Abstract
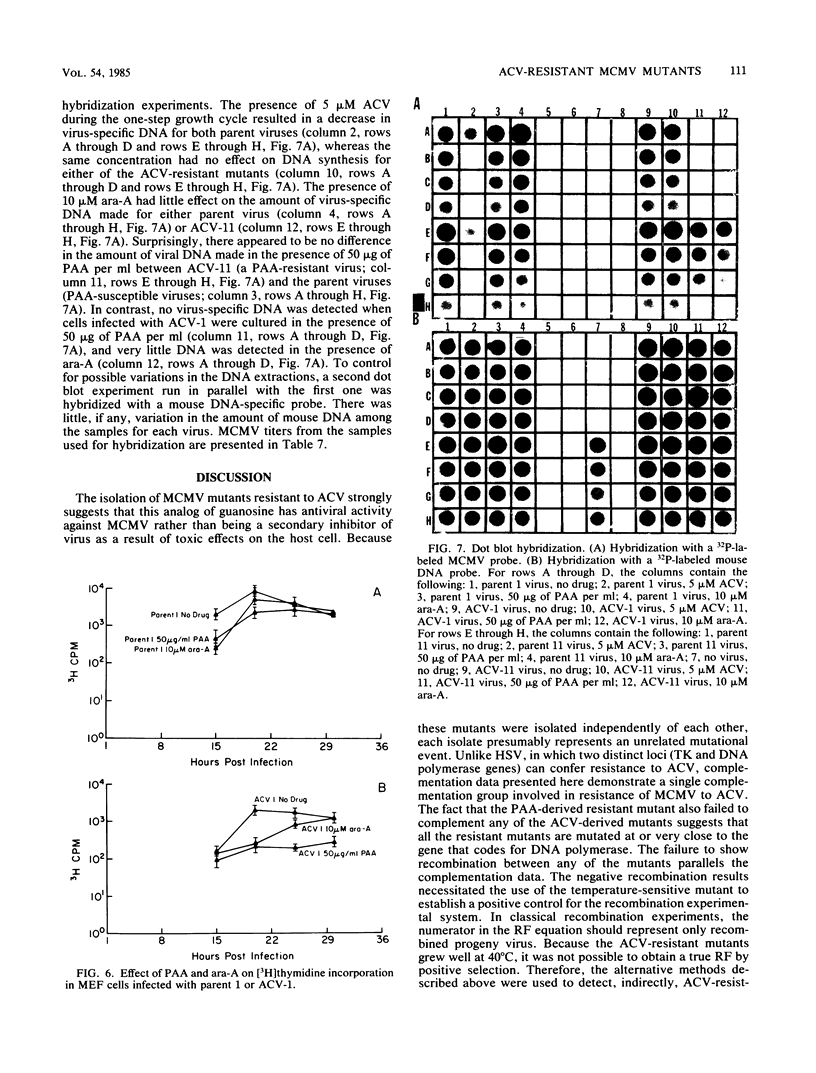
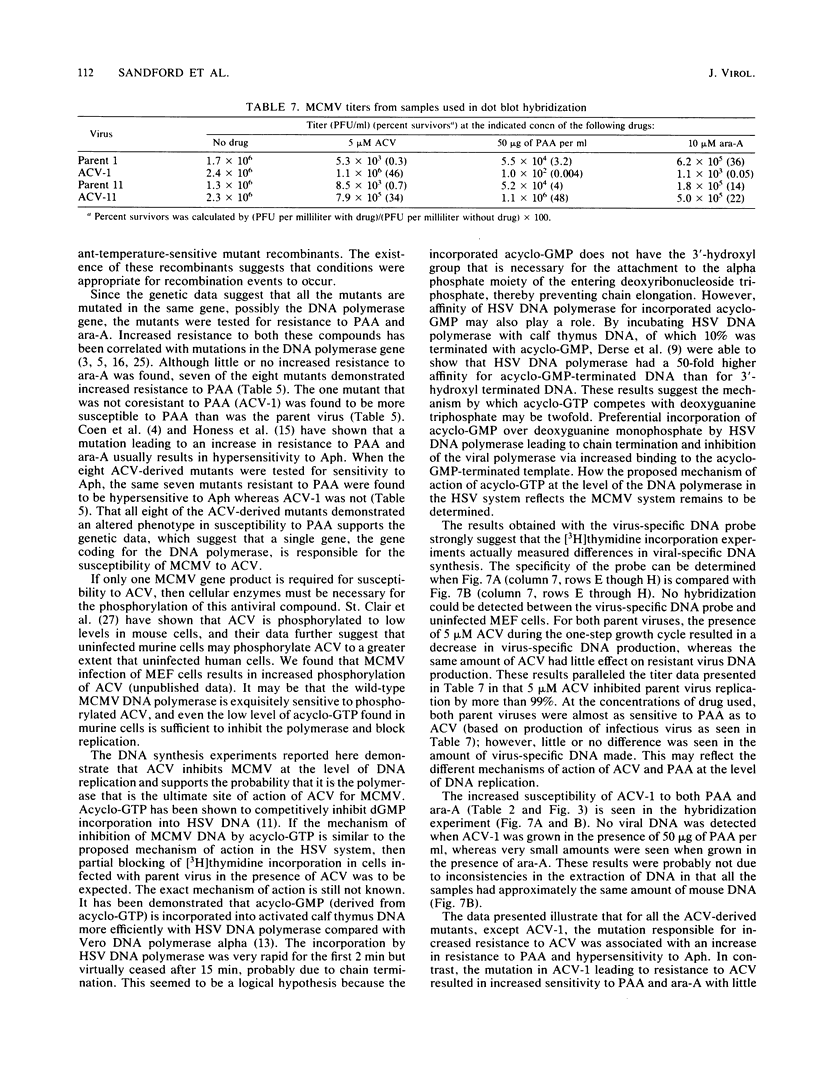
Eight independently derived mouse cytomegalovirus (MCMV) mutants resistant to acyclovir (ACV) were obtained by the sequential plating of wild-type virus in increasing concentrations of ACV. Results of complementation studies among these eight mutants suggest that all had mutations within the same or closely associated genes. A ninth MCMV mutant resistant to phosphonoacetate (PAA) derived by plating wild-type virus in the presence of 100 micrograms of PAA per ml displayed coresistance to ACV and was unable to complement any of the ACV-derived mutants. Recombination experiments among all combinations of the nine MCMV mutants were performed and supported the complementation data in that no recombination could be detected. Seven of the eight ACV-resistant mutants demonstrated cross-resistance to PAA and hypersensitivity to aphidicolin. The one mutant not coresistant to PAA was more susceptible to PAA than was the parent virus. Only a few mutants demonstrated coresistance when the mutants were tested against 9-beta-D-arabinofuranosyladenine (ara-A). The ACV mutant that demonstrated increased susceptibility to PAA was 30-fold more susceptible to ara-A but remained unchanged in susceptibility to aphidicolin. Two of the parent-mutant combinations were selected for DNA synthesis analysis in the presence of ACV (5 microM). A significant decrease in DNA synthesis was demonstrated for both parent viruses, and there was little effect on mutant virus DNA synthesis at the same drug concentration. These results suggest that susceptibility of MCMV to ACV is confined to a product of a single gene and that a mutation of this gene can lead to an altered phenotype when compared with parent virus in susceptibility of DNA synthesis to PAA, ara-A, and aphidicolin, drugs that are known to inhibit DNA polymerase activity.
Full text
PDF


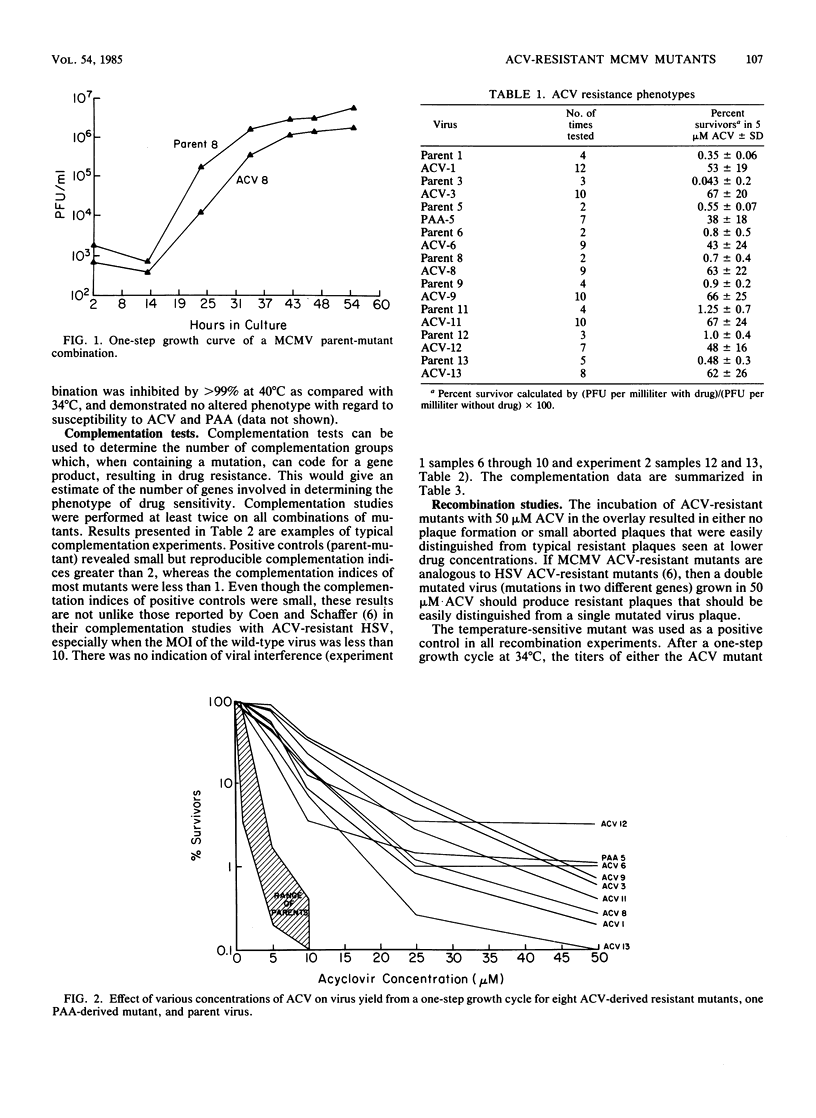
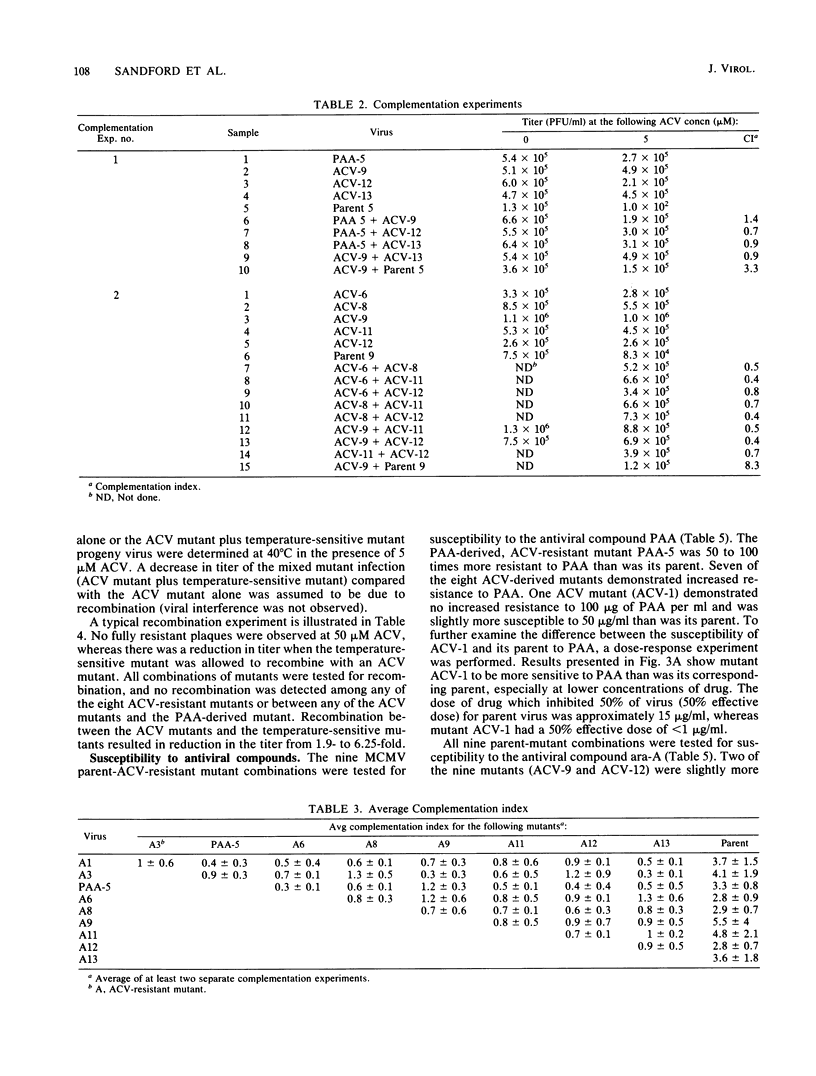
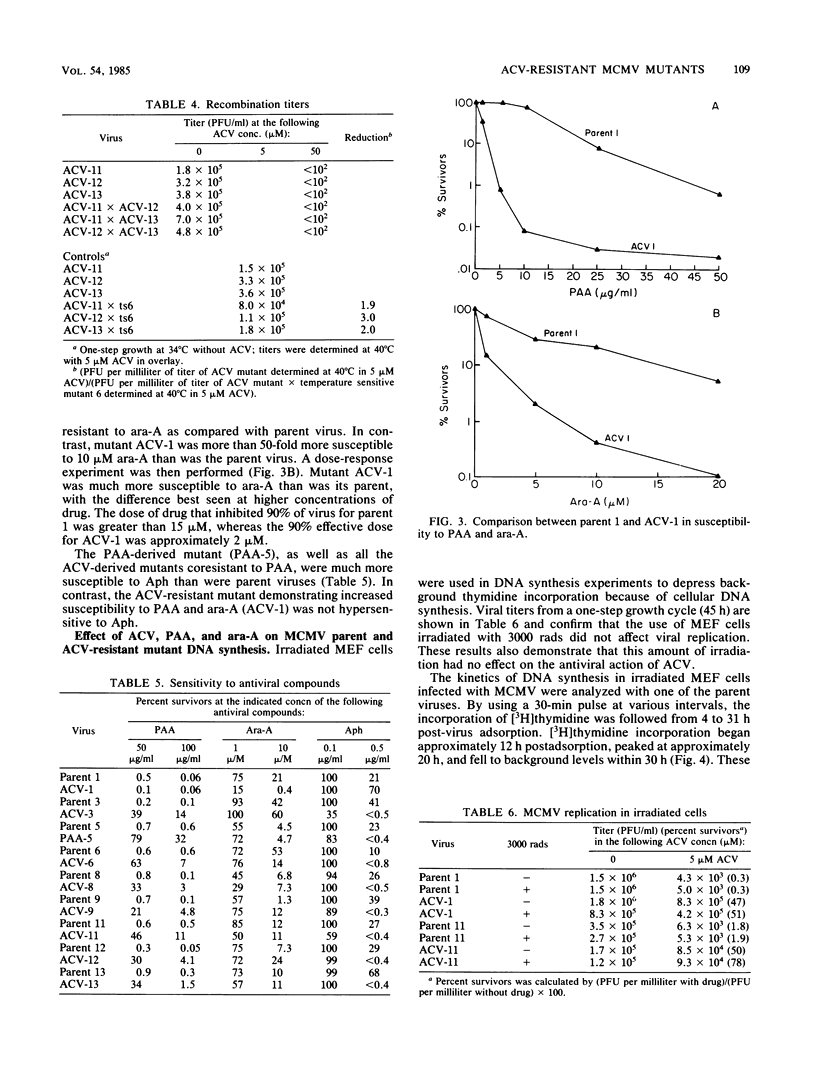
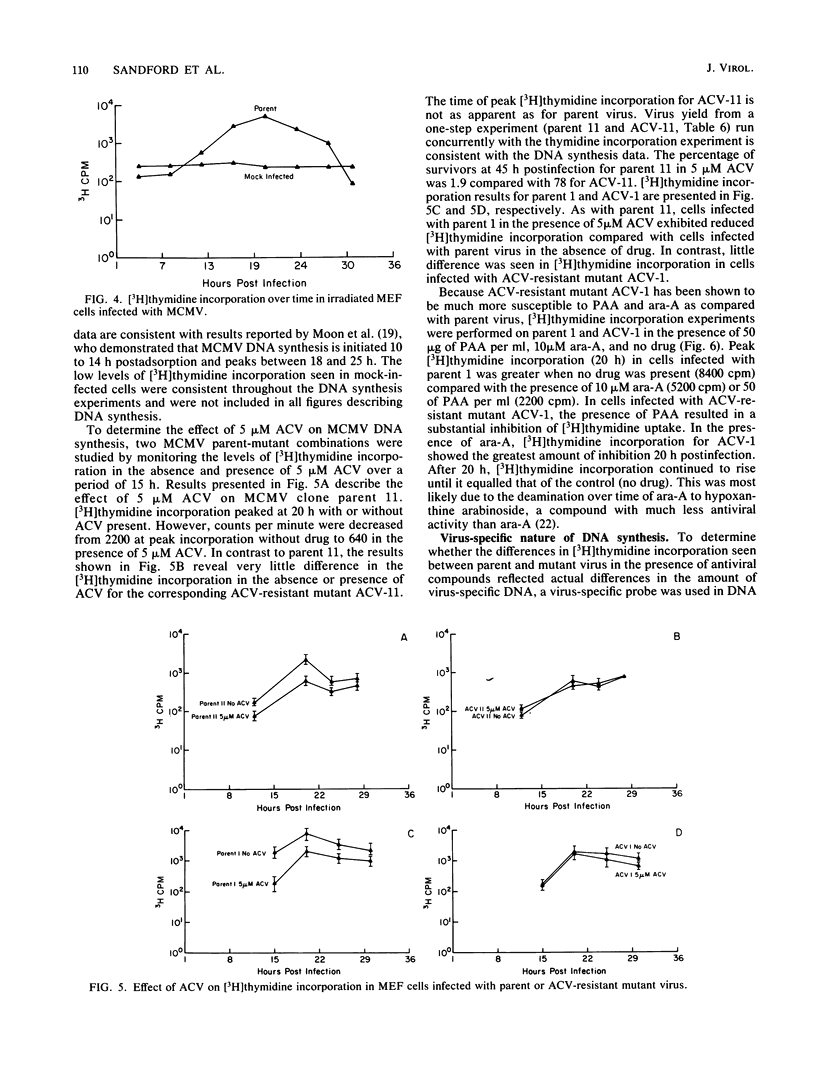

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Burns W. H., Wingard J. R., Bender W. J., Saral R. Thymidine kinase not required for antiviral activity of acyclovir against mouse cytomegalovirus. J Virol. 1981 Sep;39(3):889–893. doi: 10.1128/jvi.39.3.889-893.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand P., Stow N. D., Timbury M. C., Wilkie N. M. Physical mapping of paar mutations of herpes simplex virus type 1 and type 2 by intertypic marker rescue. J Virol. 1979 Aug;31(2):265–276. doi: 10.1128/jvi.31.2.265-276.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Aschman D. P., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene conferring hypersensitivity to aphidicolin. Nucleic Acids Res. 1983 Aug 11;11(15):5287–5297. doi: 10.1093/nar/11.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Gelep P. T., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene can confer resistance to 9-beta-D-arabinofuranosyladenine. J Virol. 1982 Mar;41(3):909–918. doi: 10.1128/jvi.41.3.909-918.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Zaia J. A., Levin M. J. Growth inhibition by acycloguanosine of herpesviruses isolated from human infections. Antimicrob Agents Chemother. 1979 May;15(5):642–645. doi: 10.1128/aac.15.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Derse D., Cheng Y. C., Furman P. A., St Clair M. H., Elion G. B. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J Biol Chem. 1981 Nov 25;256(22):11447–11451. [PubMed] [Google Scholar]
- Eizuru Y., Minamishima Y., Hirano A., Kurimura T. Replication of mouse cytomegalovirus in thymidine kinase-deficient mouse cells. Microbiol Immunol. 1978;22(12):755–764. doi: 10.1111/j.1348-0421.1978.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., Coen D. M., St Clair M. H., Schaffer P. A. Acyclovir-resistant mutants of herpes simplex virus type 1 express altered DNA polymerase or reduced acyclovir phosphorylating activities. J Virol. 1981 Dec;40(3):936–941. doi: 10.1128/jvi.40.3.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Honess R. W., Purifoy D. J., Young D., Gopal R., Cammack N., O'Hare P. Single mutations at many sites within the DNA polymerase locus of herpes simplex viruses can confer hypersensitivity to aphidicolin and resistance to phosphonoacetic acid. J Gen Virol. 1984 Jan;65(Pt 1):1–17. doi: 10.1099/0022-1317-65-1-1. [DOI] [PubMed] [Google Scholar]
- Jofre J. T., Schaffer P. A., Parris D. S. Genetics of resistance to phosphonoacetic acid in strain KOS of herpes simplex virus type 1. J Virol. 1977 Sep;23(3):833–836. doi: 10.1128/jvi.23.3.833-836.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Carp R. I. Abortive infection of human diploid cells by murine cytomegalovirus. Infect Immun. 1972 Nov;6(5):793–797. doi: 10.1128/iai.6.5.793-797.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J Biol Chem. 1980 Aug 10;255(15):7204–7207. [PubMed] [Google Scholar]
- Moon H. M., Sapienza V. J., Carp R. I., Kim K. S. DNA synthesis in mouse embryo fibroblast cells infected with murine cytomegalovirus. Virology. 1976 Dec;75(2):376–383. doi: 10.1016/0042-6822(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Mounts P., Shah K. V., Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5425–5429. doi: 10.1073/pnas.79.17.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Hudson J. B. Thymidine kinase activity in mouse 3T3 cells infected by murine cytomegalovirus (MCV). Virology. 1977 Jul 15;80(2):430–433. doi: 10.1016/s0042-6822(77)80019-6. [DOI] [PubMed] [Google Scholar]
- North T. W., Cohen S. S. Aranucleosides and aranucleotides in viral chemotherapy. Pharmacol Ther. 1979;4(1):81–108. doi: 10.1016/0163-7258(79)90016-0. [DOI] [PubMed] [Google Scholar]
- Overby L. R., Robishaw E. E., Schleicher J. B., Rueter A., Shipkowitz N. L., Mao J. C. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974 Sep;6(3):360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer G., Goodheart C. R. Growth of murine cytomegalovirus in a heterologous cell system and its enhancement by 5-iodo-2'-deoxyuridine. Infect Immun. 1974 Jul;10(1):251–256. doi: 10.1128/iai.10.1.251-256.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purifoy D. J., Lewis R. B., Powell K. L. Identification of the herpes simplex virus DNA polymerase gene. Nature. 1977 Oct 13;269(5629):621–623. doi: 10.1038/269621a0. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Aron G. M., Biswal N., Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973 Mar;52(1):57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- St Clair M. H., Furman P. A., Lubbers C. M., Elion G. B. Inhibition of cellular alpha and virally induced deoxyribonucleic acid polymerases by the triphosphate of acyclovir. Antimicrob Agents Chemother. 1980 Nov;18(5):741–745. doi: 10.1128/aac.18.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard J. R., Bender W. J., Saral R., Burns W. H. Efficacy of acyclovir against mouse cytomegalovirus in vivo. Antimicrob Agents Chemother. 1981 Aug;20(2):275–278. doi: 10.1128/aac.20.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]



