Abstract
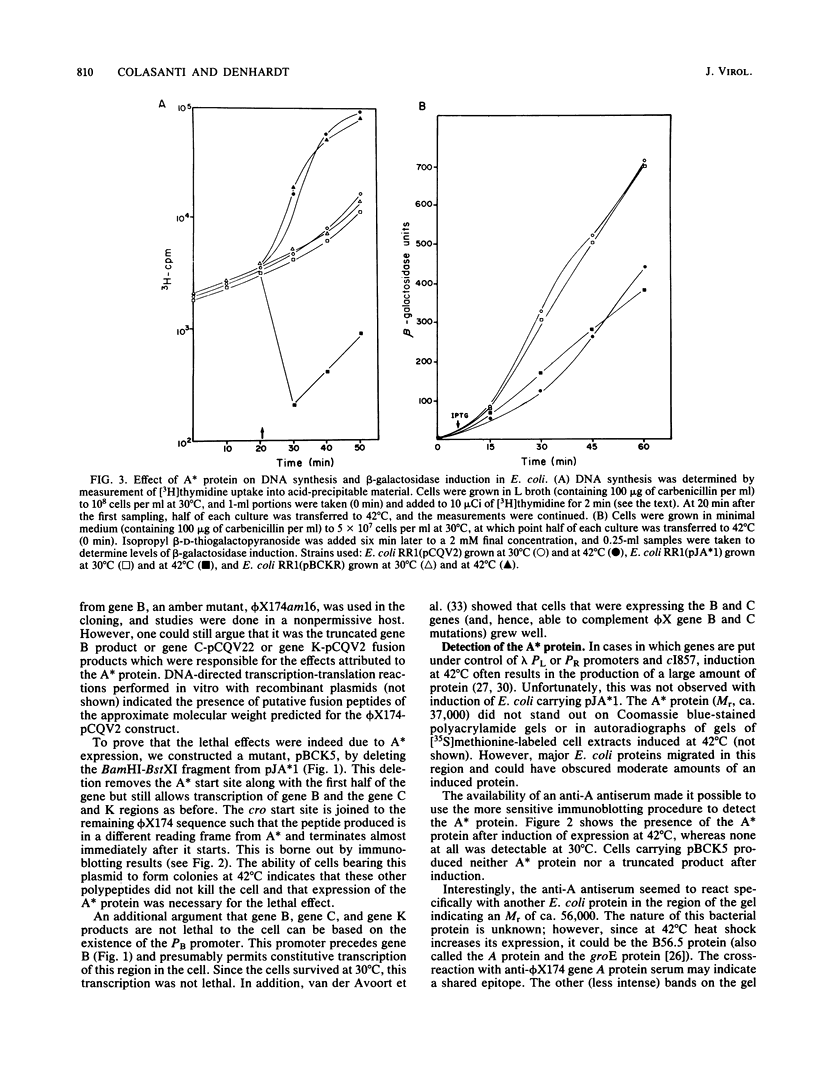
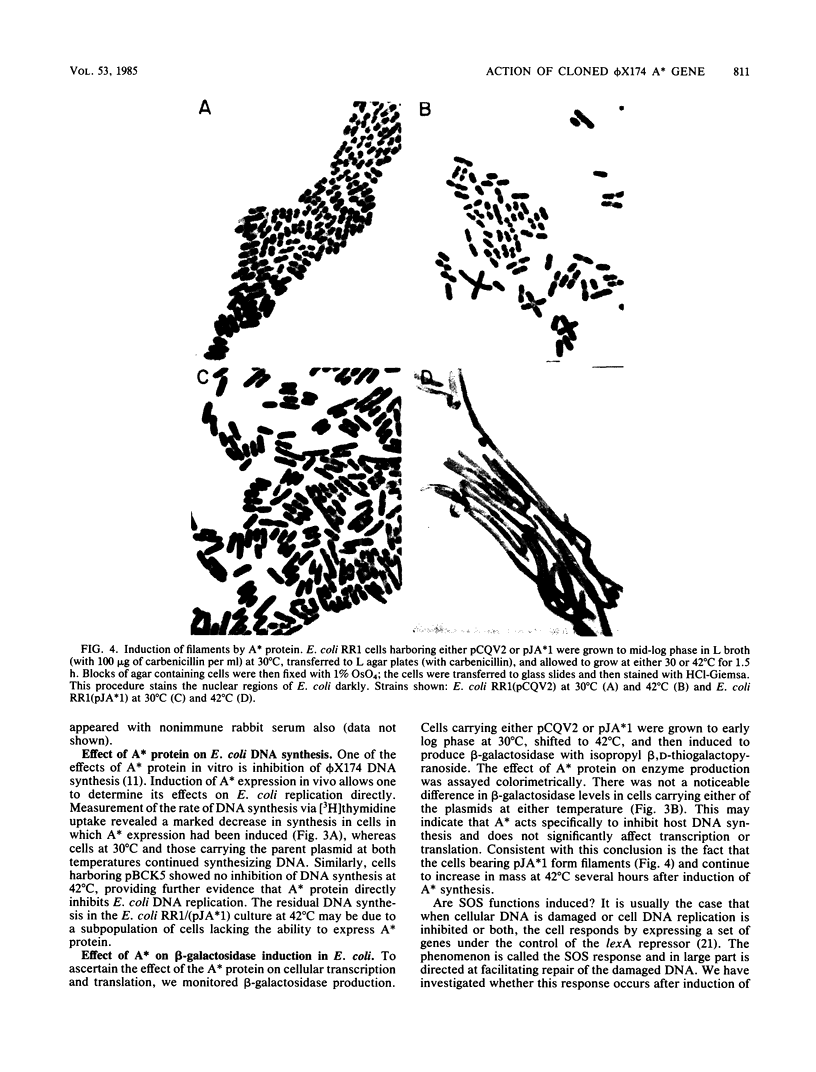
The A* gene of bacteriophage phi X174 has been cloned into the inducible expression vector pCQV2 under conditions allowing its lethal action to be controlled by the lambda cI857 repressor. Upon induction of expression, DNA synthesis in Escherichia coli carrying the recombinant plasmid is severely inhibited; however, these same cells permit beta-galactosidase induction at a rate similar to that observed in control cells at the inducing (for A*) temperature. Cells in which A* is expressed form filaments and produce more RecA protein, indicating at least a partial induction of the SOS response; however, there is no evidence of damage to the bacterial chromosome. It appears that the A* protein has as one function the inhibition of cell division and DNA replication but not transcription or protein synthesis during phage infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama A., Hamatake R. K., Hayashi M. In vitro synthesis of bacteriophage phi X174 by purified components. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4195–4199. doi: 10.1073/pnas.80.14.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bollen A., Loriau R., Herzog A., Hérion P. Expression of human alpha 1-antitrypsin in Escherichia coli. FEBS Lett. 1984 Jan 23;166(1):67–70. doi: 10.1016/0014-5793(84)80046-0. [DOI] [PubMed] [Google Scholar]
- Burton P., Holland I. B. Two pathways of division inhibition in UV-irradiated E. coli. Mol Gen Genet. 1983;190(2):309–314. doi: 10.1007/BF00330656. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. Formation of ribosylthymine in Escherichia coli. Studies on pulse labeling with thymine and thymidine. J Biol Chem. 1969 May 25;244(10):2710–2715. [PubMed] [Google Scholar]
- Dubeau L., Denhardt D. T. The mechanism of replication of phi X 174. XVIII. Gene A and A* proteins of phi X 174 bind tightly to phi X 174 replicative form DNA. Biochim Biophys Acta. 1981 Mar 26;653(1):52–60. doi: 10.1016/0005-2787(81)90103-9. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Ascarelli R. The A* protein of phi X174 is an inhibitor of DNA replication. Nucleic Acids Res. 1981 Apr 24;9(8):1991–2002. doi: 10.1093/nar/9.8.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Finer M. Cleavage and circularization of single-stranded DNA: a novel enzymatic activity of phi X174 A* protein. Nucleic Acids Res. 1980 Nov 25;8(22):5305–5315. doi: 10.1093/nar/8.22.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk F. D., Snover D. Pleiotropic effects of mutants in gene A of bacteriophage phi chi 174. J Virol. 1976 Apr;18(1):141–150. doi: 10.1128/jvi.18.1.141-150.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Poddar R. K. Reduced synthesis of beta-galactosidase in Escherichia coli infected with phage phi X 174. Can J Microbiol. 1977 Aug;23(8):1069–1077. doi: 10.1139/m77-160. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., Gottesman S. Cell-division control in Escherichia coli: specific induction of the SOS function SfiA protein is sufficient to block septation. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4490–4494. doi: 10.1073/pnas.81.14.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld S. A., van Mansfeld A. D., de Winter J. M., Weisbeek P. J. Cleavage of single-stranded DNA by the A and A* proteins of bacteriophage phi X174. Nucleic Acids Res. 1979 Dec 20;7(8):2177–2188. doi: 10.1093/nar/7.8.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld S. A., van Mansfeld A. D., van der Ende A., van de Pol J. H., van Arkel G. A., Weisbeek P. J. The nuclease specificity of the bacteriophage phi X174 A* protein. Nucleic Acids Res. 1981 Feb 11;9(3):545–562. doi: 10.1093/nar/9.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenberger J. A., Court D., Papas T. S. High-level expression in Escherichia coli of the carboxy-terminal sequences of the avian myelocytomatosis virus (MC29) v-myc protein. Gene. 1983 Jul;23(1):75–84. doi: 10.1016/0378-1119(83)90218-4. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Linney E., Hayashi M. Intragenic regulation of the synthesis of phi chi 174 gene A proteins. Nature. 1974 May 24;249(455):345–348. doi: 10.1038/249345a0. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Martin D. F., Godson G. N. Identification of a phiX174 coded protein involved in the shut-off of host DNA replication. Biochem Biophys Res Commun. 1975 Jul 8;65(1):323–330. doi: 10.1016/s0006-291x(75)80096-9. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Salas M. High level synthesis in Escherichia coli of the Bacillus subtilis phage phi 29 proteins p3 and p4 under the control of phage lambda PL promoter. Nucleic Acids Res. 1982 Oct 11;10(19):5773–5784. doi: 10.1093/nar/10.19.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Phillips T. A., VanBogelen R. A., Smith M. W., Georgalis Y., Subramanian A. R. Identity of the B56.5 protein, the A-protein, and the groE gene product of Escherichia coli. J Bacteriol. 1981 Jan;145(1):513–520. doi: 10.1128/jb.145.1.513-520.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C. A vector that uses phage signals for efficient synthesis of proteins in Escherichia coli. J Mol Appl Genet. 1983;2(1):1–10. [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Inducible high level synthesis of mature human fibroblast interferon in Escherichia coli. Nucleic Acids Res. 1983 Jul 25;11(14):4677–4688. doi: 10.1093/nar/11.14.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Simatake H., Rosenberg M. Purified lambda regulatory protein cII positively activates promoters for lysogenic development. Nature. 1981 Jul 9;292(5819):128–132. doi: 10.1038/292128a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Avoort H. G., Teertstra R., Versteeg R., Weisbeek P. J. Genes and regulatory sequences of bacteriophage phi X174. Biochim Biophys Acta. 1983 Oct 13;741(1):94–102. doi: 10.1016/0167-4781(83)90014-3. [DOI] [PubMed] [Google Scholar]
- van Mansfeld A. D., van Teeffelen H. A., Zandberg J., Baas P. D., Jansz H. S., Veeneman G. H., van Boom J. H. A protein of bacteriophage phi X174 carries an oligonucleotide which it can transfer to the 3' -OH of a DNA chain. FEBS Lett. 1982 Dec 13;150(1):103–108. doi: 10.1016/0014-5793(82)81313-6. [DOI] [PubMed] [Google Scholar]
- van der Ende A., Langeveld S. A., Teertstra R., van Arkel G. A., Weisbeek P. J. Enzymatic properties of the bacteriophage phi X174 A protein on superhelical phi X174 DNA: a model for the termination of the rolling circle DNA replication. Nucleic Acids Res. 1981 May 11;9(9):2037–2053. doi: 10.1093/nar/9.9.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende A., Langeveld S. A., Van Arkel G. A., Weisbeek P. J. The interaction of the A and A* proteins of bacteriophage phi X174 with single-stranded and double-stranded phi X DNA in vitro. Eur J Biochem. 1982 May 17;124(2):245–252. doi: 10.1111/j.1432-1033.1982.tb06584.x. [DOI] [PubMed] [Google Scholar]