alpha-haemolysin via outer membrane vesicles from Escherichia coli. Mol. Microbiol. 5999-112. [DOI] [PubMed] [Google Scholar]
- 5.Baselski, V. S., R. A. Medina, and C. D. Parker. 1979. In vivo and in vitro characterization of virulence-deficient mutants of Vibrio cholerae. Infect. Immun. 24111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennish, M. L. 1994. Cholera: pathophysiology, clinical features, and treatment, p. 229-255. In K. I. Wachsmuth, P. A. Blake, and O. Olsik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, DC.
- 7.Beveridge, T. J. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1814725-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nokleby, E. Rosenqvist, et al. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 3381093-1096. [DOI] [PubMed] [Google Scholar]
- 9.Brandtzaeg, P. 2007. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 255467-5484. [DOI] [PubMed] [Google Scholar]
- 10.Brandtzaeg, P. 2003. Mucosal immunity. Dev. Biol. (Basel) 115111-117. [PubMed] [Google Scholar]
- 11.Brown, W. R. 1996. Enteric immunization: promises and challenges. Dig. Dis. 14192-200. [DOI] [PubMed] [Google Scholar]
- 12.Butler, S. M., and A. Camilli. 2004. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 1015018-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler, S. M., E. J. Nelson, N. Chowdhury, S. M. Faruque, S. B. Calderwood, and A. Camilli. 2006. Cholera stool bacteria repress chemotaxis to increase infectivity. Mol. Microbiol. 60417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butterton, J. R., E. T. Ryan, R. A. Shahin, and S. B. Calderwood. 1996. Development of a germfree mouse model of Vibrio cholerae infection. Infect. Immun. 644373-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabrera, O., M. E. Martinez, M. Cuello, C. R. Soto, T. Valmaseda, B. Cedre, and G. S. Gonzalez. 2006. Preparation and evaluation of Vibrio cholerae O1 EL Tor Ogawa lipopolysaccharide-tetanus toxoid conjugates. Vaccine 24(Suppl. 2)S2-74-S2-75. [DOI] [PubMed] [Google Scholar]
- 16.Cash, R. A., S. I. Music, J. P. Libonati, M. J. Snyder, R. P. Wenzel, and R. B. Hornick. 1974. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J. Infect. Dis. 12945-52. [DOI] [PubMed] [Google Scholar]
- 17.Cassel, D., and T. Pfeuffer. 1978. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc. Natl. Acad. Sci. USA 752669-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee, S. N., and J. Das. 1967. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J. Gen. Microbiol. 491-11. [DOI] [PubMed] [Google Scholar]
- 19.Chiang, S. L., and J. J. Mekalanos. 1999. rfb mutations in Vibrio cholerae do not affect surface production of toxin-coregulated pili but still inhibit intestinal colonization. Infect. Immun. 67976-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemens, J. D., D. A. Sack, J. R. Harris, M. R. Khan, J. Chakraborty, S. Chowdhury, M. R. Rao, F. P. van Loon, B. F. Stanton, M. Yunus, et al. 1990. Breast feeding and the risk of severe cholera in rural Bangladeshi children. Am. J. Epidemiol. 131400-411. [DOI] [PubMed] [Google Scholar]
- 21.Coster, T. S., K. P. Kileen, M. K. Waldor, D. Beattie, D. Spriggs, J. R. Kenner, A. Trofa, J. Sadoff, J. J. Mekalanos, and D. N. Taylor. 1995. Safety, immunogenicity and efficacy of a live attenuated Vibrio cholerae O139 vaccine prototype, Bengal-15. Lancet 345949-952. [DOI] [PubMed] [Google Scholar]
- 22.Dalseg, R., E. Wedege, J. Holst, I. L. Haugen, E. A. Hoiby, and B. Haneberg. 1999. Outer membrane vesicles from group B meningococci are strongly immunogenic when given intranasally to mice. Vaccine 172336-2345. [DOI] [PubMed] [Google Scholar]
- 23.de Moraes, J. C., B. A. Perkins, M. C. Camargo, N. T. Hidalgo, H. A. Barbosa, C. T. Sacchi, I. M. Landgraf, V. L. Gattas, G. Vasconcelos Hde, et al. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 3401074-1078. [DOI] [PubMed] [Google Scholar]
- 24.Devoe, I. W., and J. E. Gilchrist. 1973. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J. Exp. Med. 1381156-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorward, D. W., and C. F. Garon. 1989. DNA-binding proteins in cells and membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 1714196-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta, S., K. Iida, A. Takade, Y. Meno, G. B. Nair, and S. Yoshida. 2004. Release of Shiga toxin by membrane vesicles in Shigella dysenteriae serotype 1 strains and in vitro effects of antimicrobials on toxin production and release. Microbiol. Immunol. 48965-969. [DOI] [PubMed] [Google Scholar]
- 27.Faruque, S. M., M. J. Albert, and J. J. Mekalanos. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 621301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiocca, R., V. Necchi, P. Sommi, V. Ricci, J. Telford, T. L. Cover, and E. Solcia. 1999. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188220-226. [DOI] [PubMed] [Google Scholar]
- 29.Fisseha, M., P. Chen, B. Brandt, T. Kijek, E. Moran, and W. Zollinger. 2005. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect. Immun. 734070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gankema, H., J. Wensink, P. A. Guinee, W. H. Jansen, and B. Witholt. 1980. Some characteristics of the outer membrane material released by growing enterotoxigenic Escherichia coli. Infect. Immun. 29704-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García, L., M. Díaz Jidy, H. García, B. L. Rodríguez, R. Fernández, G. Año, B. Cedré, T. Valmaseda, E. Suzarte, M. Ramírez, Y. Pino, J. Campos, J. Menéndez, R. Valera, D. González, I. González, O. Pérez, T. Serrano, M. Lastre, F. Miralles, J. Del Campo, J. L. Maestre, J. L. Pérez, A. Talavera, A. Pérez, K. Marrero, T. Ledón, and R. Fando. 2005. The vaccine candidate Vibrio cholerae 638 is protective against cholera in healthy volunteers. Infect. Immun. 733018-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glass, R. I., A. M. Svennerholm, B. J. Stoll, M. R. Khan, K. M. Hossain, M. I. Huq, and J. Holmgren. 1983. Protection against cholera in breast-fed children by antibodies in breast milk. N. Engl. J. Med. 3081389-1392. [DOI] [PubMed] [Google Scholar]
- 33.Guentzel, M. N., and L. J. Berry. 1974. Protection of suckling mice from experimental cholera by maternal immunization: comparison of the efficacy of whole-cell, ribosomal-derived, and enterotoxin immunogens. Infect. Immun. 10167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guentzel, M. N., L. H. Field, E. R. Eubanks, and L. J. Berry. 1977. Use of fluorescent antibody in studies of immunity to cholera in infant mice. Infect. Immun. 15539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta, R. K., S. C. Szu, R. A. Finkelstein, and J. B. Robbins. 1992. Synthesis, characterization, and some immunological properties of conjugates composed of the detoxified lipopolysaccharide of Vibrio cholerae O1 serotype Inaba bound to cholera toxin. Infect. Immun. 603201-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guthrie, T., S. Y. Wong, B. Liang, L. Hyland, S. Hou, E. A. Hoiby, and S. R. Andersen. 2004. Local and systemic antibody responses in mice immunized intranasally with native and detergent-extracted outer membrane vesicles from Neisseria meningitidis. Infect. Immun. 722528-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haneberg, B., R. Dalseg, F. Oftung, E. Wedege, E. A. Hoiby, I. L. Haugen, J. Holst, S. R. Andersen, A. Aase, L. Meyer Naess, T. E. Michaelsen, E. Namork, and L. R. Haaheim. 1998. Towards a nasal vaccine against meningococcal disease, and prospects for its use as a mucosal adjuvant. Dev. Biol. Stand. 92127-133. [PubMed] [Google Scholar]
- 38.Haneberg, B., R. Dalseg, E. Wedege, E. A. Hoiby, I. L. Haugen, F. Oftung, S. R. Andersen, L. M. Naess, A. Aase, T. E. Michaelsen, and J. Holst. 1998. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect. Immun. 661334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hang, L., M. John, M. Asaduzzaman, E. A. Bridges, C. Vanderspurt, T. J. Kirn, R. K. Taylor, J. D. Hillman, A. Progulske-Fox, M. Handfield, E. T. Ryan, and S. B. Calderwood. 2003. Use of in vivo-induced antigen technology (IVIAT) to identify genes uniquely expressed during human infection with Vibrio cholerae. Proc. Natl. Acad. Sci. USA 1008508-8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanson, L. A., Y. Hofvander, B. Lindquist, and R. Zetterstrom. 1985. Breast-feeding as a protection against gastroenteritis and other infections. Acta Paediatr. Scand. 74641-642. [DOI] [PubMed] [Google Scholar]
- 41.Hartley, D. M., J. G. Morris, Jr., and D. L. Smith. 2006. Hyperinfectivity: a critical element in the ability of V. cholerae to cause epidemics? PLoS Med. 3e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herrington, D. A., R. H. Hall, G. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin-coregulated pili, and the toxR regulation are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 1681487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoekstra, D., J. W. van der Laan, L. de Leij, and B. Witholt. 1976. Release of outer membrane fragments from normally growing Escherichia coli. Biochim. Biophys. Acta 455889-899. [DOI] [PubMed] [Google Scholar]
- 44.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1993. Evaluation of different immunization schedules for oral cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine 111007-1012. [DOI] [PubMed] [Google Scholar]
- 45.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1994. Immunological memory after immunization with oral cholera B subunit-whole-cell vaccine in Swedish volunteers. Vaccine 121078-1082. [DOI] [PubMed] [Google Scholar]
- 46.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1992. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine 10130-132. [DOI] [PubMed] [Google Scholar]
- 47.Kadurugamuwa, J. L., and T. J. Beveridge. 1999. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other gram-negative bacteria. Microbiology 1452051-2060. [DOI] [PubMed] [Google Scholar]
- 48.Kadurugamuwa, J. L., and T. J. Beveridge. 1995. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 1773998-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 848-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato, S., Y. Kowashi, and D. R. Demuth. 2002. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 321-13. [DOI] [PubMed] [Google Scholar]
- 51.Keenan, J., T. Day, S. Neal, B. Cook, G. Perez-Perez, R. Allardyce, and P. Bagshaw. 2000. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol. Lett. 182259-264. [DOI] [PubMed] [Google Scholar]
- 52.Keenan, J. I., R. A. Allardyce, and P. F. Bagshaw. 1998. Lack of protection following immunisation with H. pylori outer membrane vesicles highlights antigenic differences between H. felis and H. pylori. FEMS Microbiol. Lett. 16121-27. [DOI] [PubMed] [Google Scholar]
- 53.Kesavalu, L., J. L. Ebersole, R. L. Machen, and S. C. Holt. 1992. Porphyromonas gingivalis virulence in mice: induction of immunity to bacterial components. Infect. Immun. 601455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klose, K. E. 2001. Regulation of virulence in Vibrio cholerae. Int. J. Med. Microbiol. 29181-88. [DOI] [PubMed] [Google Scholar]
- 55.Klose, K. E. 2000. The suckling mouse model of cholera. Trends Microbiol. 8189-191. [DOI] [PubMed] [Google Scholar]
- 56.Koch, R. 1884. An address on cholera and its bacillus. Br. Med. J. 2403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kondo, K., A. Takade, and K. Amako. 1993. Release of the outer membrane vesicles from Vibrio cholerae and Vibrio parahaemolyticus. Microbiol. Immunol. 37149-152. [DOI] [PubMed] [Google Scholar]
- 58.Kossaczka, Z., J. Shiloach, V. Johnson, D. N. Taylor, R. A. Finkelstein, J. B. Robbins, and S. C. Szu. 2000. Vibrio cholerae O139 conjugate vaccines: synthesis and immunogenicity of V. cholerae O139 capsular polysaccharide conjugates with recombinant diphtheria toxin mutant in mice. Infect. Immun. 685037-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuehn, M. J., and N. C. Kesty. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 192645-2655. [DOI] [PubMed] [Google Scholar]
- 60.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 61.Levine, M. M., R. E. Black, M. L. Clements, L. Cisneros, D. R. Nalin, and C. R. Young. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143818-820. [DOI] [PubMed] [Google Scholar]
- 62.Lombardo, M. J., J. Michalski, H. Martinez-Wilson, C. Morin, T. Hilton, C. G. Osorio, J. P. Nataro, C. O. Tacket, A. Camilli, and J. B. Kaper. 2007. An in vivo expression technology screen for Vibrio cholerae genes expressed in human volunteers. Proc. Natl. Acad. Sci. USA 10418229-18234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merrell, D. S., S. M. Butler, F. Qadri, N. A. Dolganov, A. Alam, M. B. Cohen, S. B. Calderwood, G. K. Schoolnik, and A. Camilli. 2002. Host-induced epidemic spread of the cholera bacterium. Nature 417642-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merrell, D. S., and A. Camilli. 1999. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol. Microbiol. 34836-849. [DOI] [PubMed] [Google Scholar]
- 65.Miller, V. L., V. J. DiRita, and J. J. Mekalanos. 1989. Identification of toxS, a regulatory gene whose product enhances toxR-mediated activation of the cholera toxin promoter. J. Bacteriol. 1711288-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1702575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitragotri, S. 2005. Immunization without needles. Nat. Rev. Immunol. 5905-916. [DOI] [PubMed] [Google Scholar]
- 68.Nesper, J., S. Schild, C. M. Lauriano, A. Kraiss, K. E. Klose, and J. Reidl. 2002. Role of Vibrio cholerae O139 surface polysaccharides in intestinal colonization. Infect. Immun. 705990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olivier, V., N. H. Salzman, and K. J. Satchell. 2007. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect. Immun. 755043-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osorio, C. G., H. Martinez-Wilson, and A. Camilli. 2004. The ompU paralogue vca1008 is required for virulence of Vibrio cholerae. J. Bacteriol. 1865167-5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peter, G., and M. G. Myers. 2002. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics 110e67. [DOI] [PubMed] [Google Scholar]
- 72.Post, D. M., D. Zhang, J. S. Eastvold, A. Teghanemt, B. W. Gibson, and J. P. Weiss. 2005. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J. Biol. Chem. 28038383-38394. [DOI] [PubMed] [Google Scholar]
- 73.Provenzano, D., and K. E. Klose. 2000. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc. Natl. Acad. Sci. USA 9710220-10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qadri, F., M. I. Chowdhury, S. M. Faruque, M. A. Salam, T. Ahmed, Y. A. Begum, A. Saha, A. Al Tarique, L. V. Seidlein, E. Park, K. P. Killeen, J. J. Mekalanos, J. D. Clemens, and D. A. Sack. 2007. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine 25231-238. [DOI] [PubMed] [Google Scholar]
- 75.Qadri, F., M. I. Chowdhury, S. M. Faruque, M. A. Salam, T. Ahmed, Y. A. Begum, A. Saha, M. S. Alam, K. Zaman, L. V. Seidlein, E. Park, K. P. Killeen, J. J. Mekalanos, J. D. Clemens, and D. A. Sack. 2005. Randomized, controlled study of the safety and immunogenicity of Peru-15, a live attenuated oral vaccine candidate for cholera, in adult volunteers in Bangladesh. J. Infect. Dis. 192573-579. [DOI] [PubMed] [Google Scholar]
- 76.Qadri, F., E. T. Ryan, A. S. Faruque, F. Ahmed, A. I. Khan, M. M. Islam, S. M. Akramuzzaman, D. A. Sack, and S. B. Calderwood. 2003. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 714808-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qureshi, K., K. Molbak, A. Sandstrom, P. E. Kofoed, A. Rodrigues, F. Dias, P. Aaby, and A. M. Svennerholm. 2006. Breast milk reduces the risk of illness in children of mothers with cholera: observations from an epidemic of cholera in Guinea-Bissau. Pediatr. Infect. Dis. J. 251163-1166. [DOI] [PubMed] [Google Scholar]
- 78.Reidl, J., and K. E. Klose. 2002. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol. Rev. 26125-139. [DOI] [PubMed] [Google Scholar]
- 79.Richardson, S. H. 1994. Host susceptibility, p. 273-289. In I. K. Wachsmuth, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, DC.
- 80.Richie, E. E., N. H. Punjabi, Y. Y. Sidharta, K. K. Peetosutan, M. M. Sukandar, S. S. Wasserman, M. M. Lesmana, F. F. Wangsasaputra, S. S. Pandam, M. M. Levine, P. P. O'Hanley, S. J. Cryz, and C. H. Simanjuntak. 2000. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 182399-2410. [DOI] [PubMed] [Google Scholar]
- 81.Ryan, E. T., and S. B. Calderwood. 2000. Cholera vaccines. Clin. Infect. Dis. 31561-565. [DOI] [PubMed] [Google Scholar]
- 82.Sack, D. A., R. B. Sack, G. B. Nair, and A. K. Siddique. 2004. Cholera. Lancet 363223-233. [DOI] [PubMed] [Google Scholar]
- 83.Saunders, N. B., D. R. Shoemaker, B. L. Brandt, E. E. Moran, T. Larsen, and W. D. Zollinger. 1999. Immunogenicity of intranasally administered meningococcal native outer membrane vesicles in mice. Infect. Immun. 67113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schild, S., A. K. Lamprecht, C. Fourestier, C. M. Lauriano, K. E. Klose, and J. Reidl. 2005. Characterizing lipopolysaccharide and core lipid A mutant O1 and O139 Vibrio cholerae strains for adherence properties on mucus-producing cell line HT29-Rev MTX and virulence in mice. Int. J. Med. Microbiol. 295243-251. [DOI] [PubMed] [Google Scholar]
- 85.Schild, S., R. Tamayo, E. J. Nelson, F. Qadri, S. B. Calderwood, and A. Camilli. 2007. Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe 2264-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tacket, C. O., G. Losonsky, J. P. Nataro, L. Comstock, J. Michalski, R. Edelman, J. B. Kaper, and M. M. Levine. 1995. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J. Infect. Dis. 172883-886. [DOI] [PubMed] [Google Scholar]
- 87.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 842833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thelin, K. H., and R. K. Taylor. 1996. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect. Immun. 642853-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trach, D. D., P. D. Cam, N. T. Ke, M. R. Rao, D. Dinh, P. V. Hang, N. V. Hung, D. G. Canh, V. D. Thiem, A. Naficy, B. Ivanoff, A. M. Svennerholm, J. Holmgren, and J. D. Clemens. 2002. Investigations into the safety and immunogenicity of a killed oral cholera vaccine developed in Viet Nam. Bull. W. H. O. 802-8. [PMC free article] [PubMed] [Google Scholar]
- 90.Vance, R. E., J. Zhu, and J. J. Mekalanos. 2003. A constitutively active variant of the quorum-sensing regulator LuxO affects protease production and biofilm formation in Vibrio cholerae. Infect. Immun. 712571-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vesy, C. J., R. L. Kitchens, G. Wolfbauer, J. J. Albers, and R. S. Munford. 2000. Lipopolysaccharide-binding protein and phospholipid transfer protein release lipopolysaccharides from gram-negative bacterial membranes. Infect. Immun. 682410-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Viret, J. F., G. Dietrich, and D. Favre. 2004. Biosafety aspects of the recombinant live oral Vibrio cholerae vaccine strain CVD 103-HgR. Vaccine 222457-2469. [DOI] [PubMed] [Google Scholar]
- 93.Wai, S. N., B. Lindmark, T. Soderblom, A. Takade, M. Westermark, J. Oscarsson, J. Jass, A. Richter-Dahlfors, Y. Mizunoe, and B. E. Uhlin. 2003. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 11525-35. [DOI] [PubMed] [Google Scholar]
- 94.Wai, S. N., A. Takade, and K. Amako. 1995. The release of outer membrane vesicles from the strains of enterotoxigenic Escherichia coli. Microbiol. Immunol. 39451-456. [DOI] [PubMed] [Google Scholar]
- 95.Warfield, K. L., S. E. Blutt, S. E. Crawford, G. Kang, and M. E. Conner. 2006. Rotavirus infection enhances lipopolysaccharide-induced intussusception in a mouse model. J. Virol. 8012377-12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wensink, J., and B. Witholt. 1981. Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur. J. Biochem. 116331-335. [DOI] [PubMed] [Google Scholar]
- 97.WHO. 2007. Cholera, 2006. Weekly epidemiological record. World Health Organization, Geneva, Switzerland. http://www.who.int/wer/2007/wer8231.pdf.
- 98.WHO. 2005. Oral cholera vaccine use in complex emergencies: what next? Report of the WHO meeting. World Health Organization, Geneva, Switzerland. http://www3.alliance-hpsr.org/topics/cholera/publications/cholera_vaccines_emergencies_2005.pdf.
- 99.Winner, L., III, J. Mack, R. Weltzin, J. J. Mekalanos, J. P. Kraehenbuhl, and M. R. Neutra. 1991. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect. Immun. 59977-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yancey, R. J., D. L. Willis, and L. J. Berry. 1978. Role of motility in experimental cholera in adult rabbits. Infect. Immun. 22387-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshiyama, Y., and W. R. Brown. 1987. Specific antibodies to cholera toxin in rabbit milk are protective against Vibrio cholerae-induced intestinal secretion. Immunology 61543-547. [PMC free article] [PubMed] [Google Scholar]
- 102.Zollinger, W. D., J. Boslego, E. Moran, J. Garcia, C. Cruz, S. Ruiz, B. Brandt, M. Martinez, J. Arthur, P. Underwood, et al.1991. Meningococcal serogroup B vaccine protection trial and follow-up studies in Chile. NIPH Ann. 14211-213. [PubMed] [Google Scholar]