Abstract
The phosphoinositide 3-kinase (PI3K)/3-phosphoinositide-dependent protein kinase 1 (PDK1)/Akt pathway regulates various cellular functions, especially cell survival and cell cycle progression. In contrast to other survival pathways, there have been few reports of scaffold proteins that regulate signaling cascade specificity in this pathway. Here we identify a 5′ repressor element under dual-repression binding protein 1 (Freud-1)/Akt kinase-interacting protein 1 (Aki1) as a novel scaffold for the PDK1/Akt pathway. Freud-1/Aki1 (also known as CC2D1A) expression induced formation of a PDK1/Akt complex and regulated Akt activation in a concentration-dependent biphasic manner. Freud-1/Aki1 also associated with epidermal growth factor (EGF) receptor in response to EGF stimulation and was required for Akt activation induced by EGF, but not by insulin-like growth factor 1. Freud-1/Aki1 gene silencing decreased Akt kinase activity, resulting in induction of apoptosis and increased sensitivity toward chemotherapeutic agents. Our results suggest that Freud-1/Aki1 is a novel receptor-selective scaffold protein for the PDK1/Akt pathway and present a new activation mechanism of Akt.
Several growth factors and cytokines have been reported to promote cell survival and cell cycle progression. Interaction among these factors and their specific receptors triggers the activation of phosphoinositide-3-kinase (PI3K). Activated PI3K generates phosphatidylinositol 3,4,5-trisphosphate (PIP3) and phosphatidylinositol 3,4-bisphosphate (PIP2), which are phospholipid second messenger molecules (44, 46). These lipids then induce the activation of several members of the A, G, and C (AGC) family of protein kinases, including Akt/protein kinase B, p70 ribosomal protein S6 kinase (S6K), protein kinase C isoforms (PKCs), and serum- and glucocorticoid-inducible kinases (SGKs). Interaction of Akt with lipids is believed to induce conformational change in Akt. In addition, Akt is phosphorylated at two key regulatory sites, Thr308 in the activation loop of the catalytic domain and Ser473 in the COOH-terminal domain. Activated Akt prevents cells from undergoing apoptosis and mediates cell survival and cell cycle progression by phosphorylating downstream key regulatory proteins, contributing to tumor formation and progression (8, 45, 47).
3-Phosphoinositide-dependent protein kinase 1 (PDK1) was originally identified as a kinase that could phosphorylate Akt on its activation loop (residue Thr308) (1). Later work, however, showed that PDK1 is not only an Akt kinase but also a kinase responsible for phosphorylating members of the AGC family of protein kinases: S6K, SGKs, PKCs, and p90 ribosomal protein S6 kinases (RSKs) at the Thr308-equivalent residues of Akt (45). PDK1 itself is also a member of the AGC subfamily of protein kinases and is phosphorylated at the Ser241 residue (equivalent to Thr308 of Akt) in the activation loop. Because PDK1 expressed in bacteria is active and phosphorylated at Ser241, it is thought that PDK1 phosphorylates by itself at this site (11). Furthermore, mutation of Ser241 to Ala was reported to abolish PDK1 kinase activity, and insulin-like growth factor 1 (IGF-1) stimulation did not cause further activation of PDK1 (11). According to these results, PDK1 was thought to be constitutively active. Therefore, its specificity is achieved by placing the enzyme in proximity to its downstream targets (11). For example, recruiting PDK1 onto muscle A kinase anchoring protein (mAKAPα) facilitates activation and release of the downstream target RSK (28). When PIP2 and PIP3 are generated by the activated PI3K, both PDK1 and Akt are recruited to the plasma membrane, and then PDK1 activates Akt. In contrast, it was also reported that growth factor stimulation had no effect on the subcellular localization of PDK1 (14). Thus, growth factor-induced PDK1 membrane localization is controversial, and other mechanisms targeting PDK1 to Akt may also exist.
There is increasing evidence that scaffold proteins maintain signaling specificity, facilitate the activation of pathway components, and localize them to particular subcellular sites or to specific targets. Major scaffolds include axin in Wnt/β-catenin signaling (37), and KSR and JIP-1 in mitogen-activated protein kinase (MAPK) pathways (29, 49). MAPK pathways feature a conserved signaling cascade consisting of MAPK kinase kinase (MAPKKK), which phosphorylates and activates MAPK kinase (MAPKK), which then activates MAPK by phosphorylation. The components of these pathways associate with many types of scaffold proteins that can colocalize the components of the pathway and regulate their activities. However, while many components involved in the PI3K/PDK1/Akt pathway have been elucidated, little is known about how those components are coordinated by scaffold proteins.
In this study, we provide evidence that Freud-1/Aki1, a novel Akt kinase (PDK1)-interacting protein, facilitates formation of the PDK1/Akt complex. In addition, we find that Freud-1/Aki1 selectively associates with epidermal growth factor receptor (EGFR) and is essential for PDK1-mediated Akt phosphorylation in EGF signaling. Moreover, small interfering RNA (siRNA)-mediated Freud-1/Aki1 gene silencing causes cell apoptosis and increases sensitivity to chemotherapeutic drugs. These results suggest that Freud-1/Aki1 functions as a scaffold in promoting EGF-induced Akt phosphorylation and plays an important role in cell survival.
MATERIALS AND METHODS
Escherichia coli two-hybrid screening.
The BacterioMatch II two-hybrid system (Stratagene, La Jolla, CA) was used for screening, according to the manufacturer's instructions.
Cell culture conditions and reagents.
Human embryonic kidney HEK293 cells, 293T cells, and human epidermoid carcinoma A431 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Human fibrosarcoma HT1080 cells were cultured in RPMI 1640 medium supplemented with 10% FBS. Paclitaxel (Taxol) and etoposide (VP-16) were kindly provided by Bristol-Myers Squibb Co., Ltd. (Tokyo, Japan). Camptothecin (CPT) was kindly provided by Yakult Co., Ltd. (Tokyo, Japan). Geneticin, 3× FLAG peptide, LY294002, and okadaic acid were purchased from Sigma (St. Louis, MO). Recombinant human inactive Akt1, human active PDK1 (amino acids 52 to 556), and human SGK protein were purchased from Upstate Biotechnology (Lake Placid, NY). Glycogen synthase kinase 3 (GSK3) fusion protein was obtained from Cell Signaling Technology (Beverly, MA). Recombinant human EGF, IGF-1, and platelet-derived growth factor (PDGF) were purchased from Peprotech (London, United Kingdom). PIP3 was purchased from Biomol International (Plymouth Meeting, PA). The potent EGF tyrosine kinase inhibitor 4-(3-chloroanilino)-6,7-dimethoxyquinazoline (AG1478) was purchased from Calbiochem (San Diego, CA).
Plasmid construction.
Human full-length wild-type (WT) Freud-1/Aki1 cDNA in a pFLAG-CMV-2 vector was generated by PCR with an IMAGE clone (clone identification no. 6585236; Invitrogen, San Diego, CA) as the template. The sense and antisense primers used for the PCR were 5′-TGCACAAGAGGAAAGGACCCC-3′ and 5′-TCACCTGCGGAGCCGCTGCAGCTCACTCTCTACCAGATTC-3′, respectively. The PCR products were ligated to a pCRII vector (Invitrogen). Freud-1/Aki1 in the pCRII vector was digested with EcoRI and cloned into the EcoRI site of a pFLAG-CMV-2 (Sigma) or pHM6 vector (Roche Diagnostics, Indianapolis, IN). The Freud-1/Aki1 mutants were generated by PCR with pFLAG-CMV-2-WT-Freud-1/Aki1 as the template, using a QuickChange mutagenesis kit (Stratagene). Human WT, K179A-, K179A/S473A-, K179A/T308A/S473A- (AAA-), E40K-, T308D-, S473D-, and T308D/S473D-Akt1 cDNAs, the mutants of PDK1 cDNA and NH2-terminal-deleted SGK cDNA (ΔN60-SGK) in a pFLAG-CMV-2 vector, and the human WT Akt1 cDNA or S6K cDNA in a pHM6 vector were established in our laboratories (17, 39). Myc-tagged human full-length PDK1 cDNA (WT PDK1) in a pCMV3 vector was kindly provided by P. Hawkins and K. Anderson (The Babraham Institute, Cambridge, United Kingdom) (2). All plasmid DNAs for transfection were purified using a Qiagen plasmid maxikit according to the manufacturer's protocol (Qiagen, Chatsworth, CA).
Antibodies.
The following antibodies were used for immunoblotting: Freud-1/Aki1 from Bethyl Laboratories, Montgomery, TX; Akt, phospho-Thr308-Akt, phospho-Ser473-Akt, EGFR, phospho-Tyr1068-EGFR, FOXO1, phospho-Ser21-GSK3α, IGF-1R, phospho-Tyr1131-IGF-1R, cleaved poly(ADP-ribose) polymerase (PARP), PDK1, phospho-Ser241-PDK1, and SGK from Cell Signaling Technology; p27Kip1 from BD Transduction Laboratories, Lexington, KY; S6K from New England Biolabs, Beverly, MA; FOXO3a and phospho-Thr256-SGK from Upstate Biotechnology; topoisomerase IIβ from BD Biosciences-PharMingen, San Diego, CA; FLAG tag (clone M2) from Sigma; hemagglutinin (HA) tag (clone 3A10) and Myc tag (clone 9E10) from Roche Diagnostics; and β-actin (C-2) from Santa Cruz Biotechnology, Santa Cruz, CA.
Transient transfections, immunoprecipitations, and Western blot analysis.
Cells were transfected with appropriate plasmids or siRNA using Superfect transfection reagent (Qiagen) or Lipofectamine 2000 reagent (Invitrogen), according to the manufacturers' instructions.
The PDK1-2 siRNA was designed as described previously (39). Negative control siRNA, Freud-1/Aki1-1 siRNA, and Freud-1/Aki1-2 siRNA were purchased from Invitrogen. These oligonucleotides were stealth siRNAs. The coding strands of the siRNAs were GGCGCUCUAUCAGACAGCAAUUGAA (Freud-1/Aki1-1; directed to nucleotides 432 to 456) and CCCUGGCGAUCUGGAUGUCUUUGUU (Freud-1/Aki1-2; directed to nucleotides 2022 to 2046).
After transfection for the appropriate times, the culture medium was replaced with a medium containing 0.1% FBS. After incubation for 24 h, cells were treated with bovine serum albumin (BSA) as a control, 50 ng/ml IGF-1, 50 ng/ml EGF, or 100 ng/ml PDGF for 10 min. In some experiments, cells were treated with 1 μM AG1478 for 1 h prior to EGF addition or treated with 50 μM LY294002 for an appropriate time before cell harvest. Cells were then harvested and solubilized in lysis buffer (20 mM Tris-HCl [pH 7.5], 0.2% Nonidet P-40, 10% glycerol, 1 mM EDTA, 1.5 mM magnesium chloride, 137 mM sodium chloride, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 12 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, and 1 mM aprotinin) (40). Whole-cell lysates were prepared using lysis buffer containing sodium dodecyl sulfate (SDS) (1% SDS, 10% glycerol, and 100 mM Tris-HCl [pH 7.6]). In some experiments, cytoplasmic and nuclear fractions or cytoplasmic, membrane, and nuclear fractions were separated using an NE-PER extraction kit (Pierce, Rockford, IL) or compartmental protein extraction kit (Chemicon, Temecula, CA), respectively, according to the manufacturer's instructions. Tagged proteins were immunoprecipitated with an anti-HA antibody-conjugated agarose (clone HA-7; Sigma) or an anti-FLAG antibody-conjugated agarose (clone M2; Sigma). In some experiments, cell lysates were incubated with an anti-Akt antibody-conjugated agarose (C-20; Santa Cruz Biotechnology), protein A-Sepharose (Zymed Laboratories, South San Francisco, CA) that had been conjugated with normal sheep immunoglobulin G (IgG), a sheep anti-S6K antibody, a sheep anti-PDK1 antibody (Upstate Biotechnology), or anti-EGFR Ab-13 (clone 528 + 199.12; Thermo Fisher Scientific, Fremont, CA) or with protein G-Sepharose (Zymed Laboratories) that had been conjugated with an anti-Freud-1/Aki1 antibody (43). Then the immunoprecipitated proteins or the cell lysates were analyzed by immunoblotting. Blots were scanned using an Image Reader LAS-3000 mini (Fujifilm, Tokyo, Japan) and quantified using Multi Gauge software (Fujifilm).
Sequential immunoprecipitation analysis was performed as follows. 293T cells were transfected with FLAG-Freud-1/Aki1, HA-Akt, and Myc-PDK1. For the control of second immunoprecipitation, we used HA-mock vector (encoding no protein) and HA-S6K. After transfection for 24 h, the cells were solubilized in lysis buffer and the lysates were immunoprecipitated with anti-FLAG antibody-conjugated agarose for 2 h at 4°C. The FLAG-Freud-1/Aki1 complex was eluted with lysis buffer containing 200 μg/ml FLAG peptide for 1 h at 4°C. Then, the eluate was immunoprecipitated again with an anti-HA antibody-conjugated agarose for 2 h at 4°C.
Flow cytometric analysis.
Cells were harvested and fixed with 70% ice-cold ethanol for 30 min at 4°C. After washing with phosphate-buffered saline (PBS), the cells were incubated with PBS containing 1 mg/ml RNase for 30 min at 37°C. After washing with PBS again, the cells were stained in propidium iodide solution (50 μg/ml propidium iodide, 0.1% sodium citrate, 0.1% NP-40) for 30 min at 4°C. Analyses were performed on a Cytomics 500 flow cytometer (Beckman Coulter, Miami, FL) with Cytomics RXP and MultiCycler software (20).
Kinase assays.
Endogenous proteins were immunoprecipitated with an immobilized Akt antibody (1G1) (Cell Signaling Technology), control mouse IgG-conjugated agarose, or anti-PDK1 antibody-conjugated agarose (E-3) (Santa Cruz Biotechnology). FLAG-tagged proteins were immunoprecipitated with anti-FLAG antibody-conjugated agarose. Akt or PDK1 kinase activity was assayed using the Upstate Biotechnology kinase assay kit according to the manufacturer's instructions. Human recombinant SGK or GSK3 fusion protein was incubated with immunoprecipitated PDK1 or Akt in the kinase reaction buffer for PDK1 or for Akt for 30 min at 30°C, respectively. In a PDK1 kinase assay with PIP3, human recombinant PDK1, Akt, and PIP3-containing vesicles were incubated with immunoprecipitated FLAG-tagged Freud-1/Aki1 (44, 46). Reaction mixtures were electrophoresed and immunoblotted. To check the input level of GSK3 fusion protein, the SDS-polyacrylamide gel was stained with Coomassie brilliant blue after electrophoresis.
MTT assay.
To assess cell viability, we employed the MTT [3-(4,5-methylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] assay. In brief, cells were incubated with MTT for 2.5 h. Formazan products were solubilized with dimethyl sulfoxide, and optical density was measured at 525 nm, with a reference at 650 nm, using a microplate spectrophotometer (Benchmark Plus; Bio-Rad, Hercules, CA). Viability was calculated by dividing the absorbance of each sample by that of the corresponding control.
Pull-down assay.
HA-tagged recombinant human WT-Freud-1/Aki1, p27Kip1, or mouse MAPK2 protein was obtained by in vitro translation of pHM6 plasmid encoding WT Freud-1/Aki1, p27Kip1, or MAPK2 using the TNT Quick Coupled transcription/translation system (Promega, Madison, WI), according to the manufacturer's instructions. To obtain a control protein, the same experiment was done with a pHM6 vector encoding no protein. The HA-tagged proteins were immunopurified with an anti-HA agarose and then incubated with recombinant PDK1 or Akt at 4°C for 2 h. After washing, PDK1 or Akt that was associated with HA-tagged recombinant Freud-1/Aki1 was detected by immunoblot analysis.
RESULTS
Molecular identification of Freud-1/Aki1 as a PDK1-interacting protein.
To identify novel molecules that form a complex with and regulate PDK1, a HeLa cDNA library and a human fetal brain cDNA library were screened using an E. coli two-hybrid system with the NH2-terminal-deleted PDK1 (ΔN51-PDK1) as a bait. We used ΔN51-PDK1 as the bait because it has been reported to be as functional as WT PDK1 (2) and its level of expression was higher than that of WT PDK1 in cells. Eight identical clones (F1 to F8) were isolated. At that time, the F1 clone was functionally unknown. We therefore focused on and renamed F1, calling it Aki1 because it is an Akt kinase (PDK1)-interacting protein 1. Aki1 was originally designated as a hypothetical protein, FLJ20241 (Full Length cDNA Database). It is also called “coiled-coil and C2 domain containing 1A” (CC2D1A) based on its structure. Recently, it has been shown that Aki1 is identical to Freud-1 (32). Although Freud-1/Aki1 has been reported to be a transcriptional regulator involved in autosomal recessive nonsyndromic mental retardation (ARNSMR) (6, 35), there have been no reports on the function of Freud-1/Aki1 in PI3K/PDK1/Akt signaling.
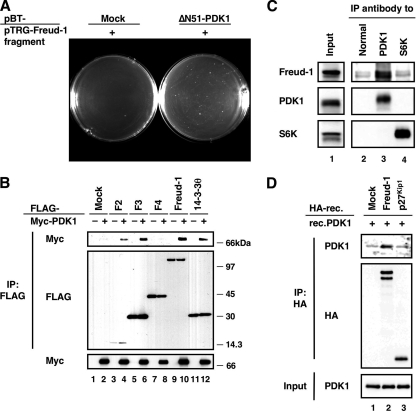
Plasmid DNAs isolated from the F1 clone (pTRG-Freud-1/Aki1 fragment) were transformed into E. coli together with a bait cDNA (pBT-ΔN51-PDK1) or a negative control cDNA (pBT-mock) to reconfirm the interaction between Freud-1/Aki1 and PDK1 in bacteria. We observed positive colonies among the Freud-1/Aki1 transformants, indicating that Freud-1/Aki1 interacts with ΔN51-PDK1 in E. coli (Fig. 1A). To examine the interaction in mammalian cells, FLAG-tagged Freud-1/Aki1 or other putative PDK1-interacting proteins (clones F2 to F8) were overexpressed together with Myc-tagged PDK1 in 293T cells. Myc-PDK1 could bind to all clones except F4 (Fig. 1B [results for F5 to F8 not shown]). The interaction of F4 with PDK1 might be inhibited by other proteins or its posttranslational modifications in mammalian cells. Because it had been reported that 14-3-3θ interacted with PDK1 (39), we used 14-3-3θ as the positive control for binding to PDK1. In addition, endogenous Freud-1/Aki1 could be detected in the anti-PDK1 immunoprecipitant but not in normal IgG or anti-S6K immunoprecipitant, indicating that Freud-1/Aki1 indeed interacts with PDK1 at their endogenous levels (Fig. 1C). The binding to PDK1 seemed to be constitutive because treatment with EGF or IGF-1 had no effect on complex formation (data not shown). Pull-down analysis with in vitro-translated recombinant Freud-1/Aki1 or p27Kip1 and recombinant PDK1 clearly indicated that Freud-1/Aki1 directly binds to PDK1 (Fig. 1D). Thus, we concluded that Freud-1/Aki1 is a novel PDK1-binding partner.
FIG. 1.
Identification of Freud-1/Aki1 as a PDK1-interacting protein. (A) BacterioMatch II validation reporter-competent cells were transformed with a pBT vector encoding no protein (Mock) or ΔN51-PDK1 together with a pTRG vector encoding the Freud-1/Aki1 fragment (+). (B) 293T cells were transfected with the indicated plasmids encoding full-length human PDK1 and putative PDK1-interacting proteins. Immunoprecipitated (IP) proteins (top and middle) and cell lysates (bottom) were electrophoresed and immunoblotted with antibodies to FLAG or to Myc. (C) 293T cell lysates were incubated with protein A-Sepharose conjugated with normal sheep IgG (Normal), an anti-PDK1 antibody, or an anti-S6K antibody. Immunoprecipitated proteins (lanes 2 to 4) and cell lysates (Input) were electrophoresed and immunoblotted with the indicated antibodies. (D) HA-tagged recombinant (HA-rec.) Freud-1/Aki1 (lane 2) or p27Kip1 (lane 3) protein was obtained by in vitro translation of a pHM6 vector encoding Freud-1/Aki1 or p27Kip1. The HA-tagged protein was obtained from a pHM6 vector encoding no protein (Mock). HA-tagged proteins were immunopurified with an anti-HA agarose and then incubated with recombinant PDK1 (rec.PDK1). The reaction mixture (Input) and agarose-bound proteins (top and middle) were electrophoresed and immunoblotted with antibodies to HA or PDK1.
Identification of the domains responsible for interactions of Freud-1/Aki1 with PDK1.
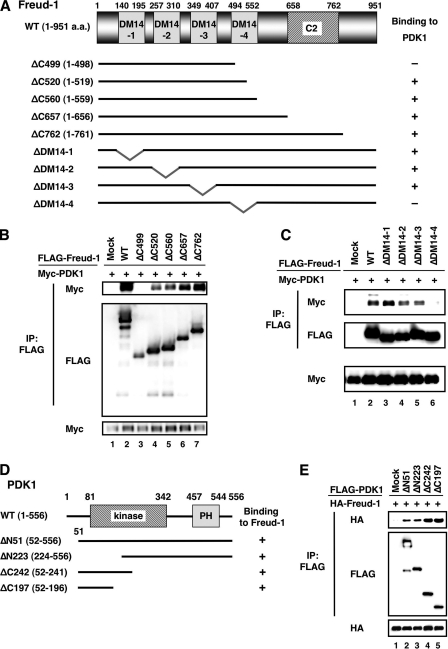
Freud-1/Aki1 contains four Drosophila melanogaster 14 (DM14) domains and one C2 domain. The DM14 domain is a 60-amino-acid repeat sequence conserved among Freud-1/Aki1 homologs, even in lower organisms such as Caenorhabditis elegans and Drosophila, but its function has never been clarified. The C2 domain is a PKC-conserved region 2, which is a Ca2+-dependent membrane-targeting domain found in many cellular proteins involved in signal transduction. To identify the domain or domains in Freud-1/Aki1 that are critical for complex formation with PDK1, we prepared several Freud-1/Aki1 deletion mutants (Fig. 2A). PDK1 formed a complex with ΔC520-, ΔC560-, ΔC657-, and ΔC762-Freud-1/Aki1, but not with ΔC499-Freud-1/Aki1 (Fig. 2B). In addition, the fourth DM14 domain (DM14-4) was found to be critical for Freud-1/Aki1-PDK1 binding (Fig. 2C). These results indicate that a region around the DM14-4 domain is associated with binding to PDK1.
FIG. 2.
Identification of the domains responsible for interactions between Freud-1/Aki1 and PDK1. (A) The structural domains of Freud-1/Aki1 and its deletion mutants used in the experiments are represented as black bars. (B and C) 293T cells were transfected with plasmids to express WT PDK1 and Freud-1/Aki1 mutants as indicated. Immunoprecipitated (IP) proteins (top and middle) and cell lysates (bottom) were electrophoresed and immunoblotted with antibodies to FLAG or to Myc. (D) Structural domains of PDK1 and its deletion mutants used in the experiments are represented as black bars. (E) 293T cells were transfected with plasmids to express Freud-1/Aki1 and PDK1 mutants as indicated. Immunoprecipitated proteins (top and middle) and cell lysates (bottom) were electrophoresed and immunoblotted with antibodies to FLAG or to HA.
To determine the Freud-1/Aki1 binding site in PDK1, we prepared PDK1 deletion mutants (Fig. 2D). When the FLAG-PDK1 deletion mutants were immunoprecipitated with an anti-FLAG antibody, HA-Freud-1/Aki1 could be detected in all of the immunoprecipitants (Fig. 2E). This result indicates that PDK1 may bind to Freud-1/Aki1 through more than one binding site.
Freud-1/Aki1 activates the PDK1/Akt signaling pathway.
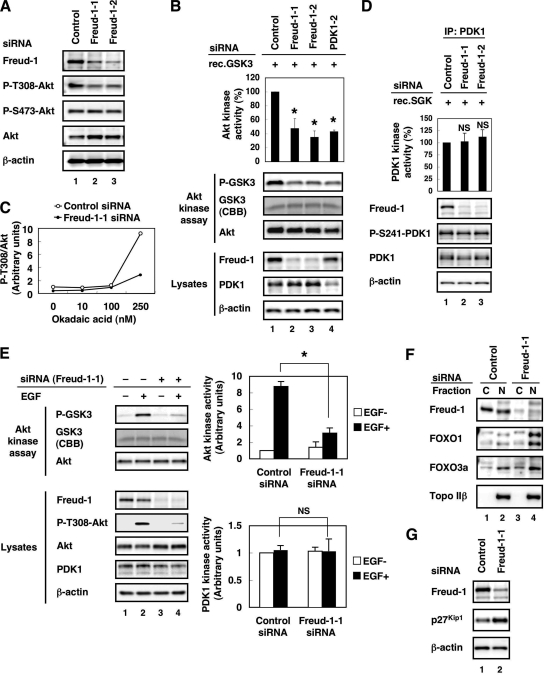
To investigate the role of endogenous Freud-1/Aki1 in PDK1/Akt signaling, we designed two Freud-1/Aki1 siRNAs. Both Freud-1/Aki1-1 and Freud-1/Aki1-2 siRNAs effectively attenuated endogenous Freud-1/Aki1 protein expression. Under these conditions, both Freud-1/Aki1 siRNAs significantly decreased the level of phospho-Thr308-Akt compared with control siRNA. Importantly, neither siRNA had a significant effect on Akt phosphorylation at Ser473 (Fig. 3A). Because Akt activity correlates with its phosphorylation levels at the Thr308 and Ser473 residues, we employed an in vitro kinase assay to confirm the downregulation of Akt kinase activity after Freud-1/Aki1 knockdown. Because Akt is known to phosphorylate GSK3α at Ser21 (13), we measured the phospho-Ser21-GSK3α level as an indication of Akt kinase activity. Endogenous Freud-1/Aki1 knockdown by Freud-1/Aki1 siRNAs and endogenous PDK1 knockdown by our previously designed PDK1-2 siRNA (39) clearly reduced Akt kinase activity. Three independent experiments confirmed a reduction of about 60% in Akt kinase activity after Freud-1/Aki1 gene silencing (Fig. 3B).
FIG. 3.
Freud-1/Aki1 positively regulates AktT308 phosphorylation and Akt kinase activity. (A) 293T cells were transfected with nonsilencing control siRNA (lane 1), Freud-1/Aki1-1 siRNA (lane 2), or Freud-1/Aki1-2 siRNA (lane 3). After transfection for 72 h, cell lysates were electrophoresed and immunoblotted with the indicated antibodies. (B) 293T cells were transfected with nonsilencing control siRNA (lane 1), Freud-1/Aki1-1 siRNA (lane 2), Freud-1/Aki1-2 siRNA (lane 3), or PDK1-2 siRNA (lane 4). Then an Akt kinase assay was performed. The Akt kinase activity was determined by quantifying the phospho-GSK3 level using Multi Gauge, as described in Materials and Methods. Error bars represent standard deviations of triplicate experiments. The Akt kinase activity of Freud-1/Aki1-1, Freud-1/Aki1-2, or PDK1-2 siRNA-transfected cells was significantly different from that of control siRNA-transfected cells (*, P < 0.025). (C) 293T cells were transfected with nonsilencing control siRNA (open circles) or Freud-1/Aki1-1 siRNA (closed circles). After transfection for 72 h, cells were treated with 0, 10, 100, or 250 nM okadaic acid. After incubation for an additional 4 h, cell lysates were electrophoresed and immunoblotted with antibodies to Akt or phospho-Thr308-Akt. Phospho-Thr308-Akt/Akt ratios were quantified using Multi Gauge, as described in Materials and Methods. (D) 293T cells were transfected with nonsilencing control siRNA (lane 1), Freud-1/Aki1-1 siRNA (lane 2), or Freud-1/Aki1-2 siRNA (lane 3). Cell lysates were incubated with an anti-PDK1 antibody-conjugated agarose. Then a PDK1 kinase assay was performed. PDK1 kinase activity was determined by quantifying the phospho-SGK level by Multi Gauge, as described in Materials and Methods. Error bars represent standard deviations of triplicate experiments. The PDK1 kinase activity of Freud-1/Aki1-1 or Freud-1/Aki1-2 siRNA-transfected cells was not significantly different (NS) from that of control siRNA-transfected cells (P > 0.25). IP, immunoprecipitated. (E) HT1080 cells were transfected with nonsilencing control siRNA (lanes 1 and 2) or Freud-1/Aki1-1 siRNA (lanes 3 and 4). Cells were treated with BSA or 50 ng/ml EGF, as described in Materials and Methods. Then an Akt or PDK1 kinase assay was performed. Error bars represent standard deviations of triplicate experiments. The Akt kinase activity of Freud-1/Aki1-1 siRNA-transfected and EGF-stimulated cells was significantly different from that of control siRNA-transfected and EGF-stimulated cells (*, P < 0.0004). The PDK1 kinase activity of Freud-1/Aki1-1 siRNA-transfected and EGF-stimulated cells was not significantly different (NS) from that of control siRNA-transfected and EGF-stimulated cells (P > 0.4). (F) 293T cells were transfected with nonsilencing control siRNA (lanes 1 and 2) or Freud-1/Aki1-1 siRNA (lanes 3 and 4). After transfection for 72 h, cytoplasmic (C) and nuclear (N) fractions were separated, electrophoresed, and immunoblotted with the indicated antibodies. (G) 293T cells were transfected with nonsilencing control siRNA (lane 1) or Freud-1/Aki1-1 siRNA (lane 2). After transfection for 72 h, cell lysates were electrophoresed and immunoblotted with the indicated antibodies.
There are two possibilities for the mechanism by which Freud-1/Aki1 regulates Akt phosphorylation at Thr308 and its kinase activity: first, Freud-1/Aki1 antagonizes phosphatase-mediated Akt dephosphorylation; second, Freud-1/Aki1 promotes PDK1-mediated Akt phosphorylation. We first examined the former possibility using a phosphatase inhibitor, okadaic acid. Okadaic acid was able to inactivate protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A), both of which are known to be associated with Akt dephosphorylation at Thr308 (40, 51). Adding okadaic acid dramatically upregulated the phospho-Thr308-Akt level in control siRNA-transfected cells but not in Freud-1/Aki1-1 siRNA-transfected cells (Fig. 3C). Because phosphatase inhibition by okadaic acid did not result in an increase in the phospho-Thr308-Akt level in Freud-1/Aki1-1 siRNA-transfected cells, we conclude that Freud-1/Aki1 gene silencing impairs the mechanism(s) of PDK1-mediated Akt phosphorylation.
Because PDK1 was reported to be constitutively active (11), we checked the change in PDK1 kinase activity after Freud-1/Aki1 gene silencing. We performed an in vitro kinase assay after Freud-1/Aki1 knockdown. Because PDK1 is known to phosphorylate SGK at the Thr256 residue (21), we measured the phospho-Thr256-SGK level as an indication of PDK1 kinase activity. Three independent experiments confirmed approximately equal PDK1 kinase activities after Freud-1/Aki1 knockdown (Fig. 3D). Because PDK1 was reported to phosphorylate by itself at Ser241 (11), we also estimated PDK1 kinase activity by checking the phospho-Ser241-PDK1 level in lysates. Consistent with the result of the in vitro PDK1 kinase assay, Freud-1/Aki1 knockdown did not affect the phospho-Ser241-PDK1 level (Fig. 3D).
Several growth factors trigger the activation of PI3K, which leads to Akt activation. Thus, we next examined the effect of Freud-1/Aki1 knockdown under the growth factor-stimulated condition. In this experiment, we used HT1080 cells, because they showed a low phospho-Akt level under the steady-state condition and were appropriate to examine the level of growth factor-induced Akt activation. EGF stimulation increased the phospho-Thr308-Akt levels and Akt kinase activity in control siRNA-treated cells. Meanwhile, Freud-1/Aki1 knockdown interfered with Akt phosphorylation at Thr308 and its activation induced by EGF (Fig. 3E). Under the same condition, Freud-1/Aki1 knockdown did not affect PDK1 kinase activity (Fig. 3E). Almost no increase was observed in PDK1 kinase activity in response to EGF. This result is consistent with the report that PDK1 is constitutively active (11).
Members of the FOXO family of transcription factors (FOXO1/FKHR, FOXO3a/FKHRL1, or FOXO4/AFX) exert their apoptosis-inducing or cell cycle-inhibitory functions by promoting the transcription of various proteins, including p27Kip1 (27). Akt-mediated phosphorylation of the FOXO family of transcription factors promotes their nuclear export, resulting in the loss of transcriptional activity (3). Akt inactivation by knockdown of Freud-1/Aki1 increased the amount of FOXO1 and FOXO3a in the nucleus (Fig. 3F) without affecting the total amount (data not shown). We suppose that the increase in FOXO1 and FOXO3a amounts in the nuclear fraction did not lead to the decrease in FOXO1 and FOXO3a amounts in the cytoplasmic fraction because the amount of nuclear protein is small compared with the amount of cytoplasmic protein. Consistent with the increase in the amount of the nuclear FOXO family of transcription factors, p27Kip1 expression was induced by Freud-1/Aki1 gene silencing (Fig. 3G). These data indicate that Freud-1/Aki1 positively regulates Akt phosphorylation and activation.
Freud-1/Aki1 is a scaffold for PDK1/Akt signaling.
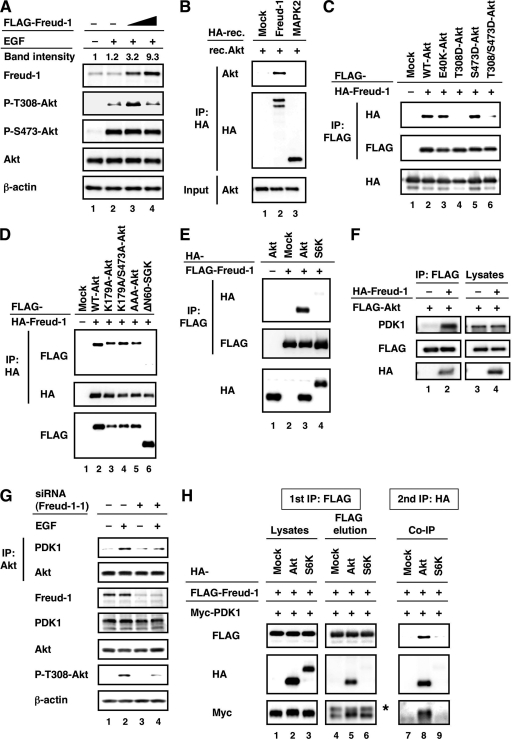
We showed that Freud-1/Aki1 is required for EGF-induced Akt phosphorylation at Thr308 (Fig. 3E); we therefore measured EGF signaling in the presence of different concentrations of Freud-1/Aki1. Freud-1/Aki1 expression modulated EGF-induced Akt phosphorylation at Thr308 in a concentration-dependent biphasic manner and had no effect on Akt phosphorylation at Ser473 (Fig. 4A). Quantitative studies of the MAPK cascade revealed that molecular scaffolds might either facilitate or inhibit signal propagation depending on their concentration (23). Previous studies reported both positive and negative regulation by KSR-1, depending on the level of protein expression (9, 22). Quantification of the band intensities of the anti-Freud-1/Aki1 immunoblot revealed that Freud-1/Aki1 acts as a positive effector when overexpressed at near physiological levels and acts as a negative effector when overexpressed much more than physiological levels (Fig. 4A). Therefore, Freud-1/Aki1 might function as a scaffold for EGFR/PDK1/Akt signaling.
FIG. 4.
Freud-1/Aki1 functions as a scaffold for the PDK1/Akt pathway. (A) HT1080 cells were transfected with increasing amounts of pFLAG-CMV-2-Freud-1/Aki1 plasmid. Cells were treated with BSA or 50 ng/ml EGF as described in Materials and Methods. Cell lysates were electrophoresed and immunoblotted with the indicated antibodies. The relative band intensities of the anti-Freud-1/Aki1 immunoblot were quantified and are indicated as described in Materials and Methods. (B) The HA-tagged recombinant Freud-1/Aki1 (lane 2) or MAPK2 (lane 3) protein was obtained by in vitro translation of a pHM6 vector encoding Freud-1/Aki1 or MAPK2. The HA-tagged protein was obtained from a pHM6 vector encoding no protein (Mock). The HA-tagged proteins were immunopurified with an anti-HA agarose and then incubated with recombinant Akt (rec.Akt). The reaction mixture (Input) or agarose-bound proteins (top and middle) were electrophoresed and immunoblotted with antibodies to HA or Akt. IP, immunoprecipitated. (C to E) 293T cells were transfected with the indicated plasmids encoding Freud-1/Aki1, WT Akt, Akt mutants, S6K, and ΔN60-SGK. Immunoprecipitated proteins (top and middle) and cell lysates (bottom) were electrophoresed and immunoblotted with antibodies to FLAG or to HA. (F) 293T cells were transfected with a pFLAG-CMV-2-WT-Akt plasmid (lanes 1 to 4) together with a pHM6 vector encoding no protein (lanes 1 and 3) or Freud-1/Aki1 (lanes 2 and 4). Immunoprecipitated proteins (lanes 1 and 2) and cell lysates (lanes 3 and 4) were electrophoresed and immunoblotted with the indicated antibodies. (G) HT1080 cells were transfected with nonsilencing control siRNA (lanes 1 and 2) or Freud-1/Aki1-1 siRNA (lanes 3 and 4). Cells were treated with BSA or 50 ng/ml EGF as described in Materials and Methods. Immunoprecipitated proteins and cell lysates were electrophoresed and immunoblotted with the indicated antibodies. (H) 293T cells were transfected with a pFLAG-CMV-2-Freud-1/Aki1 plasmid (lanes 1 to 9) and a pCMV3-PDK1 plasmid (lanes 1 to 9) together with a pHM6 vector encoding no protein (Mock), Akt, or S6K. The first immunoprecipitation was performed with an anti-FLAG antibody. The immunopurified FLAG-tagged protein was eluted with FLAG peptide, followed by a second immunoprecipitation with an anti-HA antibody. Protein samples from each step were electrophoresed and immunoblotted with the indicated antibodies. *, nonspecific band.
Freud-1/Aki1, as a scaffold protein, would form a complex with not only PDK1 but also Akt. To investigate the interaction of Freud-1/Aki1 with Akt, we employed pull-down analysis with in vitro-translated recombinant Freud-1/Aki1 and recombinant Akt. As shown in Fig. 4B, Akt directly bound to Freud-1/Aki1 but not to the control or MAPK2. We also discovered that Freud-1/Aki1 could form a complex with all Akt isoforms (Akt1, Akt2, and Akt3) in cells (data not shown). Furthermore, Freud-1/Aki1 could form a complex with a kinase-dead form of Akt (K179A-Akt), phosphorylation-deficient forms of Akt (K179A/S473A-Akt and AAA-Akt), a constitutively active form of Akt (E40K-Akt), and an S473 phosphorylation-mimic form of Akt (S473D-Akt) but not with T308 phosphorylation-mimic forms of Akt (T308D- and T308D/S473D-Akt) (Fig. 4C and D). These data suggest that formation of the complex depends on the level of phospho-Thr308-Akt. Freud-1/Aki1 may preferentially bind to Akt with a low phosphorylation level at Thr308 to enable PDK1 to phosphorylate Akt and dissociate from Akt after its phosphorylation by PDK1.
Scaffold proteins regulate signaling specificity. We therefore studied whether Freud-1/Aki1 could form a complex with other PDK1 substrates such as S6K or SGK. Immunoprecipitation studies revealed that Freud-1/Aki1 did not form a complex with S6K or SGK, even when both were overexpressed (Fig. 4D and E). To establish whether the effect of Freud-1/Aki1 knockdown is specific to Akt, we then examined the endogenous phosphorylation level of S6K at Thr252, PRK1 at Thr774, PRK2 at Thr816, and PKCζ at Thr410, which are known to be PDK1 substrates (5, 31). Immunoblot analysis suggested that Freud-1/Aki1 expression did not affect PDK1-mediated phosphorylation of these PDK1 substrates (data not shown).
If Freud-1/Aki1 is a scaffold for PDK1 and Akt, the interaction between them will be strengthened by Freud-1/Aki1 expression. In fact, Freud-1/Aki1 overexpression drastically increased the amount of endogenous PDK1 that formed a complex with FLAG-tagged Akt (Fig. 4F). Meanwhile, Freud-1/Aki1 knockdown suppressed the formation of endogenous PDK1-Akt complex in response to EGF (Fig. 4G). Moreover, to show that Freud-1/Aki1, PDK1, and Akt are present in the same protein complex, we employed sequential immunoprecipitation analysis. The results indicate that Freud-1/Aki1, PDK1, and Akt are present in the same protein complex, but S6K (a well-known PDK1 substrate) cannot form a ternary complex with Freud-1/Aki1 and PDK1 (Fig. 4H). Additionally, to further prove that the interaction between Freud-1/Aki1 and Akt is not mediated by PDK1, we investigated whether the binding of Freud-1/Aki1 to Akt could be correlated with the amount of PDK1. Overexpression of WT or kinase-dead PDK1 did not affect the interaction between Freud-1/Aki1 and Akt (data not shown).
These data indicate that Freud-1/Aki1 selectively interacts with Akt among the tested PDK1 substrates and promotes PDK1/Akt complex formation to positively regulate PDK1-mediated Akt phosphorylation.
Freud-1/Aki1 forms a multiprotein complex containing EGFR.
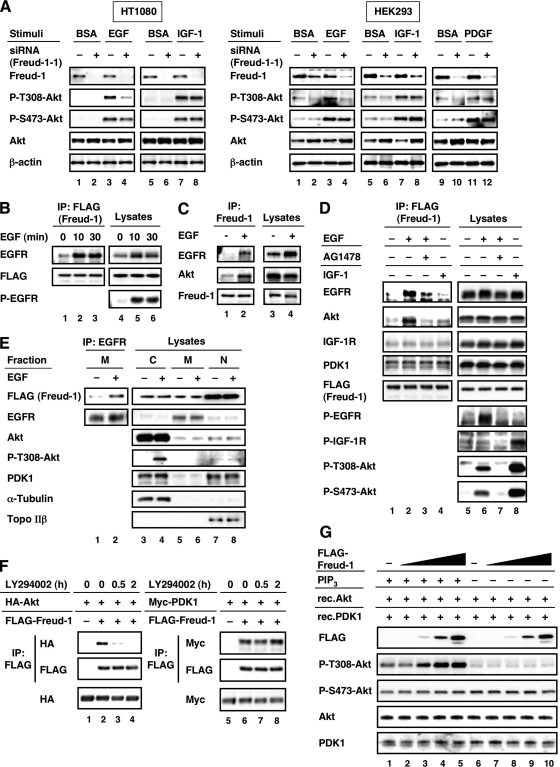
Freud-1/Aki1 is critical for EGF-induced Akt phosphorylation at Thr308 (Fig. 3E and 4A). To establish whether Freud-1/Aki1 could exhibit its ability in other growth factor signaling pathways besides EGF, we examined the effect of Freud-1/Aki1 knockdown on Akt phosphorylation in response to IGF-1 or PDGF. Interestingly, in contrast to EGF treatment, Freud-1/Aki1 knockdown did not affect the increase in the phospho-Thr308-Akt level after IGF-1 or PDGF treatment in HT1080 and HEK293 cells (Fig. 5A). The level of Akt protein expression was increased in Freud-1/Aki1 siRNA-treated HEK293 cells (Fig. 5A). The phenomenon was also observed in 293T cells (Fig. 3A). However, the increase in total Akt level was not observed in Freud-1/Aki1 siRNA-treated HT1080 cells (Fig. 3E and 5A). Because identical results were obtained in the HEK293, 293T, and HT1080 cell lines, these results seem to be independent of the Akt variation. These results suggest that Freud-1/Aki1 selectively plays a pivotal role in the regulation of PDK1-dependent Akt phosphorylation under EGF signaling.
FIG. 5.
Formation of EGFR/Freud-1/Aki1/PDK1/Akt complex in response to EGF but not IGF-1. (Left) (A) HT1080 cells were transfected with nonsilencing control siRNA (−) or Freud-1/Aki1-1 siRNA (+). Cells were treated with BSA, 50 ng/ml EGF, or 50 ng/ml IGF-1, as described in Materials and Methods. Cell lysates were electrophoresed and immunoblotted with the indicated antibodies. (Right) HEK293 cells were transfected with nonsilencing control siRNA (−) or Freud-1/Aki1-1 siRNA (+). Cells were treated with BSA, 50 ng/ml EGF or IGF-1, or 100 ng/ml PDGF, as described in Materials and Methods. Cell lysates were electrophoresed and immunoblotted with the indicated antibodies. (B) Stable Freud-1/Aki1-transfected HT1080 cells were treated with 50 ng/ml EGF for the indicated times, as described in Materials and Methods. Immunoprecipitated (IP) proteins (left) and cell lysates (right) were electrophoresed and immunoblotted with the indicated antibodies. (C) A431 cells were treated with BSA or 50 ng/ml EGF as described in Materials and Methods. Immunoprecipitated proteins (lanes 1 and 2) and cell lysates (lanes 3 and 4) were electrophoresed and immunoblotted with the indicated antibodies. (D) Stable Freud-1/Aki1-transfected HT1080 cells were treated with BSA, 50 ng/ml EGF, 50 ng/ml EGF plus 1 μM AG1478, or 50 ng/ml IGF-1 as described in Materials and Methods. Immunoprecipitated proteins (left) and cell lysates (right) were electrophoresed and immunoblotted with the indicated antibodies. (E) Stable Freud-1/Aki1-transfected HT1080 cells were treated with BSA or 50 ng/ml EGF, as described in Materials and Methods. Cytoplasmic (C), membrane (M), and nuclear (N) fractions were separated. Immunoprecipitated proteins (lanes 1 and 2) and cell lysates (lanes 3 to 8) were electrophoresed and immunoblotted with the indicated antibodies. (F) 293T cells were transfected with a pHM6-Akt plasmid (lanes 1 to 4) or a pCMV3-PDK1 plasmid (lanes 5 to 8) together with a pFLAG-CMV-2 vector encoding no protein (lanes 1 and 5) or Freud-1/Aki1 (lanes 2 to 4 and 6 to 8). Cells were treated with 50 μM LY294002 for the indicated times before cell harvest. Immunoprecipitated proteins (top and middle) and cell lysates (bottom) were electrophoresed and immunoblotted with the indicated antibodies. (G) 293T cells were transfected with increasing amounts of pFLAG-CMV-2-Freud-1/Aki1 plasmid. Then a PDK1 kinase assay with PIP3 was performed, as described in Materials and Methods.
In the case of the MAPK pathway, it has been reported that scaffold proteins promote the formation of complexes containing receptors (30, 50). Here we showed that Freud-1/Aki1 interacts with PDK1 and Akt and regulates Akt activation upon EGF signaling by functioning as a scaffold. Thus, we hypothesized that Freud-1/Aki1 may also associate with EGFR. Therefore, we established stable FLAG-WT Freud-1/Aki1-transfected HT1080 cells and assayed for protein complex formation using the cells. Because we obtained similar results both in HEK293 and in HT1080 cells (Fig. 5A) and HEK293 is resistant to Geneticin, we used HT1080 cells to establish stable cell lines. When stimulated by EGF, EGFR was coimmunoprecipitated with FLAG-Freud-1/Aki1 (Fig. 5B). The EGF-dependent Freud-1/Aki1-binding to EGFR was also found at an endogenous level in A431 cells (Fig. 5C). Furthermore, we discovered that the formation of complex between endogenous Akt and Freud-1/Aki1 was also induced by EGF treatment (Fig. 5C). We next examined whether these events were specific to EGF. EGF-induced formation of EGFR/Freud-1/Akt complex was interfered with by AG1478, a potent EGF tyrosine kinase inhibitor (Fig. 5D). Interestingly, FLAG-Freud-1/Aki1 did not form a complex with Akt or IGF-1 receptor (IGF-1R), even when cells were stimulated by IGF-1, even though IGF-I induced Akt phosphorylation at both Thr308 and Ser473 (Fig. 5D). Both EGF stimulation and IGF-1 stimulation did not affect the interaction of Freud-1/Aki1 with PDK1 (Fig. 5D). To investigate whether PDK1 interacts with Freud-1/Aki1 in both the cytoplasmic and membrane fractions and whether EGF stimulation induces membrane localization of the Freud-1/Aki1-PDK1 complex, we employed an immunoprecipitation assay using fractionated lysates (cytoplasmic, membrane, and nuclear fractions). Freud-1/Aki1 was expressed in all fractions (predominantly in the nuclear fraction), whereas PDK1 and Akt localized in the membrane fraction were barely detected regardless of EGF treatment (Fig. 5E). Freud-1/Aki1-PDK1 interaction in membrane fraction could not be detected (data not shown), which would be due to a small amount of PDK1 localized to membrane fraction. However, Freud-1/Aki1 could be detected in the anti-EGFR immunoprecipitant from the membrane fraction in an EGF-dependent manner (Fig. 5E). This result and the observation that PDK1 interacted with Freud-1/Aki1 in the absence or presence of EGF (Fig. 5D and data not shown) indicate that Freud-1/Aki1-PDK1 complex containing EGFR is formed in the membrane fraction when stimulated by EGF. Next we examined the mechanism by which Akt was recruited to Freud-1/Aki1 in response to EGF. It is known that PI3K products, including PIP3, mediate membrane recruitment of Akt, resulting in PDK1-induced Akt phosphorylation and activation (39). Therefore, we investigated the relationships of Freud-1/Aki1 with PI3K and the membrane localization of Akt. Freud-1/Aki1 knockdown did not change PIP3 production in response to EGF and IGF-1, indicating that Freud-1/Aki1 does not affect PI3K activity. Immunofluorescence analysis confirmed that the membrane localization of Akt was not affected by Freud-1/Aki1 knockdown (data not shown). However, we discovered that a PI3K inhibitor, LY294002, suppressed Freud-1/Aki1-Akt interaction but not Freud-1/Aki1-PDK1 interaction (Fig. 5F) and Freud-1/Aki1 accelerated the rate that Akt was activated by PDK1 in the presence of PIP3 (Fig. 5G). Freud-1/Aki1 did not modulate Akt phosphorylation at Thr308 in a concentration-dependent biphasic manner like Fig. 4A. Because there were an excess amount of recombinant PDK1 and Akt, immunoprecipitated Freud-1/Aki1 would not act as a negative effector but as a positive effector.
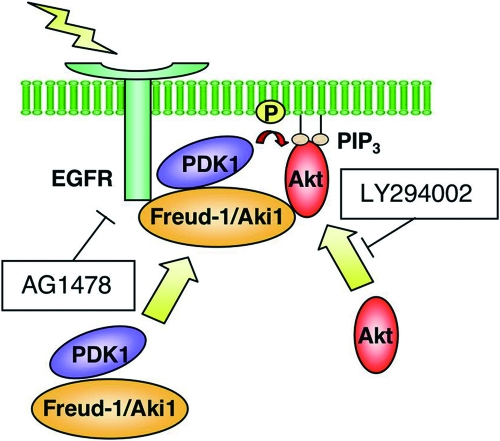
These observations suggest that Freud-1/Aki1 forms a multiprotein complex containing EGFR in response to EGF and selectively tethers PDK1/Akt signaling module to this receptor, in cooperation with PIP3 (a schematic model is shown in Fig. 7).
FIG. 7.
Schematic model of Freud-1/Aki1 functioning as a PDK1/Akt scaffold in EGF signaling. See the Discussion for further details.
Freud-1/Aki1 positively regulates cell survival and proliferation.
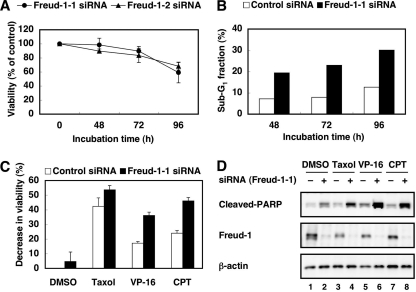
Because Freud-1/Aki1 positively regulated Akt kinase activity (Fig. 3), we thought Freud-1/Aki1 might be associated with cell survival and proliferation. The MTT assay revealed that Freud-1/Aki1 gene silencing resulted in a reduction of about 40% in the number of viable cells after 96 h of transfection compared with the control (Fig. 6A). Flow cytometric analysis demonstrated that Freud-1/Aki1 knockdown caused a two- to threefold increase in the apoptotic sub-G1 population compared with the control (Fig. 6B). In these experiments, we used 293T cells that showed the high level of Akt phosphorylation. The growth-suppressing and apoptosis-inducing effects of Freud-1/Aki1 siRNA were barely detectable in some cell lines that showed a low phospho-Akt level under the steady-state condition (e.g., HT1080 cells [data not shown]). Thus, Freud-1/Aki1 is important for cell survival and proliferation by positively regulating PDK1/Akt signaling.
FIG. 6.
Freud-1/Aki1 gene silencing induces apoptosis and enhances the sensitivity to antitumor drugs. (A) 293T cells were transfected with nonsilencing control siRNA, Freud-1/Aki1-1 siRNA (circles), or Freud-1/Aki1-2 siRNA (triangles). After transfection for 48, 72, or 96 h, the MTT assay was performed, as described in Materials and Methods. Error bars represent standard deviations of triplicate experiments. (B) 293T cells were transfected with nonsilencing control siRNA (open columns) or Freud-1/Aki1-1 siRNA (solid columns). After transfection for 48, 72, or 96 h, the number of apoptotic sub-G1 fractions was determined by flow cytometric analyses, as described in Materials and Methods. (C) 293T cells were transfected with nonsilencing control siRNA (open columns) or Freud-1/Aki1-1 siRNA (solid columns). After transfection for 72 h, cells were treated with DMSO, 10 μM Taxol, 10 μg/ml VP-16, or 10 μg/ml CPT. After incubation for an additional 24 h, the MTT assay was performed, as described in Materials and Methods. Error bars represent standard deviations of triplicate experiments. (D) 293T cells were transfected with nonsilencing control siRNA (−) or Freud-1/Aki1-1 siRNA (+). After transfection for 72 h, cells were treated with DMSO (lanes 1 and 2), 2.5 μM Taxol (lanes 3 and 4), 10 μg/ml VP-16 (lanes 5 and 6), or 10 μg/ml CPT (lanes 7 and 8). After incubation for an additional 24 h, cell lysates were electrophoresed and immunoblotted with the indicated antibodies.
We next addressed whether Freud-1/Aki1 knockdown affects sensitivity to chemotherapeutic drugs, such as Taxol, VP-16, and CPT. The MTT assay revealed that these chemotherapeutic drugs (excluding Taxol) synergistically decreased cell viability in cells transfected with Freud-1/Aki1 siRNA (Fig. 6C). Treatment of Freud-1/Aki1-1 siRNA-transfected cells with CPT decreased the number of viable cells in a dose-dependent manner (data not shown). Moreover, in the presence of CPT, Freud-1/Aki1 knockdown caused a fourfold increase in the apoptotic sub-G1 population compared with control siRNA transfectants (data not shown). To examine caspase activation in cells, we next tried to detect a PARP fragment generated by caspase-mediated cleavage. Transfection with Freud-1/Aki1 siRNA alone increased the amount of cleaved PARP fragment (Fig. 6D). Chemotherapeutic drugs, especially VP-16 and CPT, greatly enhanced caspase-mediated PARP cleavage in cells with Freud-1/Aki1 knocked down, suggesting that Freud-1/Aki1 knockdown increased sensitivity to the chemotherapeutic drugs (Fig. 6D). These results indicate that Freud-1/Aki1 is a critical regulator of PDK1/Akt signaling in the maintenance of cell survival and cell proliferation.
DISCUSSION
It is certain that PDK1 plays a central role in activating Akt and other protein kinases in the AGC subfamily (45) and then mediates intracellular signaling occurring in cell growth and survival. When stimulated by growth factors, PDK1 phosphorylates and activates Akt. Because PDK1 has been thought to be constitutively active and not to be further activated by growth factor stimulation (11), its function might be regulated by protein-protein interactions. However, little is known about the proteins that target PDK1 to Akt and contribute to cell survival and proliferation. Here, we identify Freud-1/Aki1 as a novel PDK1-interacting protein and report its role in EGFR/PDK1/Akt signaling. Three pieces of evidence support the assumption that Freud-1/Aki1 is a critical regulator of EGFR/PDK1/Akt signaling. First, Freud-1/Aki1 is required for EGF-mediated Akt phosphorylation and activation; second, Freud-1/Aki1 selectively forms an EGFR/Freud-1/PDK1/Akt complex in an EGF-dependent manner; and third, Freud-1/Aki1 knockdown downregulates Akt activity and increases cellular sensitivity to chemotherapeutic drugs.
Several PDK1-binding proteins have been reported in previous studies. However, these studies do not provide sufficient information to enable us to understand the mechanism by which PDK1 targets to Akt (12, 17, 28, 41). At least in T lymphocytes treated with glucocorticoids, Ft-1 enhances the apoptosis susceptibility by modulating the PDK1/Akt/GSK3/NF-ATc cascade (33). In our study, two-hybrid analyses revealed that Freud-1/Aki1 binds to PDK1 in E. coli (Fig. 1A). Because human PDK1 binds to human Freud-1/Aki1 expressed in bacteria (Fig. 1A), and purified recombinant PDK1 associates with in vitro-translated recombinant Freud-1/Aki1 (Fig. 1D), the interaction is direct.
The Freud-1/Aki1/CC2D1A gene family consists of two homologous genes, coding for Freud-1/Aki1/CC2D1A and Freud-2/Aki2/CC2D1B. Although the function of Freud-2/Aki2/CC2D1B is unknown, Freud-1/Aki1/CC2D1A has been shown to function as a transcriptional repressor of the serotonin-1A receptor gene that binds to a 5′ repressor element (32). The short isoform of Freud-1/Aki1/CC2D1A seems to be the predominant isoform in rodent cells, whereas the long isoform (WT Freud-1/Aki1 in our study) is more abundant in human cells, especially in the nuclear fraction. The nuclear localization of the long isoform was increased by inhibition of chromosome region maintenance 1/exportin 1-dependent nuclear export, indicating a dynamic regulation of Freud-1/Aki1 nuclear localization (34). Actually, Freud-1/Aki1 was expressed in both the cytosolic and nuclear fraction in 293T cells in our study (Fig. 3F and 5E). Freud-1/Aki1 may play a dual role depending on its localization: Freud-1/Aki1 functions as a transcriptional repressor in the nucleus, whereas it functions as a scaffold in the cytosol.
We found that the hydrophobic motif predicted to bind to PDK1 was present in Freud-1/Aki1 (amino acids 681 to 686 [FVRFDF]), although this motif, previously referred to as PIF (PDK1-interacting fragment) (4), was not required for Freud-1/Aki1 binding to PDK1 (Fig. 2B and data not shown). Because previous reports revealed that the PIF-binding pocket in PDK1 was not essential for the activation of Akt, unlike the activation of S6K or SGK (7), it is reasonable that this motif in Freud-1/Aki1 is not involved in Freud-1/Aki1-mediated activation of the PDK1/Akt pathway. Freud-1/Aki1 contains four DM14 domains and one C2 domain (Fig. 2A). The DM14-4 domain appears to be essential for the binding of Freud-1/Aki1 to PDK1 (Fig. 2B and C). In patients with ARNSMR, Freud-1/Aki1 protein was reported to be a DM14-4- and C2 domain-lacking mutant (6), suggesting the significance of Freud-1/Aki1 function to activate the PDK1/Akt pathway in the pathogenesis of such diseases. The linkage between the deletion mutation in the Freud-1/Aki1 gene and ARNSMR and the broad distribution of Freud-1/Aki1 RNA and protein in the brain suggest the involvement of Freud-1/Aki1 in brain development and cognitive function (6, 32). Akt is not only expressed in many peripheral tissues, but also highly expressed in the central nervous system. In the central nervous system, activation of Akt by growth factor stimulation has been shown to play an important role in regulating neuronal cell survival and synaptic plasticity (16, 26, 38, 48). Akt can phosphorylate GluR1, a subunit of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subtype glutamate receptors and then mediates AMPA receptor insertion during the expression of the homosynaptic hippocampal CA1 long-term potentiation (LTP), a well-characterized synaptic plasticity that has been proposed as a cellular substrate for learning and memory (25). Akt also phosphorylates the type A gamma-aminobutyric acid receptor (GABAAR). This phosphorylation induces the translocation of intracellular receptors to the plasma membrane, thereby increasing the receptor-mediated synaptic transmission in neurons. However, It is currently unclear which aspect of Freud-1/Aki1 function may be important: as a scaffold for the PDK1/Akt pathway or a transcriptional repressor of serotonin-1A receptor and dopamine-D2 receptor expression (32, 36). The deletion mutant of Freud-1/Aki1 in ARNSMR also lacks domains for its repressor function (32).
Akt is phosphorylated at the Thr308 residue in the activation loop by PDK1 and dephosphorylated by PP1 or PP2A, the Akt-Thr308 phosphatases (40, 51). Akt is also phosphorylated at the Ser473 residue in the hydrophobic motif near the COOH terminus by PDK2 and dephosphorylated by PHLPP (18). Several investigators have suggested that PDK2 is a rictor-mTOR complex (rapamycin-insensitive mTOR complex 2; mTORC2), Akt itself, or other kinases (15). Our siRNA knockdown studies showed that Freud-1/Aki1 specifically promoted Akt phosphorylation at Thr308 but not at Ser473 (Fig. 3A). Therefore, we focused on the relationship between Freud-1/Aki1 and PDK1 (or the Akt-Thr308 phosphatases), but not mTORC2. If Freud-1/Aki1 increased the level of phospho-Thr308-Akt by antagonizing the Akt-Thr308 phosphatases, Freud-1/Aki1 gene silencing might result in activation of the phosphatases. In this case, Freud-1/Aki1-1 siRNA-transfected cells would be sensitive to okadaic acid, which selectively inactivates PP1 and PP2A. However, the Freud-1/Aki1-1 siRNA transfectants exhibited resistance to okadaic acid (Fig. 3C). These facts indicate that Freud-1/Aki1 regulates PDK1-dependent Akt phosphorylation.
A remarkable feature of signaling transduction is that individual pathways depend on a group of proteins referred to as scaffolds (30). In the case of the PDK1/Akt pathway, little is known about the scaffold proteins. Freud-1/Aki1 is required for PDK1-mediated Akt phosphorylation and the interaction of PDK1 with Akt. Freud-1/Aki1 expression induced formation of a PDK1/Akt complex and regulated Akt activation in a concentration-dependent biphasic manner. In addition, Freud-1/Aki1 knockdown suppressed the PDK1-Akt interaction under EGF-stimulated conditions (Fig. 4G). These observations indicate that Freud-1/Aki1 is a scaffold between them. As the scaffold, Freud-1/Aki1 specifically and effectively activates Akt among several PDK1 substrates. Moreover, in the case of the MAPK pathway, it has been reported that scaffold proteins form complexes with receptors (30, 50). An interesting observation is that increased interactions of Freud-1/Aki1 with EGFR and Akt occurred after EGF stimulation, whereas interactions of Freud-1/Aki1 with IGF-1R and Akt were not detected after IGF-1 stimulation. To our knowledge, there has been no report on scaffold proteins forming a complex with EGFR in the PDK1/Akt pathway. Our results show for the first time that Freud-1/Aki1 forms the PDK1/Akt complex with EGFR (Fig. 7). Probably, other scaffold proteins may exist and be involved in the IGF-1R/PDK1/Akt pathway.
It has been well established that PI3K products including PIP3 mediate membrane recruitment of Akt and PDK1, resulting in PDK1-induced Akt phosphorylation and activation (39). It has also been reported that the interaction of PDK1 with its substrates was regulated by other interacting protein (28). Therefore, the relationship between PIP3-PDK1/Akt association and Freud-1/Aki1-PDK1/Akt association in Akt activation should be examined. Freud-1/Aki1 knockdown did not affect the membrane localization of Akt and PIP3 production in response to EGF and IGF-1 (data not shown). We could not detect the localization of endogenous PDK1 or Freud-1/Aki1 by immunofluorescence analysis because we could not obtain the appropriate antibodies. However, interestingly, treatment of the cells with the PI3K inhibitor LY294002 suppressed the Freud-1/Aki1-Akt interaction but not the Freud-1/Aki1-PDK1 interaction (Fig. 5F) and Freud-1/Aki1 enhanced Akt phosphorylation by PDK1 in the presence of PIP3 (Fig. 5G), indicating that PI3K-dependent conformational change in Akt and/or membrane recruitment of Akt (10) might be associated with formation of the Freud-1/Aki1-Akt complex. We therefore suppose that PIP3 facilitates Freud-1/Aki1 binding to Akt near EGFR, resulting in the PDK1-mediated phosphorylation of Akt (Fig. 7). Freud-1/Aki1-PDK1 interaction in the membrane fraction was not detected, probably due to the small amount of it (Fig. 5E and data not shown). However, taking it into consideration that Freud-1/Aki1 constitutively binds to PDK1 and forms a complex with EGFR in the membrane fraction in an EGF-dependent manner (Fig. 5D and E and data not shown), our model seems to be appropriate (Fig. 7).
The PDK1/Akt pathway is certainly important for cell survival and proliferation, and it contributes to tumorigenesis. For example, amplification of the Akt gene occurs in some tumors and increased Akt kinase activity contributes to tumor progression in prostate cancer (8, 45, 47). Loss of the tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is common in tumors, and its loss constitutively activates Akt (42). Therefore, this pathway is considered to be an attractive target for cancer chemotherapy. However, several adverse effects could result from the inhibition of other Akt-regulated cellular processes such as glucose metabolism in which inhibition of Akt potentially leads to a diabetes-like syndrome (24). Several kinase inhibitors that target PDK1 or Akt have been developed (19), but a clinically useful agent has not yet emerged. In cancer cell lines that have elevated levels of Akt phosphorylation, knockdown of Freud-1/Aki1 by siRNA reduced their viability and induced apoptosis (Fig. 6A and B and data not shown). In addition, Freud-1/Aki1 siRNA synergistically enhanced sensitivity toward chemotherapeutic agents, such as VP-16 or CPT, in tumor cells (Fig. 6C and D). Combining antitumor agents with Freud-1/Aki1 siRNA or the inhibitor(s) of EGFR/Freud-1/PDK1/Akt complex formation is expected to enhance their antitumor effects in vivo. Because our data indicate that Freud-1/Aki1 selectively promotes EGF-stimulated Akt activation, targeting Freud-1/Aki1 for cancer chemotherapy may enable us to suppress tumor growth without serious adverse effects.
In summary, Freud-1/Aki1 is the first identified receptor-selective scaffold protein of the PDK1/Akt pathway in EGF signaling. Because Freud-1/Aki1 selectively activates the PDK1/Akt pathway by forming a multiprotein complex containing EGFR and promotes cell survival, it will be a promising and attractive target for cancer chemotherapy.
Acknowledgments
We thank Emi Tokuda for technical advice on two-hybrid screening.
This study was supported in part by special grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan 17016012 and 18015008 (to T.T. and N.F.). N.F. was also supported by the National Project on Protein Structural and Functional Analyses from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by the Araki Memorial Foundation for Medical and Biochemical Research; by the Novartis Foundation (Japan) for the Promotion of Science; and by the Vehicle Racing Commemorative Foundation.
Footnotes
Published ahead of print on 28 July 2008.
REFERENCES
- 1.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 7261-269. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. E., J. Coadwell, L. R. Stephens, and P. T. Hawkins. 1998. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr. Biol. 8684-691. [DOI] [PubMed] [Google Scholar]
- 3.Arden, K. C., and W. H. Biggs III. 2002. Regulation of the FoxO family of transcription factors by phosphatidylinositol-3 kinase-activated signaling. Arch. Biochem. Biophys. 403292-298. [DOI] [PubMed] [Google Scholar]
- 4.Balendran, A., A. Casamayor, M. Deak, A. Paterson, P. Gaffney, R. Currie, C. P. Downes, and D. R. Alessi. 1999. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr. Biol. 9393-404. [DOI] [PubMed] [Google Scholar]
- 5.Balendran, A., R. Currie, C. G. Armstrong, J. Avruch, and D. R. Alessi. 1999. Evidence that 3-phosphoinositide-dependent protein kinase-1 mediates phosphorylation of p70 S6 kinase in vivo at Thr-412 as well as Thr-252. J. Biol. Chem. 27437400-37406. [DOI] [PubMed] [Google Scholar]
- 6.Basel-Vanagaite, L., R. Attia, M. Yahav, R. J. Ferland, L. Anteki, C. A. Walsh, T. Olender, R. Straussberg, N. Magal, E. Taub, V. Drasinover, A. Alkelai, D. Bercovich, G. Rechavi, A. J. Simon, and M. Shohat. 2006. The CC2D1A, a member of a new gene family with C2 domains, is involved in autosomal recessive non-syndromic mental retardation. J. Med. Genet. 43203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biondi, R. M., A. Kieloch, R. A. Currie, M. Deak, and D. R. Alessi. 2001. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 204380-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brazil, D. P., J. Park, and B. A. Hemmings. 2002. PKB binding proteins. Getting in on the Akt. Cell 111293-303. [DOI] [PubMed] [Google Scholar]
- 9.Cacace, A. M., N. R. Michaud, M. Therrien, K. Mathes, T. Copeland, G. M. Rubin, and D. K. Morrison. 1999. Identification of constitutive and Ras-inducible phosphorylation sites of KSR: implications for 14-3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol. Cell. Biol. 19229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calleja, V., D. Alcor, M. Laguerre, J. Park, B. Vojnovic, B. A. Hemmings, J. Downward, P. J. Parker, and B. Larijani. 2007. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 5e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casamayor, A., N. A. Morrice, and D. R. Alessi. 1999. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem. J. 342287-292. [PMC free article] [PubMed] [Google Scholar]
- 12.Chun, J., T. Kwon, E. J. Lee, S. Hyun, S. K. Hong, and S. S. Kang. 2005. The subcellular localization of 3-phosphoinositide-dependent protein kinase is controlled by caveolin-1 binding. Biochem. Biophys. Res. Commun. 326136-146. [DOI] [PubMed] [Google Scholar]
- 13.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378785-789. [DOI] [PubMed] [Google Scholar]
- 14.Currie, R. A., K. S. Walker, A. Gray, M. Deak, A. Casamayor, C. P. Downes, P. Cohen, D. R. Alessi, and J. Lucocq. 1999. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem. J. 337575-583. [PMC free article] [PubMed] [Google Scholar]
- 15.Dong, L. Q., and F. Liu. 2005. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am. J. Physiol. Endocrinol. Metab. 289E187-E196. [DOI] [PubMed] [Google Scholar]
- 16.Dudek, H., S. R. Datta, T. F. Franke, M. J. Birnbaum, R. Yao, G. M. Cooper, R. A. Segal, D. R. Kaplan, and M. E. Greenberg. 1997. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275661-665. [DOI] [PubMed] [Google Scholar]
- 17.Fujita, N., S. Sato, A. Ishida, and T. Tsuruo. 2002. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J. Biol. Chem. 27710346-10353. [DOI] [PubMed] [Google Scholar]
- 18.Gao, T., F. Furnari, and A. C. Newton. 2005. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol. Cell 1813-24. [DOI] [PubMed] [Google Scholar]
- 19.Hennessy, B. T., D. L. Smith, P. T. Ram, Y. Lu, and G. B. Mills. 2005. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 4988-1004. [DOI] [PubMed] [Google Scholar]
- 20.Katayama, K., N. Fujita, and T. Tsuruo. 2005. Akt/protein kinase B-dependent phosphorylation and inactivation of WEE1Hu promote cell cycle progression at G2/M transition. Mol. Cell. Biol. 255725-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi, T., and P. Cohen. 1999. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J. 339319-328. [PMC free article] [PubMed] [Google Scholar]
- 22.Kortum, R. L., and R. E. Lewis. 2004. The molecular scaffold KSR1 regulates the proliferative and oncogenic potential of cells. Mol. Cell. Biol. 244407-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levchenko, A., J. Bruck, and P. W. Sternberg. 2000. Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc. Natl. Acad. Sci. USA 975818-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo, Y., A. R. Shoemaker, X. Liu, K. W. Woods, S. A. Thomas, R. de Jong, E. K. Han, T. Li, V. S. Stoll, J. A. Powlas, A. Oleksijew, M. J. Mitten, Y. Shi, R. Guan, T. P. McGonigal, V. Klinghofer, E. F. Johnson, J. D. Leverson, J. J. Bouska, M. Mamo, R. A. Smith, E. E. Gramling-Evans, B. A. Zinker, A. K. Mika, P. T. Nguyen, T. Oltersdorf, S. H. Rosenberg, Q. Li, and V. L. Giranda. 2005. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol. Cancer Ther. 4977-986. [DOI] [PubMed] [Google Scholar]
- 25.Malenka, R. C., and R. A. Nicoll. 1999. Long-term potentiation—a decade of progress? Science 2851870-1874. [DOI] [PubMed] [Google Scholar]
- 26.Man, H. Y., Q. Wang, W. Y. Lu, W. Ju, G. Ahmadian, L. Liu, S. D'Souza, T. P. Wong, C. Taghibiglou, J. Lu, L. E. Becker, L. Pei, F. Liu, M. P. Wymann, J. F. MacDonald, and Y. T. Wang. 2003. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron 38611-624. [DOI] [PubMed] [Google Scholar]
- 27.Medema, R. H., G. J. Kops, J. L. Bos, and B. M. Burgering. 2000. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404782-787. [DOI] [PubMed] [Google Scholar]
- 28.Michel, J. J., I. K. Townley, K. L. Dodge-Kafka, F. Zhang, M. S. Kapiloff, and J. D. Scott. 2005. Spatial restriction of PDK1 activation cascades by anchoring to mAKAPalpha. Mol. Cell 20661-672. [DOI] [PubMed] [Google Scholar]
- 29.Morrison, D. K. 2001. KSR: a MAPK scaffold of the Ras pathway? J. Cell Sci. 1141609-1612. [DOI] [PubMed] [Google Scholar]
- 30.Morrison, D. K., and R. J. Davis. 2003. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 1991-118. [DOI] [PubMed] [Google Scholar]
- 31.Newton, A. C. 2003. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 370361-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ou, X. M., S. Lemonde, H. Jafar-Nejad, C. D. Bown, A. Goto, A. Rogaeva, and P. R. Albert. 2003. Freud-1: a neuronal calcium-regulated repressor of the 5-HT1A receptor gene. J. Neurosci. 237415-7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remy, I., and S. W. Michnick. 2004. Regulation of apoptosis by the Ft1 protein, a new modulator of protein kinase B/Akt. Mol. Cell. Biol. 241493-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogaeva, A., and P. R. Albert. 2007. The mental retardation gene CC2D1A/Freud-1 encodes a long isoform that binds conserved DNA elements to repress gene transcription. Eur. J. Neurosci. 26965-974. [DOI] [PubMed] [Google Scholar]
- 35.Rogaeva, A., K. Galaraga, and P. R. Albert. 2007. The Freud-1/CC2D1A family: transcriptional regulators implicated in mental retardation. J. Neurosci. Res. 852833-2838. [DOI] [PubMed] [Google Scholar]
- 36.Rogaeva, A., X. M. Ou, H. Jafar-Nejad, S. Lemonde, and P. R. Albert. 2007. Differential repression by freud-1/CC2D1A at a polymorphic site in the dopamine-D2 receptor gene. J. Biol. Chem. 28220897-20905. [DOI] [PubMed] [Google Scholar]
- 37.Salahshor, S., and J. R. Woodgett. 2005. The links between axin and carcinogenesis. J. Clin. Pathol. 58225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanna, P. P., M. Cammalleri, F. Berton, C. Simpson, R. Lutjens, F. E. Bloom, and W. Francesconi. 2002. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J. Neurosci. 223359-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato, S., N. Fujita, and T. Tsuruo. 2004. Involvement of 3-phosphoinositide-dependent protein kinase-1 in the MEK/MAPK signal transduction pathway. J. Biol. Chem. 27933759-33767. [DOI] [PubMed] [Google Scholar]
- 40.Sato, S., N. Fujita, and T. Tsuruo. 2000. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 9710832-10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato, S., N. Fujita, and T. Tsuruo. 2002. Regulation of kinase activity of 3-phosphoinositide-dependent protein kinase-1 by binding to 14-3-3. J. Biol. Chem. 27739360-39367. [DOI] [PubMed] [Google Scholar]
- 42.Sellers, W. R., and D. E. Fisher. 1999. Apoptosis and cancer drug targeting. J. Clin. Investig. 1041655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka, H., N. Fujita, and T. Tsuruo. 2005. 3-Phosphoinositide-dependent protein kinase-1-mediated IkappaB kinase beta (IkkB) phosphorylation activates NF-kappaB signaling. J. Biol. Chem. 28040965-40973. [DOI] [PubMed] [Google Scholar]
- 44.Toker, A., and L. C. Cantley. 1997. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387673-676. [DOI] [PubMed] [Google Scholar]
- 45.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346561-576. [PMC free article] [PubMed] [Google Scholar]
- 46.Vanhaesebroeck, B., S. J. Leevers, G. Panayotou, and M. D. Waterfield. 1997. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem. Sci. 22267-272. [DOI] [PubMed] [Google Scholar]
- 47.Vivanco, I., and C. L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2489-501. [DOI] [PubMed] [Google Scholar]
- 48.Wang, Q., L. Liu, L. Pei, W. Ju, G. Ahmadian, J. Lu, Y. Wang, F. Liu, and Y. T. Wang. 2003. Control of synaptic strength, a novel function of Akt. Neuron 38915-928. [DOI] [PubMed] [Google Scholar]
- 49.Whitmarsh, A. J. 2006. The JIP family of MAPK scaffold proteins. Biochem. Soc Trans. 34828-832. [DOI] [PubMed] [Google Scholar]
- 50.Willard, M. D., F. S. Willard, X. Li, S. D. Cappell, W. D. Snider, and D. P. Siderovski. 2007. Selective role for RGS12 as a Ras/Raf/MEK scaffold in nerve growth factor-mediated differentiation. EMBO J. 262029-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu, W., X. Yuan, Y. J. Jung, Y. Yang, A. Basso, N. Rosen, E. J. Chung, J. Trepel, and L. Neckers. 2003. The heat shock protein 90 inhibitor geldanamycin and the ErbB inhibitor ZD1839 promote rapid PP1 phosphatase-dependent inactivation of AKT in ErbB2 overexpressing breast cancer cells. Cancer Res. 637777-7784. [PubMed] [Google Scholar]