Abstract
Selective DNA extraction and hybridization procedures were used to estimate the relative number of covalently closed circular viral genomes in cultures of Epstein-Barr virus (EBV)-transformed cells. In virus-producing P3HR-1 cultures that were exposed for 11 days to phosphonoacetic acid or to acyclovir, the content of covalently closed circular EBV DNA was reduced ca. 70% relative to a control culture without drug. The EBV plasmid content of Raji, a virus nonproducer cell line, was not reduced by exposure to these compounds. When P3HR-1 cultures were exposed to 12-O-tetradecanoylphorbol-13-acetate, the number of circular genomes per cell increased. These findings indicate that two enzyme activities synthesize circular EBV DNA and that the virus-associated DNA polymerase synthesizes most of the circular EBV DNA in a virus producer culture. It is suggested that the circular genomes synthesized by the viral enzyme are intermediates in the syntheses of linear virus DNA.
Full text
PDF
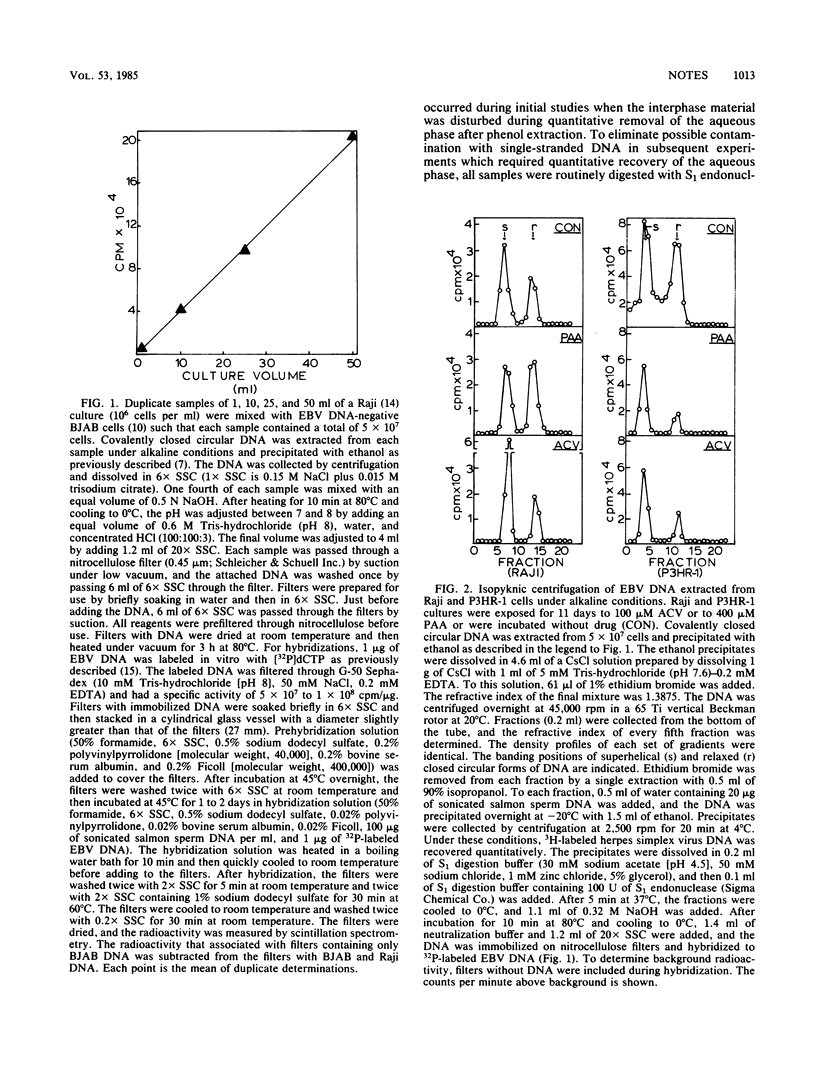
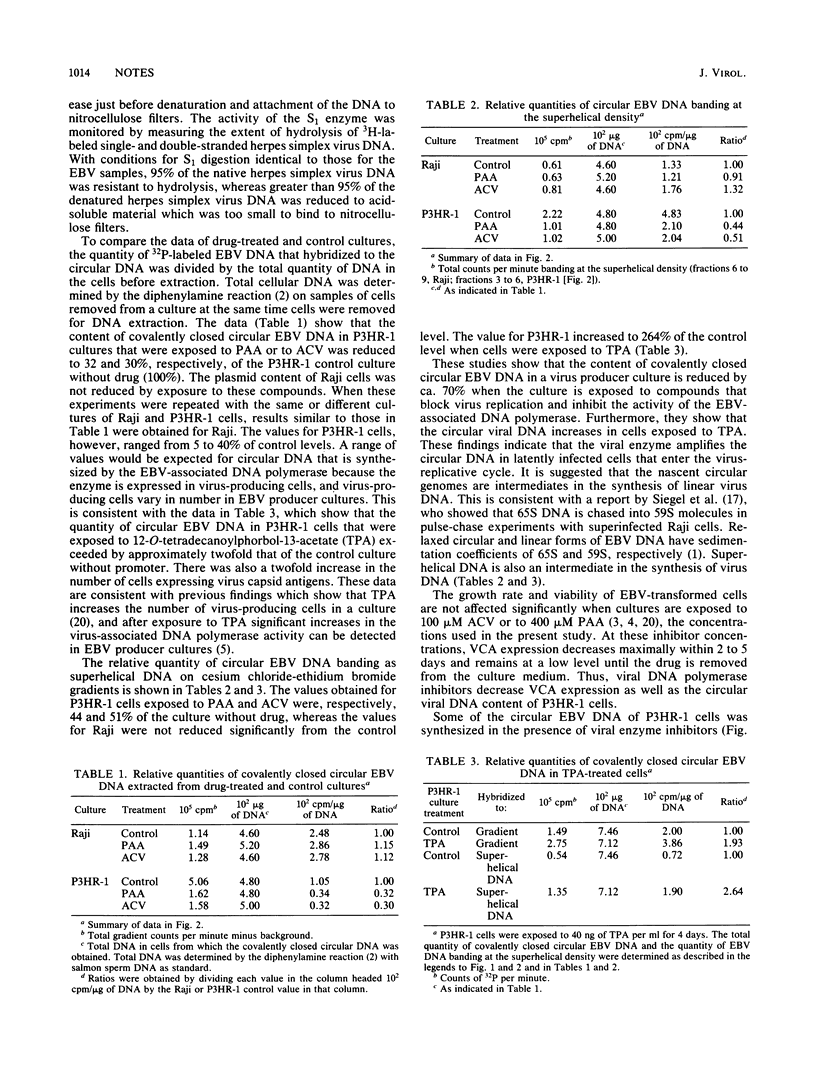

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby B. M., Shaw J. E., Datta A. K., Pagano J. S. Replication of Epstein-Barr virus DNA in lymphoblastoid cells treated for extended periods with acyclovir. Am J Med. 1982 Jul 20;73(1A):77–81. doi: 10.1016/0002-9343(82)90068-7. [DOI] [PubMed] [Google Scholar]
- Colby B. M., Shaw J. E., Elion G. B., Pagano J. S. Effect of acyclovir [9-(2-hydroxyethoxymethyl)guanine] on Epstein-Barr virus DNA replication. J Virol. 1980 May;34(2):560–568. doi: 10.1128/jvi.34.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Feighny R. J., Pagano J. S. Induction of Epstein-Barr virus-associated DNA polymerase by 12-O-tetradecanoylphorbol-13-acetate. Purification and characterization. J Biol Chem. 1980 Jun 10;255(11):5120–5125. [PubMed] [Google Scholar]
- Goodman S. R., Prezyna C., Benz W. C. Two Epstein-Barr virus-associated DNA polymerase activities. J Biol Chem. 1978 Dec 10;253(23):8617–8628. [PubMed] [Google Scholar]
- Griffin B. E., Björck E., Bjursell G., Lindahl T. Sequence complexity of circular Epstein-Bar virus DNA in transformed cells. J Virol. 1981 Oct;40(1):11–19. doi: 10.1128/jvi.40.1.11-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussander E., Adams A. Intracellular state of Epstein-Barr virus DNA in producer cell lines. J Gen Virol. 1979 Nov;45(2):331–340. doi: 10.1099/0022-1317-45-2-331. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R., Pendersen M., Kieff E. Complexity of EBV homologous DNA in continous lymphoblastoid cell lines. Virology. 1976 Oct 1;74(1):227–231. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shaw J. E., Levinger L. F., Carter C. W., Jr Nucleosomal structure of Epstein-Barr virus DNA in transformed cell lines. J Virol. 1979 Feb;29(2):657–665. doi: 10.1128/jvi.29.2.657-665.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel P. J., Clough W., Strominger J. L. Sedimentation characteristics of newly synthesized Epstein-Barr viral DNA in superinfected cells. J Virol. 1981 Jun;38(3):880–885. doi: 10.1128/jvi.38.3.880-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima Y., Tanaka A., Nonoyama M. Inhibition of productive replication of Epstein-Barr virus DNA by phosphonoacetic acid. Virology. 1976 May;71(1):352–354. doi: 10.1016/0042-6822(76)90119-7. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]


