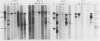Abstract
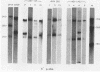
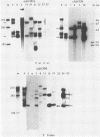
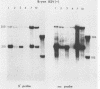
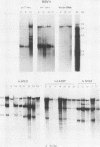
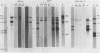
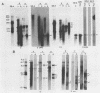
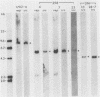
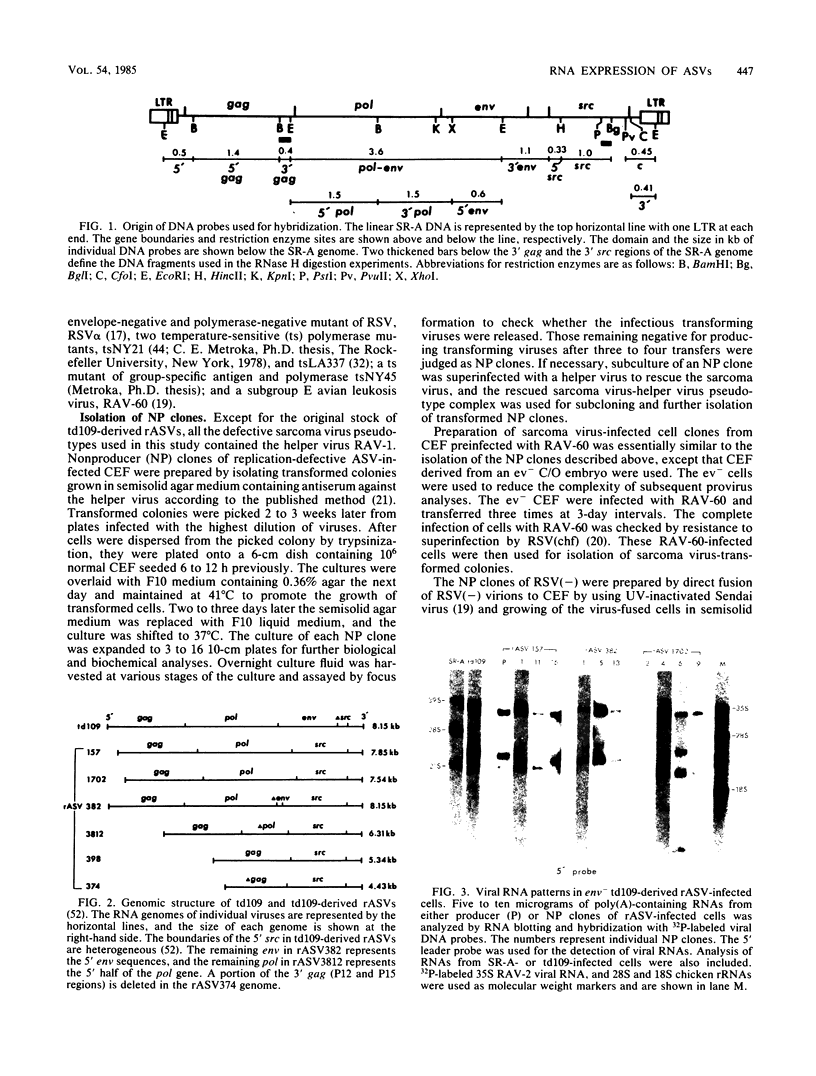
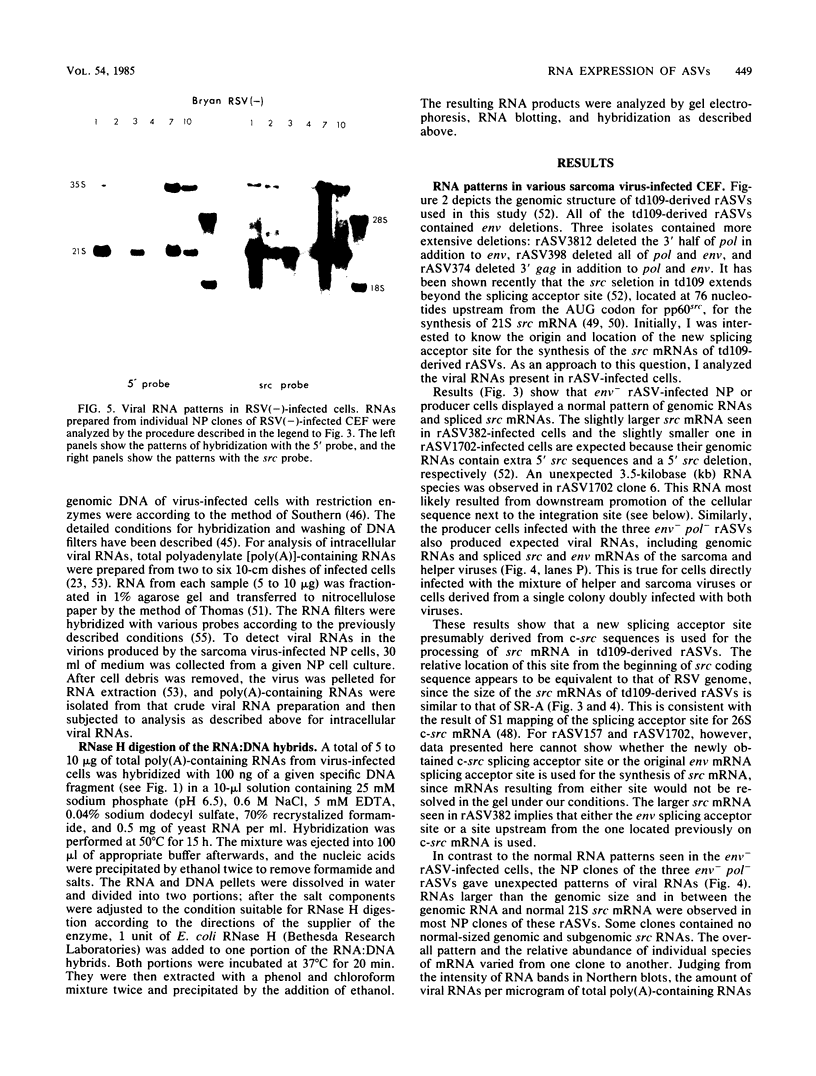
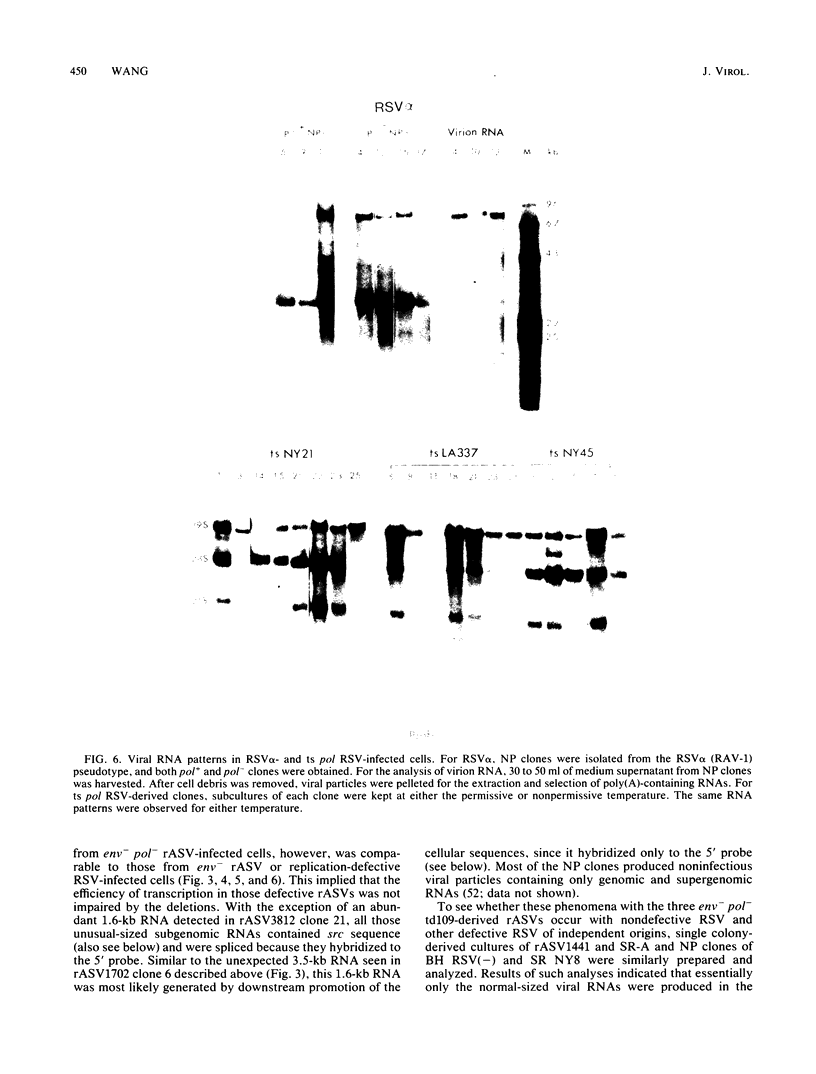
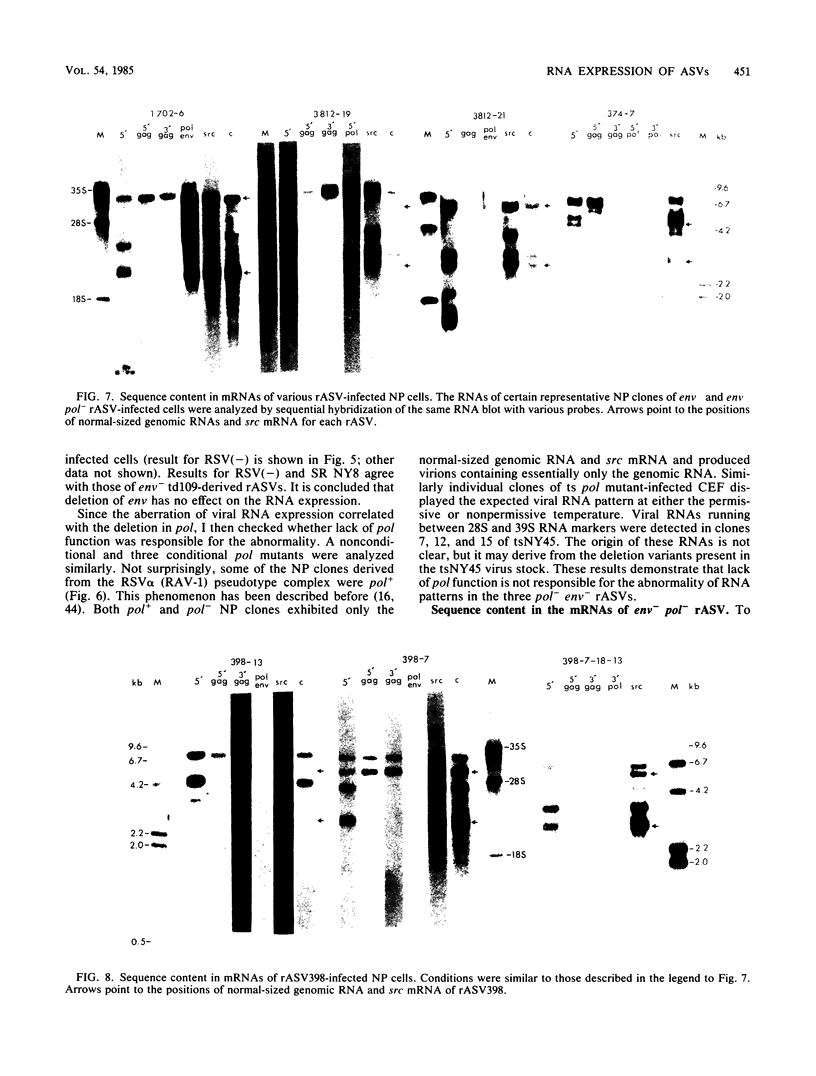
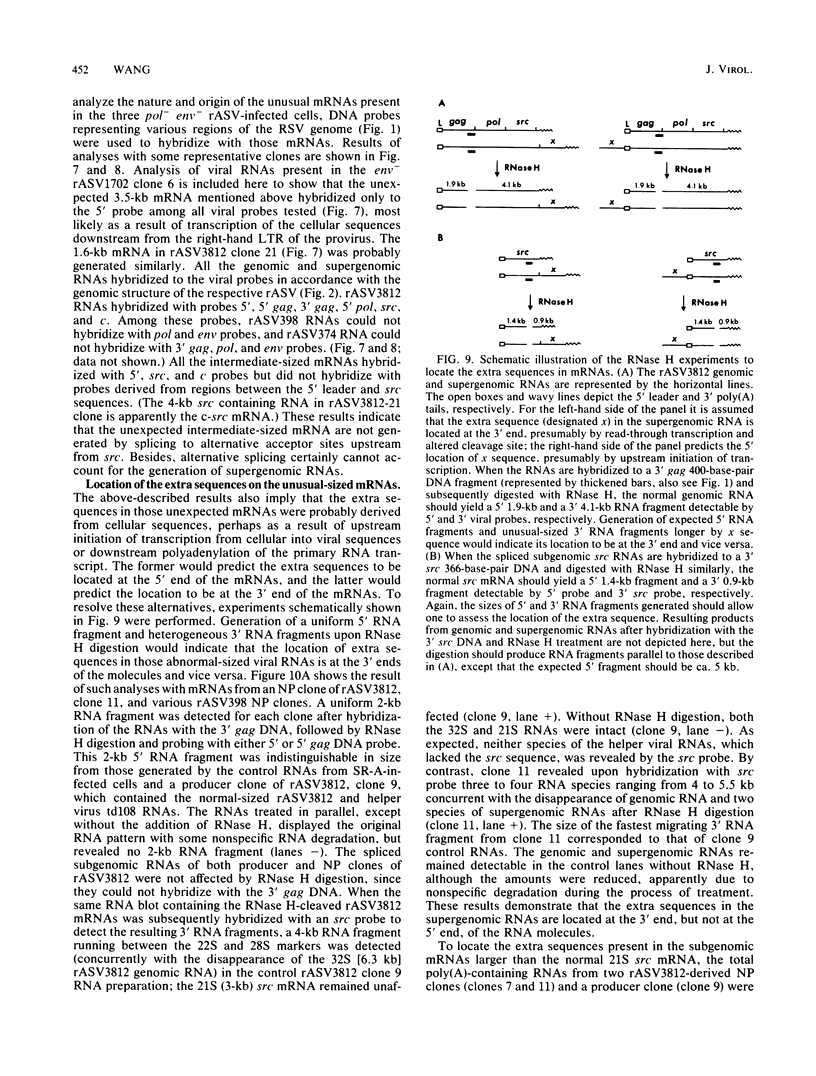
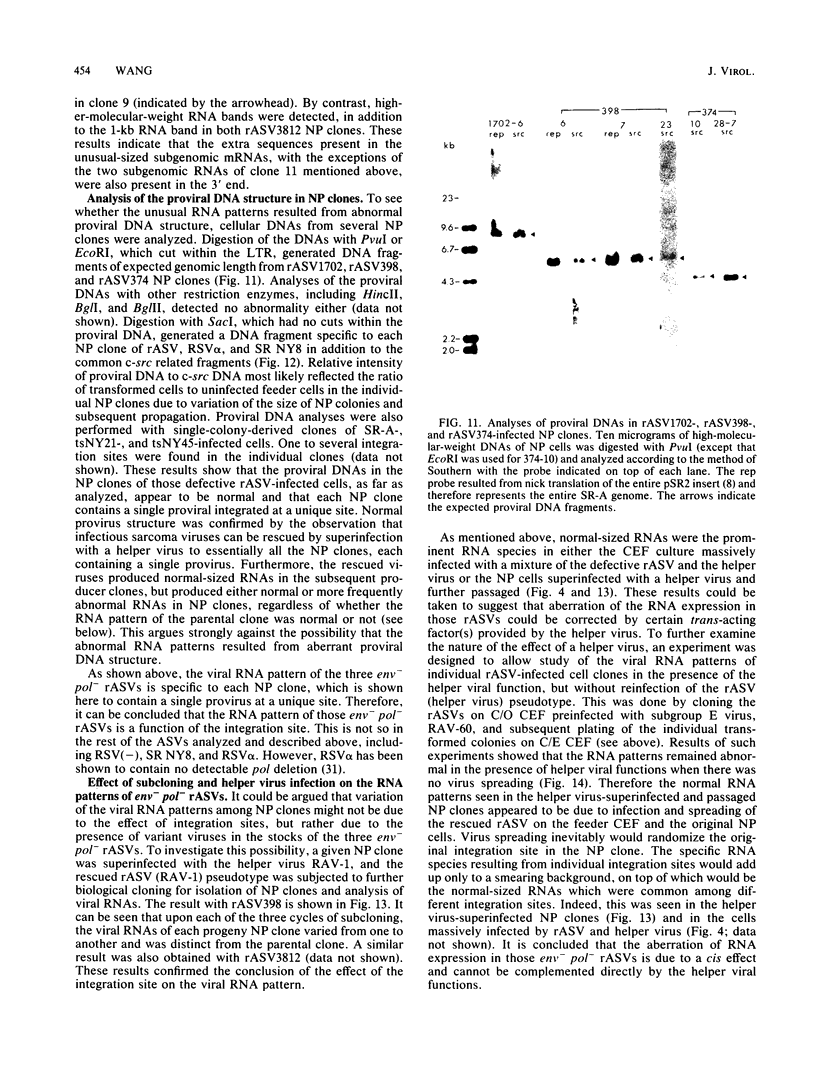
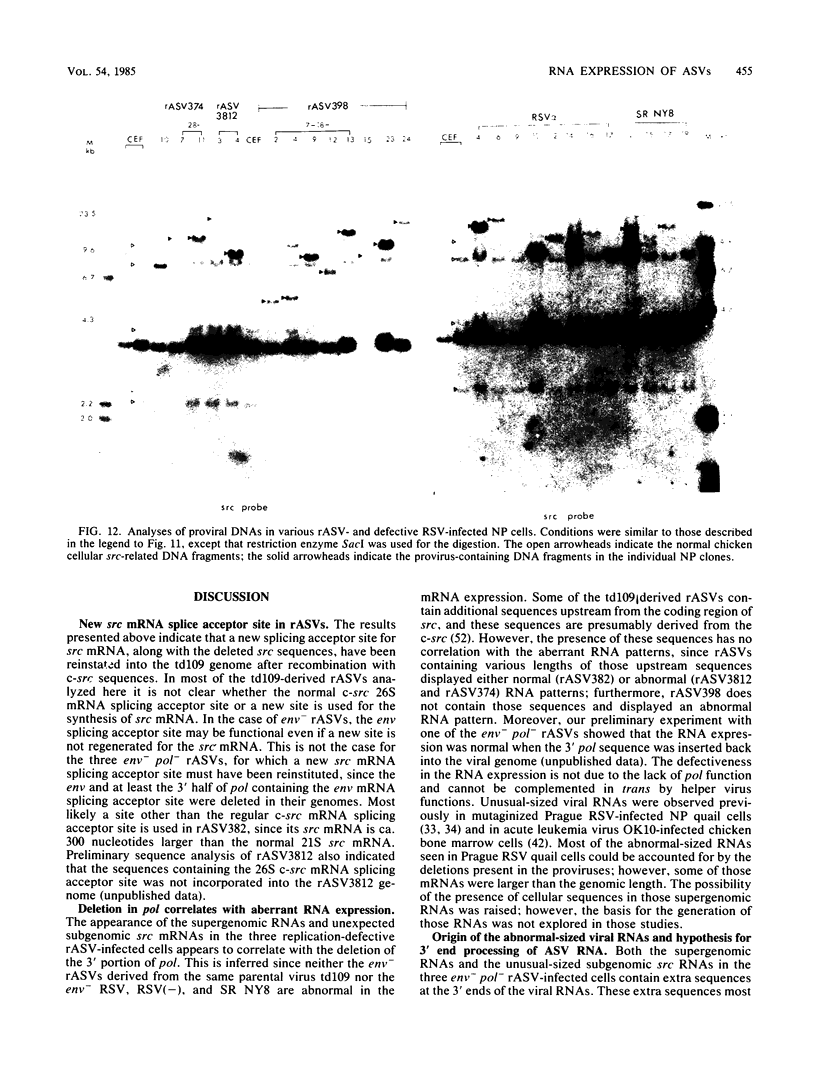
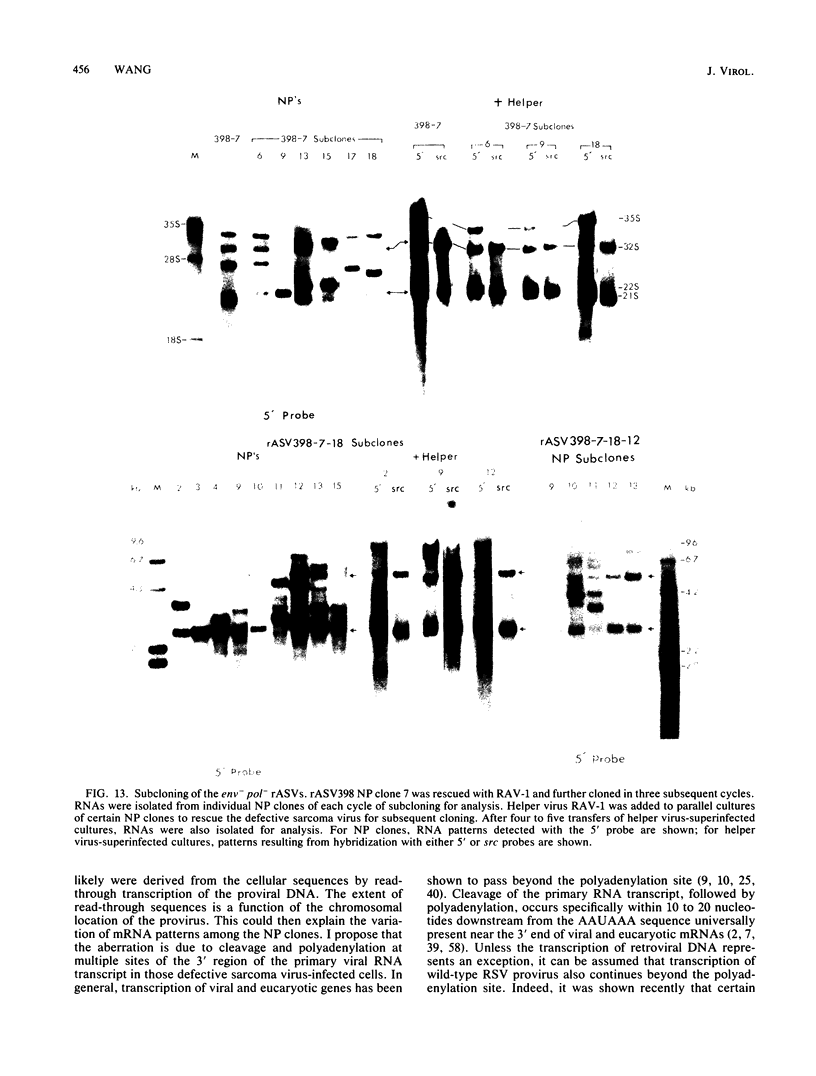
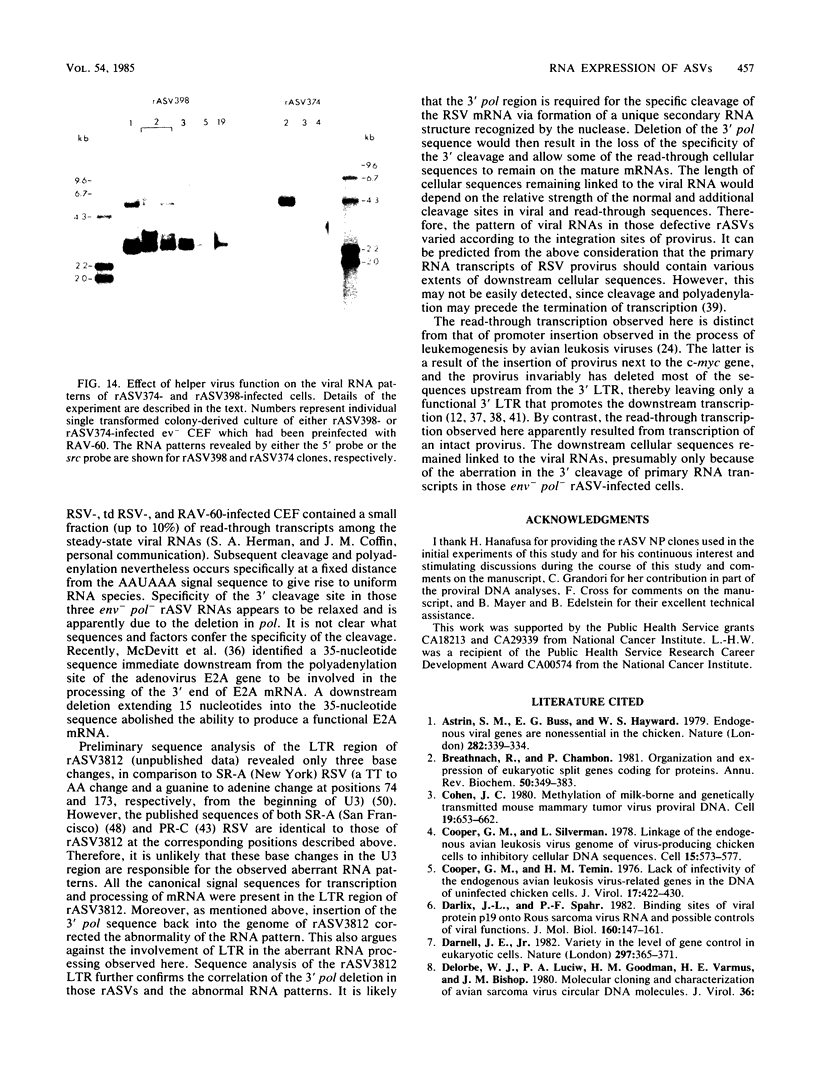
The RNA expression of a series of replication-defective recovered avian sarcoma viruses (rASVs) were studied. Abnormal-sized viral RNAs, both larger and smaller than the genome, were observed in the nonproducer cells infected with rASVs containing env and pol deletions. Each nonproducer clone contained a single provirus integrated at a unique site and expressed a unique RNA pattern. Upon rescuing of the sarcoma virus with a helper virus and subsequent cloning, the RNA pattern of individual nonproducer clones again displayed variation according to the integration sites. This was not seen in nondefective rASV or in rASVs containing only an env deletion. The aberrant RNA expression did not result from the lack of reverse transcriptase activity per se, since neither nonconditional nor temperature-sensitive mutants of RSV expressed abnormal viral RNAs in the absence of a functional reverse transcriptase. The abnormal RNA patterns could not be corrected in trans by helper virus functions. The unusual-sized RNAs in env- pol- rASV-infected cells are not due to splicing to alternative acceptor sites for src mRNA because there are no extra viral sequences between the 5' leader and the src sequences; instead, they are due to the presence of extra sequences, most likely of cellular origin, at the 3' ends of the viral RNAs. Based upon the extent of deletions in the viral genomes, the data suggest that deletion in the 3' pol region of those rASVs results in a cis effect on the transcription and processing of the 3' ends of viral RNAs. The unusual-sized viral RNAs are most likely due to read-through transcription from the right-hand terminus of provirus into downstream cellular sequences, followed by cleavage and polyadenylation at multiple sites of the 3' region of the RNA transcripts. The extent of read-through transcription appears to depend on the chromosomal location of the provirus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Silverman L. Linkage of the endogenous avian leukosis virus genome of virus-producing chicken cells to inhibitory cellular DNA sequences. Cell. 1978 Oct;15(2):573–577. doi: 10.1016/0092-8674(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Lack of infectivity of the endogenous avian leukosis virus-related genes in the DNA of uninfected chicken cells. J Virol. 1976 Feb;17(2):422–430. doi: 10.1128/jvi.17.2.422-430.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F. Binding sites of viral protein P19 onto Rous sarcoma virus RNA and possible controls of viral functions. J Mol Biol. 1982 Sep 15;160(2):147–161. doi: 10.1016/0022-2836(82)90172-3. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Ford J. P., Hsu M. T. Transcription pattern of in vivo-labeled late simian virus 40 RNA: equimolar transcription beyond the mRNA 3' terminus. J Virol. 1978 Dec;28(3):795–801. doi: 10.1128/jvi.28.3.795-801.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. W., Nevins J. R., Ziff E., Darnell J. E., Jr The major late adenovirus type-2 transcription unit: termination is downstream from the last poly(A) site. J Mol Biol. 1979 Apr 25;129(4):643–656. doi: 10.1016/0022-2836(79)90474-1. [DOI] [PubMed] [Google Scholar]
- Fuhrman S. A., Van Beveren C., Verma I. M. Identification of a RNA polymerase II initiation site in the long terminal repeat of Moloney murine leukemia viral DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5411–5415. doi: 10.1073/pnas.78.9.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin G. M., Parsons J. T. Identification of transcriptional elements within the long terminal repeat of Rous sarcoma virus. Mol Cell Biol. 1983 Oct;3(10):1834–1845. doi: 10.1128/mcb.3.10.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Rao P. Y., Mitsialis S. A., Katz R. Modification of avian sarcoma proviral DNA sequences in nonpermissive XC cells but not in permissive chicken cells. J Virol. 1980 May;34(2):569–572. doi: 10.1128/jvi.34.2.569-572.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Further studies on RSV production form transformed cells. Virology. 1968 Apr;34(4):630–636. doi: 10.1016/0042-6822(68)90084-6. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T., Fleissner E. Existence and expression of tumor virus genes in chick embryo cells. Virology. 1972 Feb;47(2):475–482. doi: 10.1016/0042-6822(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T. Recovery of a new virus from apparently normal chick cells by infection with avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1797–1803. doi: 10.1073/pnas.67.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Miyamoto T., Hanafusa H. A type of chick embryo cell that fails to support formation of infectious RSV. Virology. 1970 Jan;40(1):55–64. doi: 10.1016/0042-6822(70)90378-8. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Glover C., Weiss R. A., Arrand J. R. Differences between the endogenous and exogenous DNA sequences of Rous-associated virus-O. Cell. 1979 Nov;18(3):803–815. doi: 10.1016/0092-8674(79)90133-8. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Endogenous retroviruses. Cell. 1983 Jan;32(1):5–6. doi: 10.1016/0092-8674(83)90491-9. [DOI] [PubMed] [Google Scholar]
- Ju G., Skalka A. M. Nucleotide sequence analysis of the long terminal repeat (LTR) of avian retroviruses: structural similarities with transposable elements. Cell. 1980 Nov;22(2 Pt 2):379–386. doi: 10.1016/0092-8674(80)90348-7. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Scheible P., Smith R. E. Correlation of RNA binding affinity of avian oncornavirus p19 proteins with the extent of processing of virus genome RNA in cells. J Virol. 1980 Sep;35(3):722–731. doi: 10.1128/jvi.35.3.722-731.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner T. L., Skalka A. M., Hanafusa H. Integration of Rous sarcoma virus DNA into chicken embryo fibroblasts: no preferred proviral acceptor site in the DNA of clones of singly infected transformed chicken cells. J Virol. 1981 Nov;40(2):421–430. doi: 10.1128/jvi.40.2.421-430.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Mason W. S. Characterization of two conditional early mutants of Rous sarcoma virus. Virology. 1973 May;53(1):258–273. doi: 10.1016/0042-6822(73)90484-4. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Radke K., Hughes S., Quintrell N., Bishop J. M., Varmus H. E. Mutants of Rous sarcoma virus with extensive deletions of the viral genome. Virology. 1979 Jul 30;96(2):530–546. doi: 10.1016/0042-6822(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Mason W. S., Linial M., Hsu T. W., Eisenman R. N., Townsend J., Mark G. E., Seal G., Aldrich C., Taylor J. M. Alterations in the genomes of avian sarcoma viruses. Virology. 1982 Mar;117(2):456–474. doi: 10.1016/0042-6822(82)90484-6. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Shibuya M., Samarut J., Hanafusa H. Revertants and partial transformants of rat fibroblasts infected with Fujinami sarcoma virus. J Virol. 1984 May;50(2):325–334. doi: 10.1128/jvi.50.2.325-334.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt M. A., Imperiale M. J., Ali H., Nevins J. R. Requirement of a downstream sequence for generation of a poly(A) addition site. Cell. 1984 Jul;37(3):993–999. doi: 10.1016/0092-8674(84)90433-1. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Neiman P., Beemon K., Luce J. A. Independent recombination between avian leukosis virus terminal sequences and host DNA in virus-induced proliferative disease. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1896–1900. doi: 10.1073/pnas.78.3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Saule S., Sergeant A., Torpier G., Raes M. B., Pfeifer S., Stehelin D. Subgenomic mRNA in OK10 defective leukemia virus-transformed cells. J Virol. 1982 Apr;42(1):71–82. doi: 10.1128/jvi.42.1.71-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Rettenmier C. W., Hanafusa H. Formation of Rous associated virus-60: origin of the polymerase gene. J Virol. 1979 Mar;29(3):856–862. doi: 10.1128/jvi.29.3.856-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Wang L. H., Hanafusa H. Molecular cloning of the Fujinami sarcoma virus genome and its comparison with sequences of other related transforming viruses. J Virol. 1982 Jun;42(3):1007–1016. doi: 10.1128/jvi.42.3.1007-1016.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stuhlmann H., Jähner D., Jaenisch R. Infectivity and methylation of retroviral genomes is correlated with expression in the animal. Cell. 1981 Oct;26(2 Pt 2):221–232. doi: 10.1016/0092-8674(81)90305-6. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., DeLorbe W. J., Bishop J. M., Varmus H. E. Nucleotide sequence of cloned unintegrated avian sarcoma virus DNA: viral DNA contains direct and inverted repeats similar to those in transposable elements. Proc Natl Acad Sci U S A. 1981 Jan;78(1):124–128. doi: 10.1073/pnas.78.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Beckson M., Anderson S. M., Hanafusa H. Identification of the viral sequence required for the generation of recovered avian sarcoma viruses and characterization of a series of replication-defective recovered avian sarcoma viruses. J Virol. 1984 Mar;49(3):881–891. doi: 10.1128/jvi.49.3.881-891.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Edelstein B., Mayer B. J. Induction of tumors and generation of recovered sarcoma viruses by, and mapping of deletions in, two molecularly cloned src deletion mutants. J Virol. 1984 Jun;50(3):904–913. doi: 10.1128/jvi.50.3.904-913.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Feldman R., Shibuya M., Hanafusa H., Notter M. F., Balduzzi P. C. Genetic structure, transforming sequence, and gene product of avian sarcoma virus UR1. J Virol. 1981 Oct;40(1):258–267. doi: 10.1128/jvi.40.1.258-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Halpern C. C., Nadel M., Hanafusa H. Recombination between viral and cellular sequences generates transforming sarcoma virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5812–5816. doi: 10.1073/pnas.75.12.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., de Crombrugghe B., Pastan I. Identification of a functional promoter in the long terminal repeat of Rous sarcoma virus. Cell. 1980 Dec;22(3):787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]