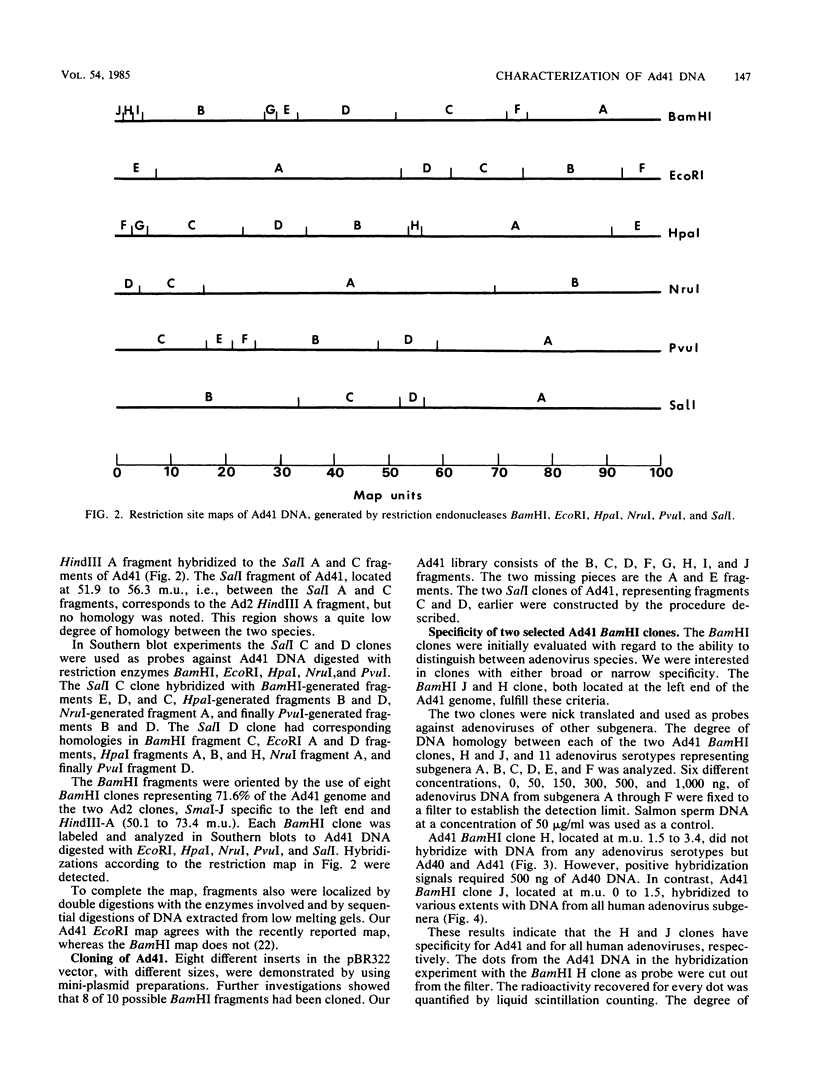
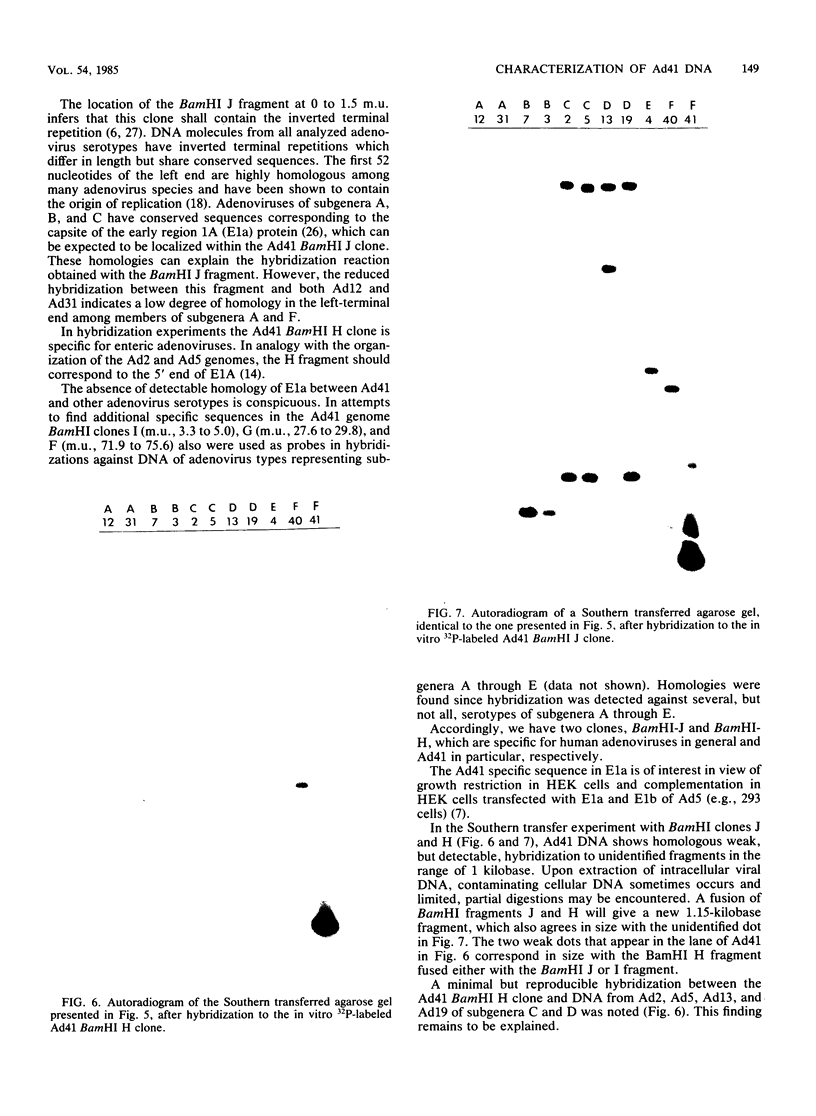
Abstract
Enteric adenovirus type 40 (Ad40) and Ad41 form the sixth subgenus of human adenoviruses. They are associated with infantile diarrhea but cannot be isolated in conventional cell cultures. The genome of the fastidious enteric Ad41 has been cloned, and the cleavage sites of the genome produced by restriction endonucleases BamHI, EcoRI, HpaI, NruI, PvuI, and SalI have been mapped. To develop useful hybridization methods for direct detection of adenoviruses, a restriction fragment library containing Ad41 DNA, with plasmid pBR322 as vector, has been constructed. Clones have been isolated which contain 8 of 10 possible BamHI fragments of Ad41, inserted into the BamHI cleavage site of the vector. Two of these clones are particularly useful for the detection of adenoviruses. One clone detects members of all human adenovirus subgenera, and the second clone is specific for enteric adenoviruses, in particular Ad41. A conspicuous absence of detectable homology was noted at 1.5 to 3.3 map units of the Ad41 genome in hybridizations against other serotypes of adenoviruses, including the closely related enteric Ad40. This sequence corresponds to the 5' portion of early region Ia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Yolken R. H., Kapikian A. Z., Arrobio J. O., Rodriguez W. J., Wyatt R. G., Chanock R. M., Parrott R. H. Comparative epidemiology of two rotavirus serotypes and other viral agents associated with pediatric gastroenteritis. Am J Epidemiol. 1979 Sep;110(3):243–254. doi: 10.1093/oxfordjournals.aje.a112809. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Stålhandske P., Vainionpä R., Pettersson U. Detection of enteroviruses by spot hybridization. J Clin Microbiol. 1984 Mar;19(3):436–438. doi: 10.1128/jcm.19.3.436-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd A. H., Banatvala J. E., de Jong J. C. Antibodies to fastidious faecal adenoviruses (species 40 and 41) in sera from children. J Med Virol. 1983;11(4):333–341. doi: 10.1002/jmv.1890110409. [DOI] [PubMed] [Google Scholar]
- Kidd A. H. Genome variants of adenovirus 41 (subgroup G) from children with diarrhoea in South Africa. J Med Virol. 1984;14(1):49–59. doi: 10.1002/jmv.1890140108. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Madeley C. R. In vitro growth of some fastidious adenoviruses from stool specimens. J Clin Pathol. 1981 Feb;34(2):213–216. doi: 10.1136/jcp.34.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Seed B. Two-dimensional agarose gel electrophoresis "SeaPlaque" agarose dimension. Methods Enzymol. 1980;65(1):358–363. [PubMed] [Google Scholar]
- Ranki M., Virtanen M., Palva A., Laaksonen M., Pettersson R., Käriäinen L., Halonen P., Söderlund H. Nucleic acid sandwich hybridization in adenovirus diagnosis. Curr Top Microbiol Immunol. 1983;104:307–318. doi: 10.1007/978-3-642-68949-9_20. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sleigh M., Engler J. A., Broker T. R. The evolution of the adenoviral genome. Ann N Y Acad Sci. 1980;354:426–452. doi: 10.1111/j.1749-6632.1980.tb27983.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stålhandske P., Hyypiä T., Gadler H., Halonen P., Pettersson U. The use of molecular hybridization for demonstration of adenoviruses in human stools. Curr Top Microbiol Immunol. 1983;104:299–306. doi: 10.1007/978-3-642-68949-9_19. [DOI] [PubMed] [Google Scholar]
- Svensson L., Wadell G., Uhnoo I., Johansson M., Von Bonsdorff C. H. Cross-reactivity between enteric adenoviruses and adenovirus type 4: analysis of epitopes by solid-phase immune electron microscopy. J Gen Virol. 1983 Nov;64(Pt 11):2517–2520. doi: 10.1099/0022-1317-64-11-2517. [DOI] [PubMed] [Google Scholar]
- Takiff H. E., Reinhold W., Garon C. F., Straus S. E. Cloning and physical mapping of enteric adenoviruses (candidate types 40 and 41). J Virol. 1984 Jul;51(1):131–136. doi: 10.1128/jvi.51.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiff H. E., Straus S. E., Garon C. F. Propagation and in vitro studies of previously non-cultivable enteral adenoviruses in 293 cells. Lancet. 1981 Oct 17;2(8251):832–834. doi: 10.1016/s0140-6736(81)91104-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984 Sep;20(3):365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Adenovirus-2 DNA contains an inverted terminal repetition. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3054–3057. doi: 10.1073/pnas.69.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Kidd A. H., Wadell G., Kapsenberg J. G., Muzerie C. J., Wermenbol A. G., Firtzlaff R. G. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11(3):215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]
- van Ormondt H., Galibert F. Nucleotide sequences of adenovirus DNAs. Curr Top Microbiol Immunol. 1984;110:73–142. doi: 10.1007/978-3-642-46494-2_4. [DOI] [PubMed] [Google Scholar]