Abstract
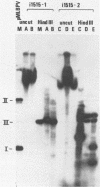
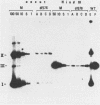
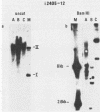
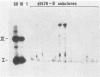
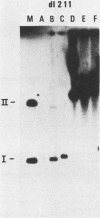
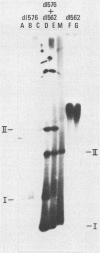
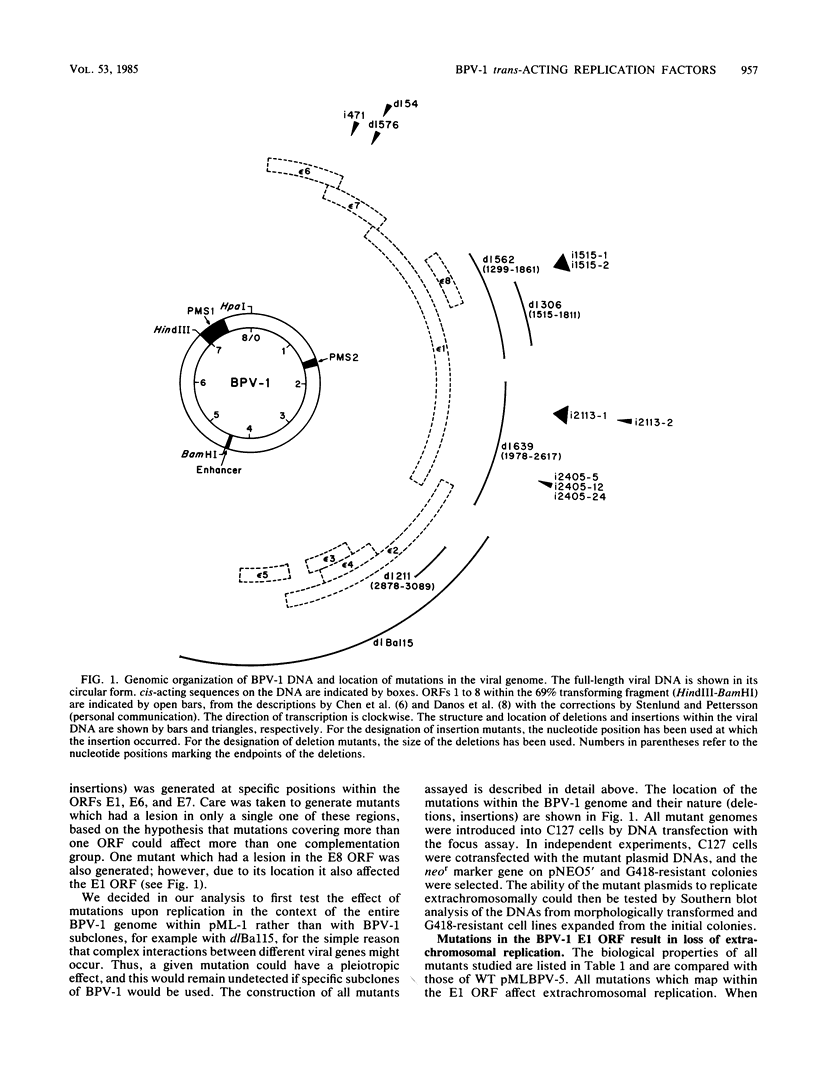
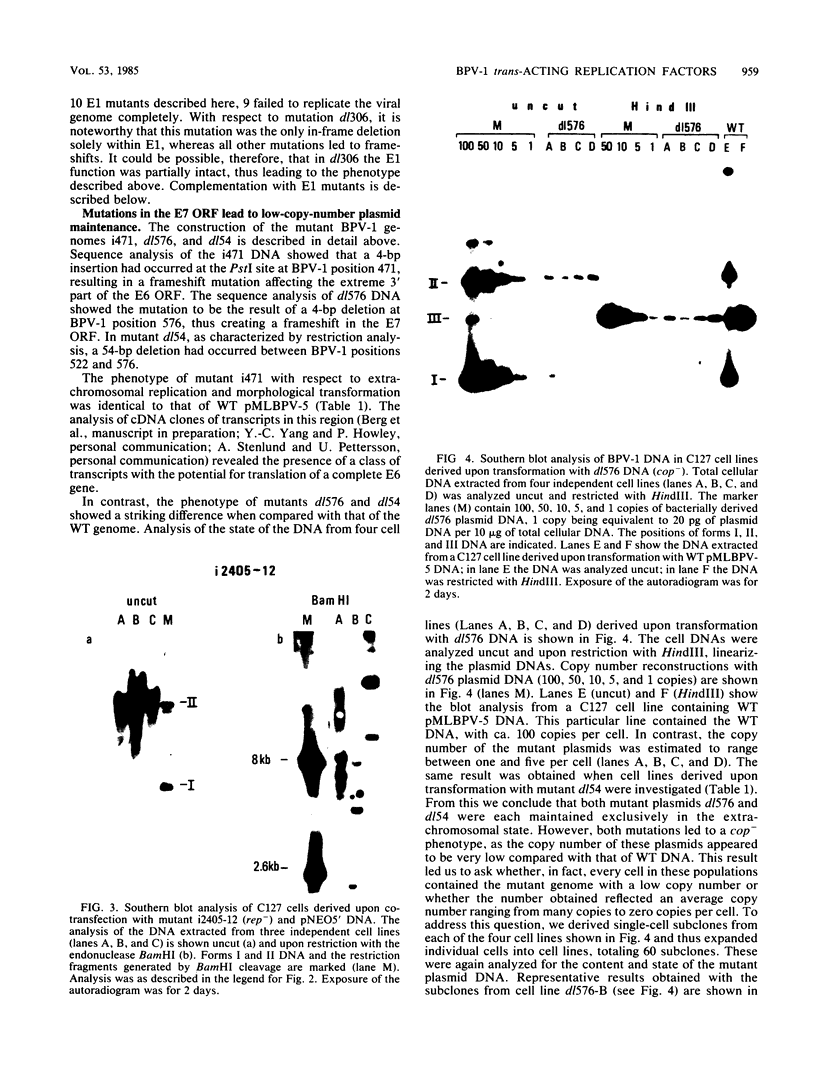
The establishment of bovine papillomavirus type 1 in somatic mammalian cells is mediated by extrachromosomal replication and stable maintenance of the viral genome as a multicopy nuclear plasmid. Previous studies indicated the requirement of viral gene expression for bovine papillomavirus type 1 replication and plasmid maintenance (M. Lusky and M. R. Botchan, Cell 36:391-401, 1984; Turek et al., Proc. Natl. Acad. Sci. U.S.A. 79:7914-7918, 1982). To define the viral genes which are necessary for this process, we constructed a series of specific mutations within the viral genome and assayed the resulting mutants for their ability to replicate extrachromosomally in mouse C127 cells. We report here that the bovine papillomavirus type 1 trans-acting replication factors were encoded by at least two distinct viral genes since the mutants fell into two complementation groups, rep and cop. Mutants (rep-) affecting the E1 open reading frame (ORF) failed to replicate bovine papillomavirus type 1 DNA extrachromosomally and would integrate into chromosomal DNA. We suggest that this gene product is one of the factors required to specifically preclude the integration event. Mutants (cop-) affecting the E7 ORF were maintained in the extrachromosomal state; however, the copy number of the mutant genomes was reduced 100-fold compared with that of wild-type DNA. Analysis of single-cell subclones showed that each cell contained the mutant genomes at a copy number of one to two, indicating that the cop- phenotype did not reflect a simple segregation defect. We propose that the gene defined by mutations in the E7 ORF played a crucial role in stably maintaining the copy number of the viral plasmid at high levels. Genomes with mutations in the cop and rep complementation groups, when cotransfected, rescued the wild-type phenotype, extrachromosomal replication with a high, stable copy number for both types of plasmids. Therefore, the gene products acted in trans, and the mutations were recessive to the wild-type functions. One specific rep- mutant showed a 30-fold-increased transformation efficiency when compared with that of the wild-type genome. In addition, morphological transformation mediated by the cop- mutants appeared to be unstable. These results imply that either or both of the replication functions played some role in regulating the expression of the viral transforming functions.
Full text
PDF


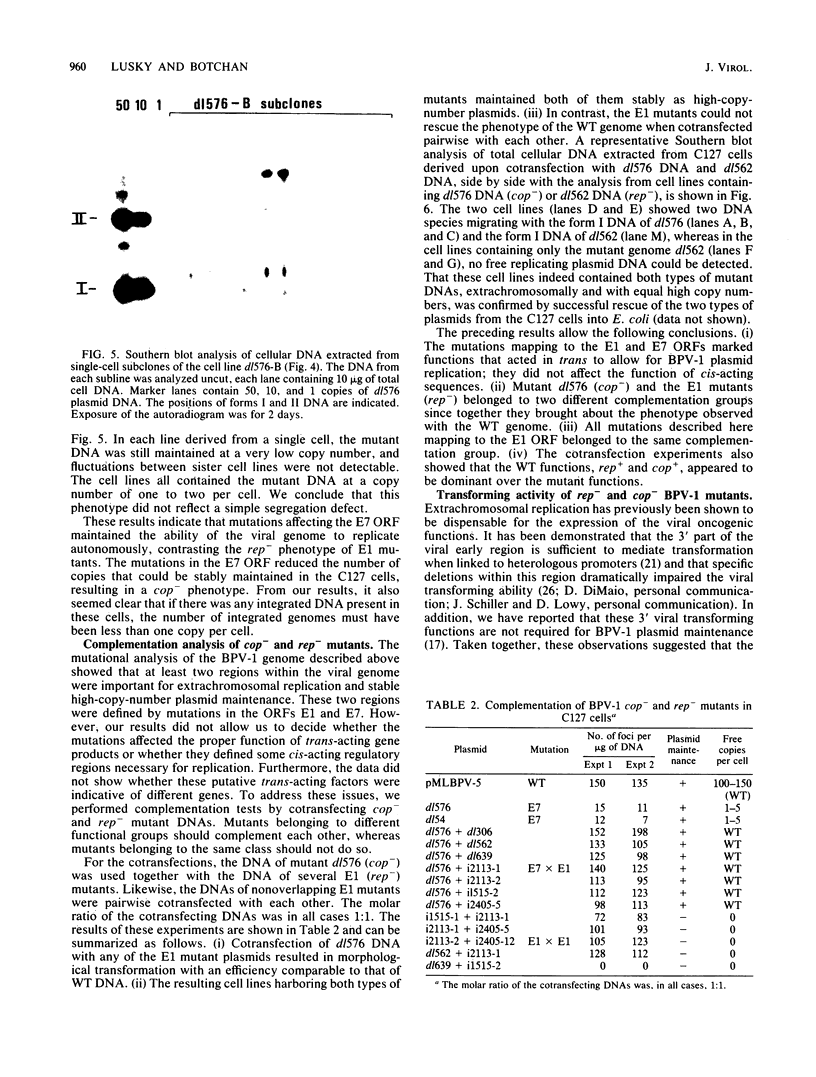
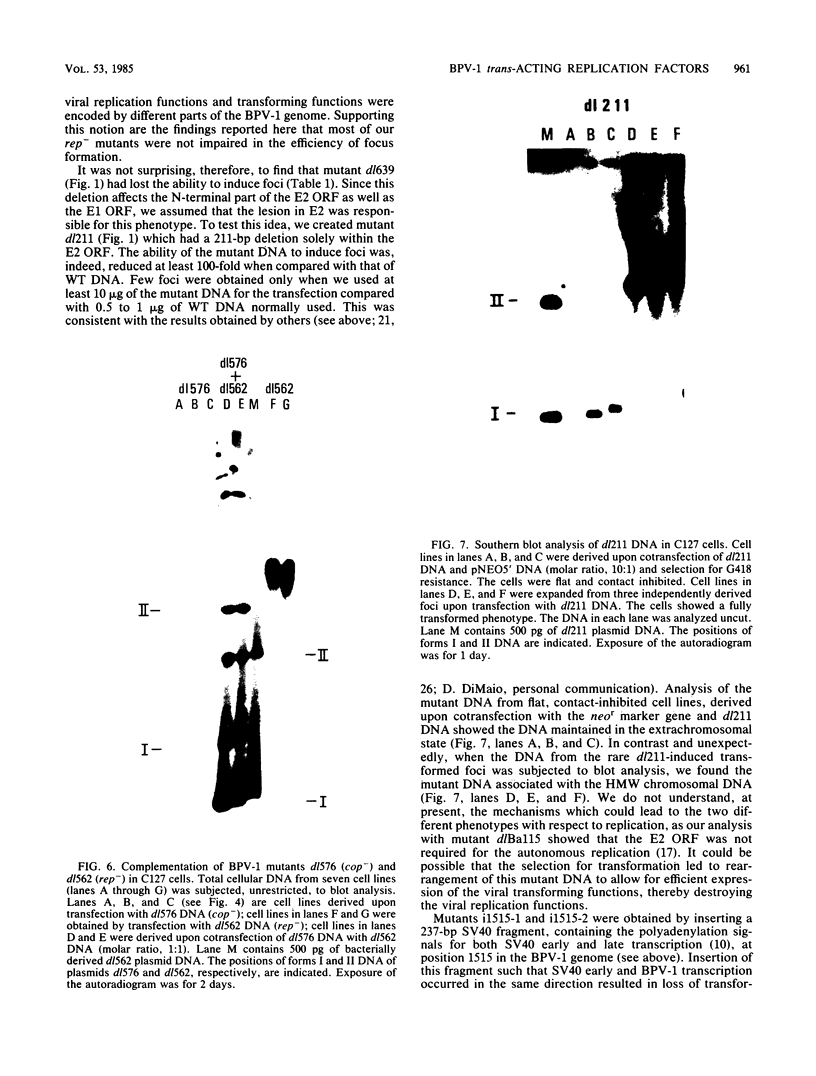




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amtmann E., Sauer G. Bovine papilloma virus transcription: polyadenylated RNA species and assessment of the direction of transcription. J Virol. 1982 Jul;43(1):59–66. doi: 10.1128/jvi.43.1.59-66.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitburd F., Favre M., Zoorob R., Fortin D., Orth G. Detection and characterization of viral genomes and search for tumoral antigens in two hamster cell lines derived from tumors induced by bovine papillomavirus type 1. Int J Cancer. 1981 May 15;27(5):693–702. doi: 10.1002/ijc.2910270517. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Danos O., Engel L. W., Chen E. Y., Yaniv M., Howley P. M. Comparative analysis of the human type 1a and bovine type 1 papillomavirus genomes. J Virol. 1983 May;46(2):557–566. doi: 10.1128/jvi.46.2.557-566.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Heilman C. A., Engel L., Lowy D. R., Howley P. M. Virus-specific transcription in bovine papillomavirus-transformed mouse cells. Virology. 1982 May;119(1):22–34. doi: 10.1016/0042-6822(82)90061-7. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D. Apparent lack of integration of bovine papillomavirus DNA in virus-induced equine and bovine tumor cells and virus-transformed mouse cells. Virology. 1981 Jan 30;108(2):251–255. doi: 10.1016/0042-6822(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Berg L., Weiher H., Botchan M. Bovine papilloma virus contains an activator of gene expression at the distal end of the early transcription unit. Mol Cell Biol. 1983 Jun;3(6):1108–1122. doi: 10.1128/mcb.3.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Tjian R. Construction and analysis of simian virus 40 origins defective in tumor antigen binding and DNA replication. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6491–6495. doi: 10.1073/pnas.77.11.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi Y., Chattopadhyay S. K., Lowy D. R. The transforming function of bovine papillomavirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5832–5836. doi: 10.1073/pnas.80.19.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Rabson M. S., Yang Y. C., Byrne J. C., Howley P. M. Localization and analysis of bovine papillomavirus type 1 transforming functions. J Virol. 1984 Nov;52(2):377–388. doi: 10.1128/jvi.52.2.377-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek L. P., Byrne J. C., Lowy D. R., Dvoretzky I., Friedman R. M., Howley P. M. Interferon induces morphologic reversion with elimination of extrachromosomal viral genomes in bovine papillomavirus-transformed mouse cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7914–7918. doi: 10.1073/pnas.79.24.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein F. O., Stevens J. G. Variable-sized free episomes of Shope papilloma virus DNA are present in all non-virus-producing neoplasms and integrated episomes are detected in some. Proc Natl Acad Sci U S A. 1982 Feb;79(3):790–794. doi: 10.1073/pnas.79.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]