Abstract
Crg1 and Crg2 are regulators of G-protein signaling homologs found in the human fungal pathogen Cryptococcus neoformans. Crg1 negatively regulates pheromone responses and mating through direct inhibition of Gα subunits Gpa2 and Gpa3. It has also been proposed that Crg2 has a role in mating, as genetic crosses involving Δcrg2 mutants resulted in formation of hyperfilaments. We found that mutation of Gpa2 and Gpa3 partially suppressed the hyperfilamentation, mutation of Gpa3 alleviated Δcrg2-specfic cell swelling, and mutation of the mitogen-activated protein kinase Cpk1 blocked both processes. These findings indicate that Gpa2 and Gpa3 function downstream of Crg2 and that Gpa3 is also epistatic to Crg2 in a Cpk1-dependent morphogenesis process linked to mating. Significantly, we found that Δcrg2 mutants formed enlarged capsules that mimic cells expressing a constitutively active GPA1(Q284L) allele and that the levels of intracellular cyclic AMP (cAMP) were also elevated, suggesting that Crg2 also negatively regulates the Gpa1-cAMP signaling pathway. We further showed that Crg2 interacted with Gpa3 and Gpa1, but not Gpa2, in a pulldown assay and that Crg2 maintained a higher in vitro GTPase-activating protein activity toward Gpa3 and Gpa1 than to Gpa2. Finally, we found that dysregulation of cAMP due to the Crg2 mutation attenuated virulence in a murine model of cryptococcosis. Taken together, our study reveals Crg2 as an RGS (regulator of G-protein signaling) protein of multiregulatory function, including one that controls mating distinctly from Crg1 and one that serves as a novel inhibitor of Gpa1-cAMP signaling.
Regulators of G-protein signaling (RGS) are a family of proteins that share the highly conserved RGS domain of about 125 amino acid residues and play an important role in cell communication among eukaryotic organisms. The RGS domain allows it to interact specifically with the cognate Gα to promote signaling inactivation through acceleration of Gα-bound GTP to GDP hydrolysis (7, 8, 10, 15, 21, 34). In humans, more than 20 RGS proteins have been reported, which are also known to have at least 20 Gα subunits, 6 Gβ subunits, and 11 Gγ subunits (20, 31, 43). In the lower eukaryotes, such as the budding yeast Saccharomyces cerevisiae that contains two Gα subunits, one Gβ subunit, and one Gγ subunit, four RGS and RGS-like proteins, including Sst2, Rgs2, Rax1, and Mdm1, have been described. Sst2 is an archetypical RGS protein that functions as a GTPase-activating protein (GAP) to inhibit Gα Gpa1 and promote pheromone desensitization (3, 9), whereas Rgs2, identified through a screen for multicopy suppressors of Gpa2-mediated glucose-induced loss of heat resistance, was shown to act as a negative regulator of Gpa2 and glucose-induced cyclic AMP (cAMP) signaling (38). Although Rax1 (return to axial) and Mdm1, a multidomain-containing protein similar to mammalian sorting nexin 13, contain the conserved RGS and RGS-like domains, there is no evidence demonstrating their direct involvement in G-protein signaling (13, 27, 28).
The encapsulated yeast-like fungus Cryptococcus neoformans is a human pathogen primarily infecting immunocompromised individuals to cause life-threatening meningoencephalitis (see the review by Mitchell and Perfect [30]). Previous studies have indicated that there are three Gα subunits (Gpa1, Gpa2, and Gpa3), one Gβ subunit (Gpb1), and two Gγ subunits (Gpg1 and Gpg2) in this fungus (18, 25, 37, 42). Gpa1 governs a conserved cAMP-dependent signaling pathway that is also linked to the production of virulence traits, such as melanin production, capsule formation, and virulence (1, 2, 11, 44). Gpa2 and Gpa3 exhibit shared and distinct functions to collectively regulate pheromone responses, mating, and virulence (18, 25). In addition, Gpa2 regulates mating through a conserved mechanism by forming a classic heterotrimeric G-protein complex with Gβ Gpb1 and Gγ Gpg1 or Gpg2, whereas the mechanism by which Gpa3 regulates pheromone production and is required for conjugation tube formation remains unclear (25). We have previously characterized an RGS protein homolog, Crg1 (for cryptococcal regulator of G-protein signaling), as a key regulator of mating and virulence, and have also provided evidence demonstrating that Crg1 functions as a GAP for Gpa2 and Gpa3 (25, 40).
The completed C. neoformans genome-sequencing project has facilitated the identification of two additional RGS and RGS-like proteins, Crg2 (GenBank accession no. XP_570417) and Crg3 (GenBank accession no. XP_569481). A role for Crg2 in mating has been inferred by the observation that a Δcrg2 mutant produced highly branched and elongated filaments (hyperfilaments) in genetic crosses, and Crg2 interacted with the pheromone receptor homolog Ste3α in a split-ubiquitin two-hybrid assay (18). In contrast, the role of Crg3, a homolog of human sorting nexin 13 and S. cerevisiae Mdm1, has not been established.
Given that Crg1 is an RGS protein specific for Gpa2- and Gpa3-mediated pheromone responses and mating, and an RGS protein specific for Gpa1 has been found, we hypothesize that Crg2 regulates the Gpa1-cAMP signaling pathway by inhibiting Gpa1. In this study, we provide genetic and biochemical evidence demonstrating that Crg2 indeed downregulates Gpa1-cAMP signaling as a GAP of Gpa1, in addition to its regulatory role in pheromone signaling. Moreover, we provide evidence indicating that dysregulated cAMP signaling due to a Crg2 mutation resulted in significant attenuation of fungal virulence. Our studies shed new insights in understanding the complexity of distinct G-protein signaling pathways and their cross talk in C. neoformans.
MATERIALS AND METHODS
Strains, media, and plasmids.
C. neoformans var. grubii MATα strains H99 and F99 (H99 ura5) and MATa strains KN99a and F99a (KN99a ura5) were as described previously (25, 32, 40). These and additional strains used in this study are listed in Table S1 in the supplemental material. The genotypes of all strains were verified by PCR and Southern blot hybridization analyses following standard protocols (35). The DNA primers used in this study are listed in Table S2 in the supplemental material.
Yeast extract-peptone-dextrose (YPD), synthetic medium (SD), 10% V8 agar (pH 5.0) for mating, filament agar for conjugation tube formation, Niger seed agar for melanin production, and Dulbecco's modified Eagle's medium (DMEM) with calcium bicarbonate for capsule induction were prepared following standard protocols or as described previously (40, 42).
The Crg2 coding domain was verified by sequencing the 5′- and 3′-end rapid amplification of cDNA end products and products of reverse transcription-PCR. The full-length cDNA was cloned into plasmid pGADT7 to be used in a yeast two-hybridization assay. cDNA and cDNA specific for the RGS domain (Rgs2) were inserted into plasmid pET41a (+) for the production of Crg2-GST (glutathione-S-transferase) fusion proteins in Escherichia coli.
Construction of Δcrg2 single and double mutants.
The CRG2 gene was identified from the C. neoformans var. grubii-specific (H99) database (http://cneo.genetics.duke.edu/), and the sequence was verified by DNA sequencing. Primers PW359 and PW181 were used to obtain the full-length gene, while primers PW402 and PW403 were used to amplify the RGS-specific Rgs2 domain. The Δcrg2::URA5 mutant allele was obtained by incorporating a SmaI restriction site in the coding region by use of primers PW227 and PW228 and by insertion of the URA5 gene into the site through blunt-end ligation. To make certain that the genotype of Δcrg2 is not due to the marker genes used, a Δcrg2::NAT knockout allele containing a nourseothricin resistance marker (NAT [29]) was also created by a similar method. The mutant alleles were introduced into the recipient strains by biolistic transformation (36). The crg2::NAT allele was also introduced into Δgpa1::URA5, Δgpa2::URA5, Δgpa3::URA5, Δgpa2::URA5 Δgpa3::URA5, Δpk1a::URA5, and Δcpk1::URA5 strains to obtain double mutant strains.
The Δcrg2::URA5 mutant strain was complemented by reintroducing a 2.7-kb fragment containing the full-length wild-type CRG2 gene, including a 1.1-kb region preceding the start codon containing the assumptive promoter region. A constitutively active Gpa1 allele, GPA1(Q284L), which corresponds to constitutively active S. cerevisiae Gpa2(Q300L), was amplified by PCR incorporating primers JH12499 and JH12500 (44). Mutants and complemented strains were screened by PCR and use of gene-specific primers, and confirmation was done by Southern blot hybridization analysis.
Phenotypic characterization.
Cells were streaked onto YPD, yeast nitrogen base (YNB), synthetic low ammonium dextrose (SLAD), and filament agar overnight, and growth was monitored daily by microscopic observation. Confrontation tests were performed in petri dishes and on glass coverslips on which 2 drops of melted filament agar were first placed. Formation of conjugation tubes was observed daily, and representative sections were photographed between 48 and 60 h of incubation by use of an Olympus digital camera attached to the microscope. Mating assays were performed by patching a mixture of α and a cells on standard V8 agar, and plates were incubated in darkness at room temperature for up to 2 weeks.
Melanin was induced by growing cells on standard Niger seed agar at 30°C for 48 h, and the polysaccharide capsule was induced by incubating cells in DMEM with glucose and calcium bicarbonate at 30°C for 48 h as described previously (39, 40). Virulence tests were performed using 4- to 6-week-old female A/JCR mice, and fungal challenge doses were administrated by inhalation. Mouse survival was analyzed by the Kaplan-Meier method using Prism 4.0 software (Graphpad Software, Inc.) as described previously (40). Mice were sacrificed through CO2 asphyxiation at predetermined time points or when moribund, and organ tissues were collected. The tissue was homogenized in a Dounce homogenizer in phosphate-buffered saline containing 1 μg/ml chloramphenicol, and the homogenates were stained with India ink to visualize C. neoformans cells under the microscope. Animal testing was approved by the institutional animal care and use committee.
Protein-protein interaction and GAP activity assays.
Recombinant fusion proteins were expressed in either S. cerevisiae or E. coli and purified using affinity chromatography as described previously (25). Two versions of the recombinant Crg2 proteins were produced: an RGS-domain-only polypeptide (Rgs2) used in the protein pulldown assay and a full-length protein (Crg2) used for assaying GAP activities. The fusion proteins were verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting by use of the anti-Xpress and anti-GST antibodies as described previously (25). Protein binding conditions and detection methods were similar to those described previously (25). Methods of measuring the rates of Crg2 accelerating the hydrolysis of GTP-bound Gα to GDP through the steady-state method were similar to those of previous studies (25, 38). Briefly, the His6-tagged Gpa1, Gpa2, or Gpa3 proteins (200 nM) and [γ-32P]GTP (7,500 cpm/pmol; final concentration of 2 μM) were mixed in 2× GTP buffer (50 mM Na-HEPES [pH 8.0], 1 mM dithiothreitol, 1 mM EDTA, 2 μM [γ-35S]GTP, and 10 mM MgCl2) for 2 min at 25°C. Crg2 (GST-Crg2) was added, and partial reaction mixtures were withdrawn at intervals of 2, 4, 6, and 8 min. The reaction was done in triplicate. The withdrawn samples were quickly mixed with 5% charcoal (50 mM NaH2PO4 [pH 2.0]) on ice and precipitated by centrifugation at 13,000 rpm for 15 min. Supernatants (400 μl) were transferred to scintillation vials, and the release of radioactive phosphate was measured using a liquid scintillation counter (Cobra II; Packard). The mean values were plotted with error bars representing the standard errors of the means. Statistical significance was calculated by the use of Student's t test (Prism 4.0, Graphpad Software, Inc.). Assays were repeated at least twice.
RESULTS
Crg2 regulates mating differently than Crg1 does and plays a role in Gpa3/Cpk1-dependent morphogenesis.
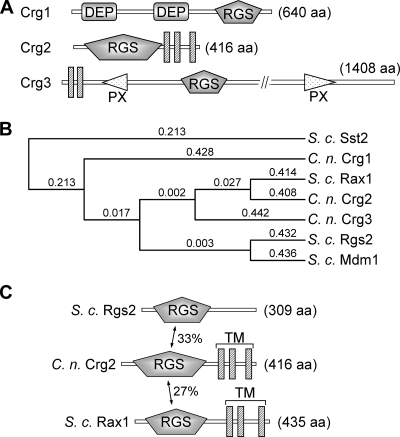
In S. cerevisiae, the archetypical RGS protein Sst2 contains two N-terminal DEP (Disheveled, EGL-10, Pleckstrin) homology domains and a C-terminal RGS domain, Rgs2 has a single N-terminal RGS domain, and Rax1 has an N-terminal RGS domain and three C-terminal membrane-spanning motifs. In comparison, the domain architecture and function of C. neoformans Crg1 are analogous to those of Sst2, whereas Crg2 shares higher sequence homology and domain architecture to Rax1 than to Rgs2 (Fig. 1A and B). However, Crg2 shares ∼33% amino acid sequence identity to Rgs2 within the RGS domain, which is higher than that with Rax1 (27%) (Fig. 1C). Yeast Rgs2 functions as a GAP to inhibit Gpa2-cAMP signaling, whereas Rax1 is required for the establishment of the bipolar budding pattern in diploid yeast cells (13, 38). A recent study has suggested that both proteins may interact with Gpa1 to control mating efficiency, but the role was far less prominent or direct than that of Sst2 (5).
FIG. 1.
C. neoformans encodes three RGS protein homologs, Crg1, Crg2, and Crg3. (A) Schematic representations of three RGS proteins in C. neoformans. DEP, domains found in Dishevelled, Egl-10, and pleckstrin; PX, domains that bind to phosphoinositides; aa, amino acids. (B) The alignment of C. neoformans (C. n.) and S. cerevisiae (S. c.) RGS proteins indicates higher amino acid sequence similarity between Crg2 and Rax1. Protein sequences were aligned, and the phylogenic tree was drawn using MacVector software (MacVector, Inc.) with the following parameters: open gap penalty of 10.0, extend gap penalty of 0.2, delay divergence of 30%, gap distance of 4, and similarity matrix blosum. (C) Comparison of Crg2 with S. cerevisiae (S. c.) Rgs2 and Rax1 proteins. Crg2 contains an N-terminal RGS domain and three C-terminal transmembrane domains (TM). The GenBank accession numbers for Crg1, Crg2, Crg3, Sst2, Rgs2, Rax1, and Mdm1 are AAR06255, XP_570417, XP_569481, NP_013557, NP_014750.1, NP_014945.1, and NP_013603.1, respectively.
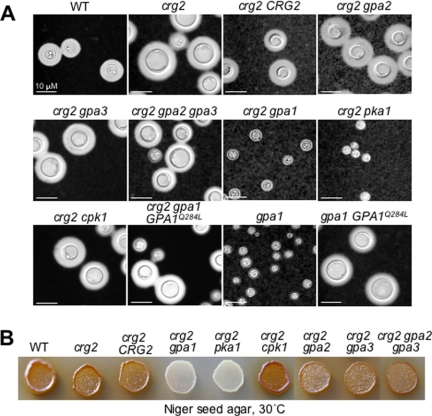
Others have provided evidence suggesting that Crg2 is involved in the mating pathway of C. neoformans, since a genetic cross involving the Δcrg2 mutant formed highly branched and elongated dikaryotic filaments (hyperfilamentation), and the cross also resulted in reduced basidiospore production and viability (18). Crg2 was shown to interact with pheromone receptor Ste3α, but the expression of Crg2 is not responsive to pheromone stimulation (18). Consistent with this study, we have found that both Δcrg2::URA5 and Δcrg2::NAT mutant strains exhibit formation of similarly elongated and highly branched dikaryotic filaments when crossed with a Δcrg1a strain, which was suppressed by the reintroduction of the wild-type CRG2 (Fig. 2, top row). Disruption of the GPA2 and GPA3 genes in the Δcrg2 mutant (Δcrg2 Δgpa2 and Δcrg2 Δgpa3) partially suppressed the formation of the hyperfilaments, and disruption of both GPA2 and GPA3 genes (Δcrg2 Δgpa2 Δgpa3) did not eliminate filament formation (Fig. 2, top and middle rows), indicating that both Gpa2 and Gpa3 function downstream from Crg2 in the mating pathway, and an additional regulatory element(s) may be present controlling filament formation. The Δcrg2 Δgpa1 and Δcrg2 Δpka1 mutant strains produced sparse patches of filaments that were also elongated and highly branched (Fig. 2, middle row), and Δgpa1 and Δcrg2 Δgpa1 mutants expressing a constitutively active GPA1(Q284L) allele exhibited hyperfilamentation similar to that of Δcrg2 (Fig. 2, bottom row). These findings suggest that the filamentation process during a genetic cross is also controlled by cAMP signaling. Finally, the Δcrg2 Δcpk1 mutant was sterile (Fig. 2, middle row), which was consistent with previous findings for the role of the mitogen-activated protein kinase cascade in filamentation and mating (6, 42).
FIG. 2.
The C. neoformans Crg2 protein regulates mating in a manner different from that of Crg1. The Δcrg2, Δcrg2 Δgpa2, Δcrg2 Δgpa3, Δcrg2 Δgpa2 Δgpa3, Δcrg2 Δcpk1, Δcrg2 Δgpa1, Δcrg2 pka1, Δcrg2 Δgpa1 GPA1(Q284L), and Δgpa1 GPA1(Q284L) mutant strains were crossed with a MATa Δcrg1 strain on V8 agar plates and incubated in the dark at room temperature for 1 week. Cross with the Δcrg2 mutant resulted in elongated and profusely branched dikaryotic filaments (hyperfilaments [18]). The wild-type (WT) strain H99, Δgpa1, Δgpa2, Δgpa3, and Δgpa2 Δgpa3 mutant strains were included as controls. The assay was repeated multiple times with representative sections shown.
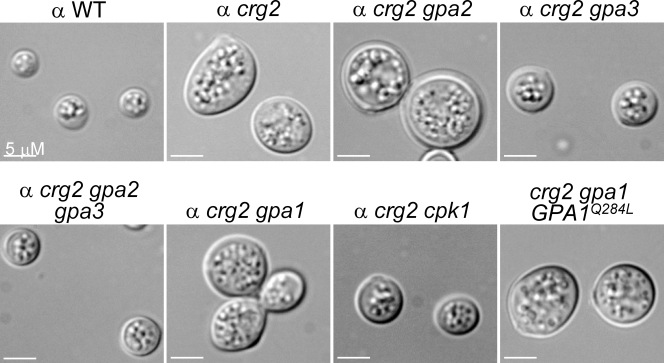
Confrontation tests for conjugation tube formation, a pheromone response, did not reveal a role for Crg2 (data not shown). However, microscopic examination revealed that Δcrg2 cells are enlarged, particularly in medium, such as filament agar, in which cells appeared to undergo an isotropic expansion to double in size (Fig. 3). This phenomenon is independent of opposite mating partners, although their presence did cause certain cells to appear elliptical, instead of spherical, but the elliptical cells did not always orient toward the mating partner (Fig. 3, see Δcrg2, top panel; also data not shown). The process is unlike the shmoo phenomenon described for S. cerevisiae, in which the haploid yeast cells project toward the direction of the mating partner. Interestingly, disruption of Gpa3, but not Gpa2, and also disruption of the mitogen-activated protein kinase homolog Cpk1 blocked this swelling process (Fig. 3). Swollen cells were seen in Δcrg2 Δgpa1 and Δcrg2 Δgpa1 GPA1(Q284L) mutants (Fig. 3, bottom row), suggesting that Crg2 differentially regulates Gpa3 to mediate this unique Cpk1-dependent morphogenesis process.
FIG. 3.
C. neoformans Crg2 regulates a unique morphogenesis event. The cell size of the Δcrg2 mutant strain is enlarged, which is independent of pheromone stimulation but dependent on Gpa3 and Cpk1. The phenomenon is most prominent in poor nutrient media, such as filament agar and YNB. Cells were streaked on filament agar medium in either petri dishes or coverslips and incubated in the dark for 3 days at room temperature. α WT, mating type α wild type. Bars, 5 μm.
Crg2 downregulates Gpa1-cAMP signaling.
In S. cerevisiae, mating and cAMP signaling are regulated by two G-protein signaling pathways complete with the corresponding RGS proteins Sst2 and Rgs2. Given that a similar cAMP signaling pathway functions in C. neoformans but that an RGS protein for Gpa1 has not been found, we sought to test whether Crg2 plays an inhibitory role in Gpa1-cAMP signaling. In the process of characterizing specialized traits, such as melanin production and capsule formation, which are known to be the targets of cAMP signaling, we have found that the Δcrg2 cells were enlarged with thick capsules, mimicking cells that express a constitutively active GPA1(Q284L) allele (Fig. 4A). In addition, the Δcrg2 Δgpa1 and Δcrg2 Δpka1 mutant strains were acapsular, similar to Δgpa1 and Δpka1 single mutant strains, indicating that Gpa1 and Pka1 are epistatic to Crg2 in the regulation of capsule formation (Fig. 4A). Consistent with this interpretation, the Δcrg2 Δgpa2, Δcrg2 Δgpa3, Δcrg2 Δgpa2 Δgpa3, and Δcrg2 Δcpk1 mutant strains did not exhibit a capsular trait significantly different from that of the Δcrg2 mutant strain (Fig. 4A).
FIG. 4.
Impact of C. neoformans Crg2 mutation on capsule production and melanin formation. (A) Crg2 negatively regulates the production of polysaccharide capsule in vitro. Cells were inoculated in liquid DMEM medium with calcium carbonate and incubated at 30°C for 2 days. Cells were then washed, stained with India ink, and examined under a microscope (Olympus BX50) equipped with a digital camera. WT, wild type. Bars, 10 μm. (B) Crg2 exhibits no detectable role in the formation of melanin in vitro. Cells were spotted on standard Niger seed agar and placed at 30°C for 2 days. Dark pigment indicates normal melanin formation, whereas white opaque colonies indicate melanin defects.
No change was found in the production of melanin pigment on Niger seed agar for the Δcrg2 mutant, and the melanin phenotype exhibited by Δcrg2 double mutants was also consistent with the proposed epistatic role of Gpa1 (Fig. 4B).
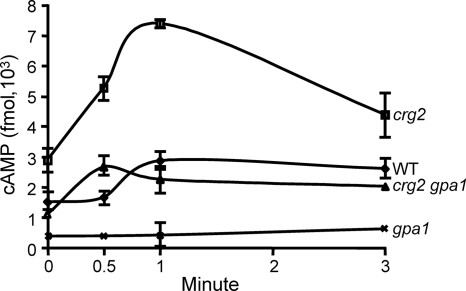
To test whether disruption of the CRG2 gene modulates intracellular cAMP, we measured the basal and transient levels of cAMP following glucose starvation and triggering. Higher levels of cAMP was seen in the Δcrg2 mutant strain and when the cells were starved and then stimulated with glucose (Fig. 5). At 0, 0.5, 1, and 3 min after glucose stimulation, cAMP levels were 2.9 × 103, 5.3 × 103, 7.4 × 103, and 4.4 × 103 fmol, respectively, in the Δcrg2 mutant strain, which were higher than those of the Δgpa1 mutant strain (0.3 × 103, 0.5 × 103, 0.6 × 103, and 0.7 × 103 fmol; P < 0.001) and the wild-type strain (1.5 × 103, 1.7 × 103, 2.9 × 103, and 2.6 ×103 fmol; P < 0.001) (Fig. 5). The levels of cAMP in the Δcrg2 Δgpa1 strain (1.0 × 103, 2.8 × 103, 2.3 × 103, and 2.2 × 103 fmol) resembled those of the wild-type strain (P > 0.05) (Fig. 5), which may indicate the existence of an additional mechanism(s) that maintains the cAMP level. Nevertheless, increases of the cAMP levels in the Δcrg2 strain provided direct evidence for a negative role of Crg2 in the regulation of intracellular cAMP.
FIG. 5.
Crg2 negatively regulates the intracellular cAMP level in C. neoformans. Cells were grown in YPD overnight and washed twice with distilled sterile water before being subjected to glucose starvation. Cell extraction and cAMP measurement were carried out as previously described (32). The assay was carried out in triplicate, and the mean values were plotted against time. The experiment was repeated twice with similar results. WT, wild type.
Crg2 exhibits in vitro GAP activity.
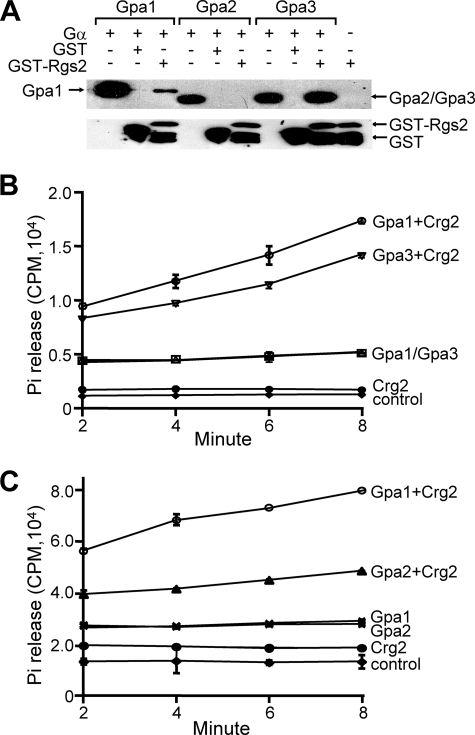
The RGS protein is known to interact with its cognate Gα and enhance its intrinsic GTPase activity by accelerating Gα-bound GTP to GDP hydrolysis. To test whether Crg2 indeed inhibits the functions of Gpa1, Gpa2, or Gpa3 as an RGS protein, we first performed assays testing protein-protein interactions. In vivo tests via the yeast two-hybrid assay were inconclusive, since Crg2 did not interact with any of the three Gα subunits (data not shown). However, in a protein pulldown assay, Crg2 consistently bound with Gpa1 and Gpa3 when GDP-AlF4 was included in the assay buffer to simulate the transition state of Gαs (Fig. 6A). No interaction was detected between Crg2 and Gpa2, indicating that associations between Crg2 and the two Gα subunits are specific.
FIG. 6.
C. neoformans Crg2 directly interacts with Gpa1 and Gpa3. (A) The protein pulldown assay results indicate that Crg2 physically interacts with Gpa1 and Gpa3, but not Gpa2. The His6- and Xpress-tagged Gpa1, Gpa2, and Gpa3 proteins were prepared as described previously (25). The GST fusion proteins were absorbed to glutathione Sepharose beads and washed, and Gpa1, Gpa2, and Gpa3 were then added (+). Bound proteins were separated and analyzed by blotting with either the anti-Xpress antibody (top blot) or the anti-GST antibody (bottom blot). The first lane of each grouping is the direct input control (20 μl). (B and C) Crg2 exhibits GAP activity toward Gpa1, Gpa2, and Gpa3. The steady-state method that measures the accumulation of free phosphate (Pi) over a period of 8 min following Crg2 addition was performed. The release of free phosphate is indicated by counts per minute (CPM). Sampling was carried out in triplicate, and the assay was repeated at least once.
To demonstrate if Crg2 functions as a GAP, we assayed the GTPase activities of Gpa1 and Gpa3 in a steady-state method that measures the accumulation of free phosphate (Pi) following the addition of Crg2. We also included Gpa2 in the same assay. Gpa1 and Gpa3 exhibited similar basal levels of GTPase activity of ∼0.5 × 104 counts per minute (cpm), whereas the addition of Crg2 resulted in ∼2- to 3.5-fold of increase in the rate of Pi accumulation by either Gpa1 or Gpa3 in 8 min, indicating that Crg2 can accelerate the hydrolysis of GTP bound to both Gα proteins (Fig. 6B). Surprisingly, even though the two proteins failed to interact with each other in a pulldown assay, Crg2 did exhibit a GAP function upon Gpa2, albeit less than that upon Gpa1 or Gpa3. In this assay, Crg2 increased the rate of Pi accumulation released from Gpa2 by ∼1.6-fold, whereas the increase for Gpa1 was ∼2.7-fold (Fig. 6C).
Mutation of Crg2 resulted in attenuated virulence.
Gpa1 and the Gpa1-mediated cAMP signaling pathway play a major role in virulence trait expression, since Δgpa1 and Δpka1 mutant strains were defective in melanin production and capsule formation and severely attenuated in animal virulence (1, 11). On the other hand, the Δpkr1 mutant that lacked the regulatory subunit for protein kinase A exhibited a noticeable increase in capsule size and, correspondingly, a reduction in mouse survival (11), suggesting that upregulation of protein kinase A (PKA) increases virulence traits and virulence.
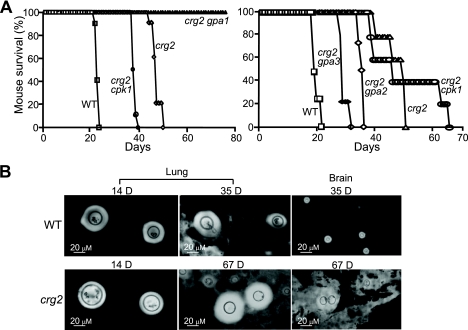
To detect the impact on virulence caused by the CRG2 gene disruption, we infected 4- to 6-week-old A/JCR female mice through the intranasal route. We have included Δcrg2, Δcrg2 Δgpa1, Δcrg2 Δgpa2, Δcrg2 Δgpa3, and Δcrg2 Δcpk1 mutant strains. In the first test, mice (groups of 10) infected with the Δcrg2 mutant survived 50 days, in contrast to mice infected with the wild-type H99 strain that survived 22 days (P < 0.0001) (Fig. 7A). The mice infected with the Δcrg2 Δgpa1 mutant strain did not show any symptoms by day 76 (P < 0.0001 versus wild type), indicating that its virulence is severely attenuated or avirulent. Mice infected with the Δcrg2 Δcpk1 strain survived 40 days (P < 0.0001) (Fig. 7A). In the second test, the survival times of mice (groups of five) infected with the Δcrg2 mutant and the wild-type strain were similar to those of the previous testing, 53 and 21 days, respectively (P = 0.0004) (Fig. 7A). Mice infected with the Δcrg2 Δgpa2 and Δcrg2 Δgpa3 strains exhibited survival rates between those of the wild-type and Δcrg2 strains, 38 days (P = 0.0004) and 33 days (P = 0.0004), respectively (Fig. 7A). Additionally, the survival times of mice infected with the Δcrg2 Δcpk1 strain were similar to those of the first testing until 39 days postinfection, when it took an additional 3 weeks for the two remaining mice to become moribund (P = 0.0004) (Fig. 7A). In the third test, which employed 20 mice per group for monitoring fungal distribution and burden within the host, mice infected with the wild-type strain survived 35 days, and mice infected with the Δcrg2 strain survived 67 days (data not shown). Staining of tissue smears with India ink showed that the Δcrg2 mutant did not reach the brain by day 35, whereas the wild-type strain did (Fig. 7B). At day 67, however, the Δcrg2 mutant was found to reach the mouse brain (Fig. 7B). Both mutant and wild-type strains were persistently found in the lung tissue at all monitored time points.
FIG. 7.
Dysregulation of cAMP due to Crg2 mutation attenuates fungal virulence. (A) In the first test, a Δcrg2 mutant strain and Δcrg2 Δgpa1 and Δcrg2 Δcpk1 double mutant strains were inoculated via nasal cavities into 10 A/JCR mice. In the second test, Δcrg2, Δcrg2 Δgpa2, Δcrg2 Δgpa3, and Δcrg2 Δcpk1 mutant strains were inoculated via the same infection route into five A/JCR mice. Mouse survival was monitored twice daily and plotted against time. The discrepancy in the survival times of mice infected with the Δcrg2 mutant strain in the two tests likely reflects variation in culture conditions, inoculation conditions, and mouse condition. WT, wild type. (B) C. neoformans cells were visible in lung and brain homogenates. The mouse tissue was homogenized with a Dounce homogenizer 2 weeks (14 days [14 D]) or 35 or 67 days (D) postinfection and stained with India ink. Please note that the cells are significantly larger than those seen in Fig. 4. Bars, 20 μm.
DISCUSSION
Crg2 is a multifunctional RGS protein.
Regulation of G-protein signaling by an RGS protein is thought to be a highly specific process that involves a specific interaction between the RGS protein and its cognate Gα(s). Our studies of Crg1 and Crg2 and their interactions with Gαs in C. neoformans have suggested that both specific and cross interactions do occur. Mutation of the C. neoformans RGS protein Crg1 resulted in enhanced pheromone responses and mating, and Crg1 functioned as a GAP for both Gpa2 and Gpa3 (25, 40). In contrast, Crg2 is multifunctional. First, genetic crosses involving the Δcrg2 mutant resulted in hyperfilamentation but with reduced fertility, suggesting a role in pheromone responses and/or mating that is also distinct from the role of Crg1. Second, the Δcrg2 mutant undergoes near isotropic expansion, which is mediated by Gpa3 and Cpk1, indicating that the phenotype could be linked to its role in mating. Last, the Δcrg2 mutant exhibited elevated levels of cAMP, directly implicating a role in the regulation of Gpa1-mediated cAMP signaling. Biochemical characterization has demonstrated that Crg2 functions as a GAP to differentially inhibit the functions of Gpa1, Gpa3, and Gpa2.
Our observations are consistent with recent findings indicating that cross interactions do occur between RGS and Gα proteins and that the spatial and temporal relationships between the RGS and Gα proteins determine the signaling intensity and/or specificity. For example, both human Rgs4 and Rgs19/GAIP proteins can attenuate Gi-mediated inhibition of cAMP synthesis and Gq-mediated activation of phospholipase Cβ. However, Rgs4 was more effective than Rgs19 (10-fold) in modulating these activities (16, 19). In S. cerevisiae, all four RGS and RGS-like proteins exhibited interaction with Gpa1 in vitro; however, only Sst2 remained as the principal protein inhibiting Gpa1 and pheromone signaling (5). In the rice blast fungal pathogen Magnaporthe grisea, the RGS protein Rgs1 regulates several cellular events, such as appressorium formation, thigmotropism, and asexual reproduction, likely through its interactions with multiple Gα subunits (26).
Crg2 is a novel inhibitor of the Gpa1-cAMP signaling pathway.
The Gα protein Gpa1 was the first G-protein subunit identified in C. neoformans (37). Additional studies have shown that Gpa1 regulates a cAMP-dependent signaling pathway that includes conserved adenylyl cyclase Cac1 and the cAMP-dependent protein kinase A catalytic and regulatory subunits Pka1 and Pkr1 (1, 2, 11). Additionally, Aca1, an accessory protein of Cac1, and phosphodiesterases Pde1 and Pde2 have also been characterized (4, 17). A recent study has demonstrated that one of the G-protein-coupled receptor homologs, Gpr4, functions in the Gpa1-cAMP pathway by sensing nutrients, such as methionine (44). All of these findings indicate that the Gpa1/cAMP-dependent pathway is analogous to that outlined in S. cerevisiae and Schizosaccharomyces pombe. Our finding that Crg2 inhibits Gpa1 function and negatively regulates the Gpa1-cAMP signaling pathway further highlights the conserved nature of cAMP signaling in evolution.
The protein pulldown and GAP assays have provided compelling evidence supporting that the multifunctional nature of Crg2 is mediated through its interaction with multiple Gα proteins. Why did Crg2 fail to interact with Gpa3 and Gpa1 in the yeast two-hybrid assay but did so in the in vitro pulldown assay and why did it fail to interact with Gpa2 in this assay but exhibited GAP function towards Gpa2? We suspect that the yeast host environment may not duplicate the environment where Gαs are in the specific transition state required for binding to Crg2. We also suggest that the in vitro GAP assay condition fosters an environment where Crg2 can exhibit GAP function upon Gpa2. All of these observations have indicated that Crg2 preferably interacts with Gpa3 and Gpa1 to regulate filament formation, cell swelling, and cAMP signaling. Interaction between Crg2 and Gpa2 could also occur, adding an additional regulatory element to pheromone-responsive mating. Clearly, further studies are needed to reveal the functional significance of the bindings and their specificities between Crg2 and these three Gα proteins.
Crg2 links mating and cAMP signaling.
The two G-protein signaling pathways in C. neoformans are distinct and specific. For example, Gpb1 specifically couples with Gpa2, but not Gpa1 or Gpa3, and the Gβ-like/RACK1 homolog protein Gib2 interacts with only Gpa1, but not Gpa2 nor Gpa3 (25, 32). Surprisingly, the phenotypic characteristics affected by the two pathways are often intertwined, suggesting the existence of pathway cross talk between pheromone-responsive mating and cAMP signaling in this fungus. For example, Gpa1 is the primary regulator of the cAMP pathway that governs the production of the virulence factors melanin and capsule. The Δgpa1 mutant strain was also attenuated in mating (1). Mutation of the Gpb1-mediated mating pathway components, such as Gpa2 and Gpa3 in conjunction or the Ste20/PAK kinase homolog Ste20α, attenuates mating, but the Δgpa2 Δgpa3 and Δste20α mutant strains exhibited attenuated virulence (25, 41). Similar cross talk between mating and cAMP signaling has been observed previously in maize pathogen Ustilago maydis, in which one of the four Gα proteins, Gpa3, regulates the cAMP signaling pathway, but deletion of it resulted in sterility (22).
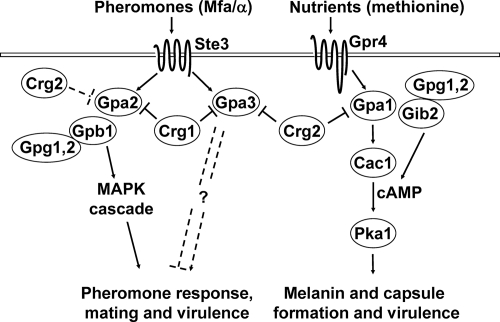
The discovery that Crg2 regulates mating and cAMP signaling implicates Crg2 as the molecule linking these two pathways. We hypothesize that the presence of pheromones is not sufficient to activate the Gpa2/Gpa3-mediated signaling pathways for mating. Additional stimuli or conditions, such as nutrient deprivation sensed by the Gpa1-cAMP signaling pathway, may feed to the mating pathway through the common negative regulator Crg2. The activated Gpa2/Gpa3/Gpb1 signaling pathways regulate events, such as the formation of conjugation tubes, cell fusion, formation of dikaryotic filaments, and meiosis (Fig. 8), whereas Gpa1 signaling provides an auxiliary role in mating. Conversely, Crg2 interacts with Gpa1, leading to its major role in the regulation of Gpa1-cAMP signaling. How Crg2 exerts such signaling specificities and strengths between Gpa1, Gpa2, and Gpa3 pathways is currently unknown but is likely to involve the regulatory mechanism of protein spatial and temporal localization, similar to those suggested for mammals and S. cerevisiae (5, 16, 19).
FIG. 8.
The RGS protein Crg2 links the mating and cAMP signal transduction pathways in C. neoformans. The pheromone-responsive mating pathway is regulated by the pheromone receptor Ste3α, heterotrimeric G-protein Gpa2/Gpb1/Gpg1/2, and the RGS protein Crg1. The G-protein-coupled receptor homolog Gpr4, Gpa1/Gib2/Gpg1/Gpg2, and Crg2 govern the conserved cAMP signaling pathway to regulate differentiation and the production of virulence factors. The Gα protein Gpa3 mediates a distinct signaling pathway in mating that is negatively regulated by both Crg1 and Crg2. Both the mating and cAMP signaling pathways are important for fungal virulence. MFa/α, mating type a or α; MAPK, mitogen-activated protein kinase.
Dysregulation of cAMP attenuates virulence.
C. neoformans has a predilection for the central nervous system, often causing fatal meningoencephalitis in immunocompromised individuals. The Gpa1-cAMP signaling pathway has a profound role in the regulation of virulence traits, such as melanin production and capsule formation. Attenuation in either trait, such as that seen in Δgpa1 and Δpka1 mutants, attenuated virulence in experimental animal models (1, 11). On the other hand, mutation of Pkr1, removing the inhibitory effect on Pka1, resulted in an increase in virulence (11). The Δpkr1 mutant exhibited enlarged capsules, but an increase in intracellular levels of cAMP was not apparent (11). Disruption of the CRG2 gene resulted in similarly enlarged capsules, and the production of melanin appeared not to be affected; however, the Δcrg2 mutant was slower in reaching the mouse brain and was attenuated in virulence. This apparent discrepancy suggests that dysregulated cAMP due to a Crg2 mutation likely affects other targets that render the fungus less pathogenic to the host. A possible explanation is that dysregulated cAMP signaling affects stress tolerance. Indeed, we have observed that the Δcrg2 mutant strain was sensitive to 39°C in contrast to the wild-type H99 and the reconstituted Δcrg2 CRG2 strains (data not shown).
Virulence attenuation because of alteration in the cAMP signaling pathway is not unique to C. neoformans. For example, in U. maydis, disruption of Gpa3, adenylyl cyclase uac1, and the PKA catalytic subunit adr1 all resulted in defective cAMP signaling, and as a consequence, the cells became filamentous rather than yeast-like (12, 14, 33). Mutation of the PKA regulatory subunit ubc1 suppressed the filamentous phenotype and altered virulence expression to cause localized lesions, instead of systemic infection as caused by the wild-type strain (14). A constitutively active allele of Gpa3 [Gpa3(Q206L)] caused smaller tumors on corn (23). These findings are consistent with ours in that manipulation of the cAMP signaling pathway alters virulence expression. It is feasible that dysregulated cAMP, whether it is down or up, results in alteration of cellular growth, stress tolerance, and attenuated formation of the specialized virulence traits that ultimately alter virulence expression.
It is interesting to note that the crg2 gpa1 mutant strain is nearly avirulent, which is distinct from the Δgpa1 mutant strain with attenuated virulence (1). This could imply that Crg2 employs an additional virulence-regulating mechanism(s). Whether and how this mechanism is mediated through Gpa2 and Gpa3 mating pathways remains to be investigated. It is known, however, that virulence was partially restored in the Δcrg2 Δgpa2, Δcrg2 Δgpa3, and Δcrg2 Δcpk1 mutant strains, which is also consistent with previous findings that (i) the mating pathway plays a role in virulence, (ii) strains with reduced mating are often associated with virulence reduction, and (iii) factors that restore mating often result in similar effects on virulence (24, 25, 40, 41).
Supplementary Material
Acknowledgments
We thank Z. G. Zhang and J. K. Thompson for early contributions toward this project, J. E. Cutler and D. S. Fox for critical comments, and T. Swayze III for technical assistance. We also thank J. Heitman and C. Y. Xue for DNA primers JH12499 and JH12599.
This research was supported in part by funds from the National Institute of Health (R01 AI054958) and the Research Institute for Children, Children's Hospital, New Orleans, LA.
Footnotes
Published ahead of print on 25 July 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Alspaugh, J. A., J. R. Perfect, and J. Heitman. 1997. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 113206-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alspaugh, J. A., R. Pukkila-Worley, T. Harashima, L. M. Cavallo, D. Funnell, G. M. Cox, J. R. Perfect, J. W. Kronstad, and J. Heitman. 2002. Adenylyl cyclase functions downstream of the Gα protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 175-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apanovitch, D. M., K. C. Slep, P. B. Sigler, and H. G. Dohlman. 1998. Sst2 is a GTPase-activating protein for Gpa1: purification and characterization of a cognate RGS-Galpha protein pair in yeast. Biochemistry 374815-4822. [DOI] [PubMed] [Google Scholar]
- 4.Bahn, Y. S., J. K. Hicks, S. S. Giles, G. M. Cox, and J. Heitman. 2004. Adenylyl cyclase-associated protein Aca1 regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase A cascade. Eukaryot. Cell 31476-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chasse, S. A., P. Flanary, S. C. Parnell, N. Hao, J. Y. Cha, D. P. Siderovski, and H. G. Dohlman. 2006. Genome-scale analysis reveals Sst2 as the principal regulator of mating pheromone signaling in the yeast Saccharomyces cerevisiae. Eukaryot. Cell 5330-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson, R. C., C. B. Nichols, G. M. Cox, J. R. Perfect, and J. Heitman. 2003. A MAP kinase cascade composed of cell type specific and non-specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 49469-485. [DOI] [PubMed] [Google Scholar]
- 7.De Vries, L., and M. G. Farquhar. 1999. RGS proteins: more than just GAPs for heterotrimeric G proteins. Trends Cell Biol. 9138-144. [DOI] [PubMed] [Google Scholar]
- 8.De Vries, L., B. Zheng, T. Fischer, E. Elenko, and M. G. Farquhar. 2000. The regulator of G protein signaling family. Annu. Rev. Pharmacol. Toxicol. 40235-271. [DOI] [PubMed] [Google Scholar]
- 9.Dietzel, C., and J. Kurjan. 1987. Pheromonal regulation and sequence of the Saccharomyces cerevisiae SST2 gene: a model for desensitization to pheromone. Mol. Cell. Biol. 74169-4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dohlman, H. G., and J. Thorner. 1997. RGS proteins and signaling by heterotrimeric G proteins. J. Biol. Chem. 2723871-3874. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza, C. A., J. A. Alspaugh, C. Yue, T. Harashima, G. M. Cox, J. R. Perfect, and J. Heitman. 2001. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 213179-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durrenberger, F., K. Wong, and J. W. Kronstad. 1998. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc. Natl. Acad. Sci. USA 955684-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita, A., M. Lord, T. Hiroko, F. Hiroko, T. Chen, C. Oka, Y. Misumi, and J. Chant. 2004. Rax1, a protein required for the establishment of the bipolar budding pattern in yeast. Gene 327161-169. [DOI] [PubMed] [Google Scholar]
- 14.Gold, S. E., S. M. Brogdon, M. E. Mayorga, and J. W. Kronstad. 1997. The Ustilago maydis regulatory subunit of a cAMP-dependent protein kinase is required for gall formation in maize. Plant Cell 91585-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hepler, J. R. 1999. Emerging roles for RGS proteins in cell signalling. Trends Pharmacol. Sci. 20376-382. [DOI] [PubMed] [Google Scholar]
- 16.Hepler, J. R., D. M. Berman, A. G. Gilman, and T. Kozasa. 1997. RGS4 and GAIP are GTPase-activating proteins for Gq alpha and block activation of phospholipase C beta by gamma-thio-GTP-Gq alpha. Proc. Natl. Acad. Sci. USA 94428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hicks, J. K., Y. S. Bahn, and J. Heitman. 2005. Pde1 phosphodiesterase modulates cyclic AMP levels through a protein kinase A-mediated negative feedback loop in Cryptococcus neoformans. Eukaryot. Cell 41971-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsueh, Y. P., C. Xue, and J. Heitman. 2007. G protein signaling governing cell fate decisions involves opposing Galpha subunits in Cryptococcus neoformans. Mol. Biol. Cell 183237-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, C., J. R. Hepler, A. G. Gilman, and S. M. Mumby. 1997. Attenuation of Gi- and Gq-mediated signaling by expression of RGS4 or GAIP in mammalian cells. Proc. Natl. Acad. Sci. USA 946159-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurowitz, E. H., J. M. Melnyk, Y. J. Chen, H. Kouros-Mehr, M. I. Simon, and H. Shizuya. 2000. Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res. 7111-120. [DOI] [PubMed] [Google Scholar]
- 21.Koelle, M. R. 1997. A new family of G-protein regulators—the RGS proteins. Curr. Opin. Cell Biol. 9143-147. [DOI] [PubMed] [Google Scholar]
- 22.Kruger, J., G. Loubradou, E. Regenfelder, A. Hartmann, and R. Kahmann. 1998. Crosstalk between cAMP and pheromone signalling pathways in Ustilago maydis. Mol. Gen. Genet. 260193-198. [DOI] [PubMed] [Google Scholar]
- 23.Kruger, J., G. Loubradou, G. Wanner, E. Regenfelder, M. Feldbrugge, and R. Kahmann. 2000. Activation of the cAMP pathway in Ustilago maydis reduces fungal proliferation and teliospore formation in plant tumors. Mol. Plant-Microbe Interact. 131034-1040. [DOI] [PubMed] [Google Scholar]
- 24.Kwon-Chung, K. J., J. C. Edman, and B. L. Wickes. 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, L., G. Shen, Z. G. Zhang, Y. L. Wang, J. K. Thompson, and P. Wang. 2007. Canonical heterotrimeric G proteins regulating mating and virulence of Cryptococcus neoformans. Mol. Biol. Cell 184201-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, H., A. Suresh, F. S. Willard, D. P. Siderovski, S. Lu, and N. I. Naqvi. 2007. Rgs1 regulates multiple Galpha subunits in Magnaporthe pathogenesis, asexual growth and thigmotropism. EMBO J. 26690-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McConnell, S. J., L. C. Stewart, A. Talin, and M. P. Yaffe. 1990. Temperature-sensitive yeast mutants defective in mitochondrial inheritance. J. Cell Biol. 111967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McConnell, S. J., and M. P. Yaffe. 1992. Nuclear and mitochondrial inheritance in yeast depends on novel cytoplasmic structures defined by the MDM1 protein. J. Cell Biol. 118385-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDade, H. C., and G. M. Cox. 2001. A new dominant selectable marker for use in Cryptococcus neoformans. Med. Mycol. 39151-154. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell, T. G., and J. R. Perfect. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neves, S. R., P. T. Ram, and R. Iyengar. 2002. G protein pathways. Science 2961636-1639. [DOI] [PubMed] [Google Scholar]
- 32.Palmer, D. A., J. K. Thompson, L. Li, A. Prat, and P. Wang. 2006. Gib2, a novel Gbeta-like/RACK1 homolog, functions as a Gbeta subunit in cAMP signaling and is essential in Cryptococcus neoformans. J. Biol. Chem. 28132596-32605. [DOI] [PubMed] [Google Scholar]
- 33.Regenfelder, E., T. Spellig, A. Hartmann, S. Lauenstein, M. Bölker, and R. Kahmann. 1997. G proteins in Ustilago maydis: transmission of multiple signals? EMBO J. 161934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross, E. M., and T. M. Wilkie. 2000. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69795-827. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 36.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 1751405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolkacheva, T., P. McNamara, E. Piekarz, and W. Courchesne. 1994. Cloning of a Cryptococcus neoformans gene, GPA1, encoding a G-protein α-subunit homolog. Infect. Immun. 622849-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Versele, M., J. H. de Winde, and J. M. Thevelein. 1999. A novel regulator of G protein signaling in yeast, Rgs2, down regulates glucose-activation of the cAMP pathway through direct inhibition of Gpa2. EMBO J. 185577-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, P., G. M. Cox, and J. Heitman. 2004. A Sch9 protein kinase homologue controlling virulence independently of the cAMP pathway in Cryptococcus neoformans. Curr. Genet. 46247-255. [DOI] [PubMed] [Google Scholar]
- 40.Wang, P., J. E. Cutler, J. King, and D. Palmer. 2004. Mutation of the regulator of G protein signaling Crg1 increases virulence in Cryptococcus neoformans. Eukaryot. Cell 31028-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, P., C. B. Nichols, K. B. Lengeler, M. E. Cardenas, G. M. Cox, J. R. Perfect, and J. Heitman. 2002. Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryot. Cell 1257-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, P., J. R. Perfect, and J. Heitman. 2000. The G-protein β subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol. Cell. Biol. 20352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie, G. X., and P. P. Palmer. 2007. How regulators of G protein signaling achieve selective regulation. J. Mol. Biol. 366349-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue, C., Y. S. Bahn, G. M. Cox, and J. Heitman. 2006. G protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol. Biol. Cell 17667-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.