Abstract
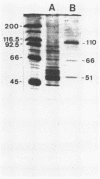
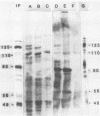
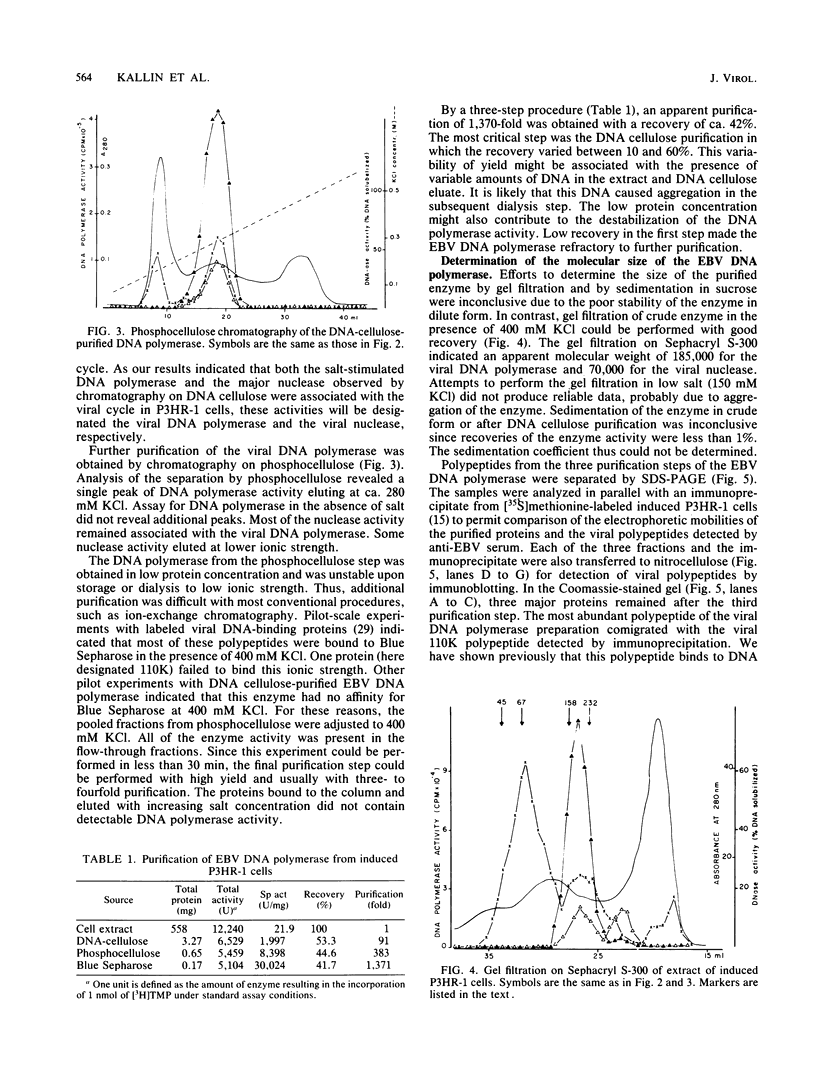
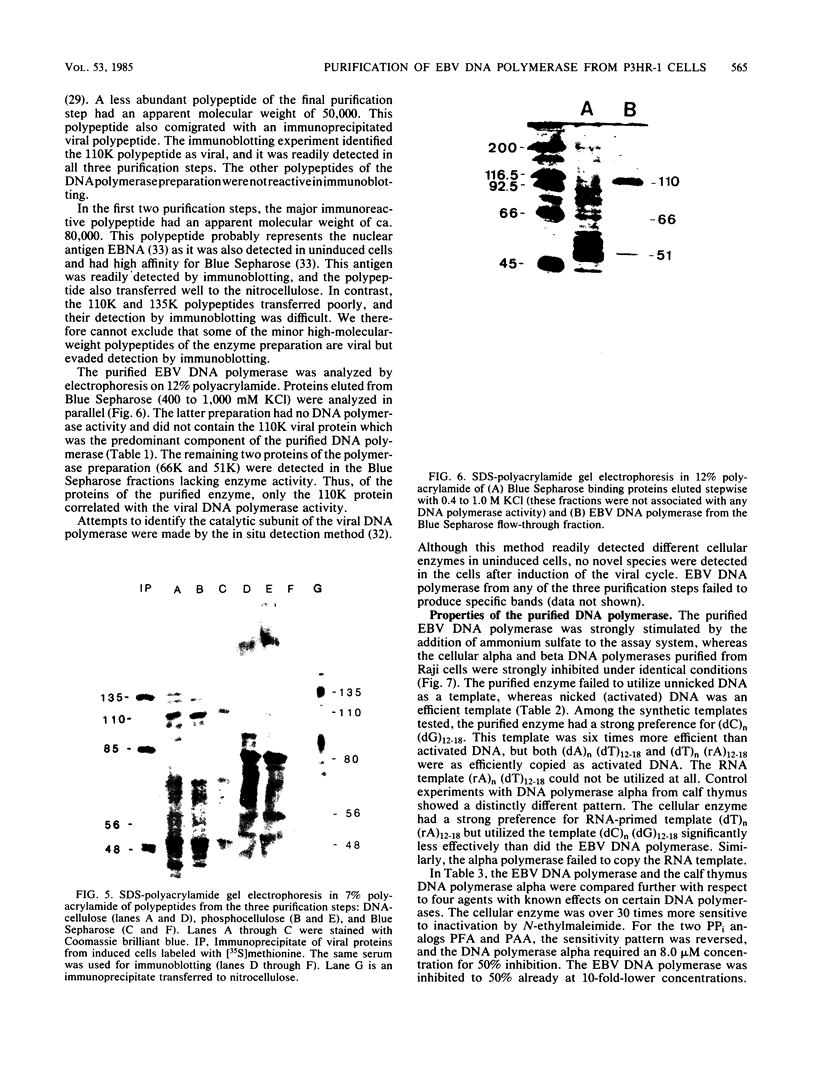
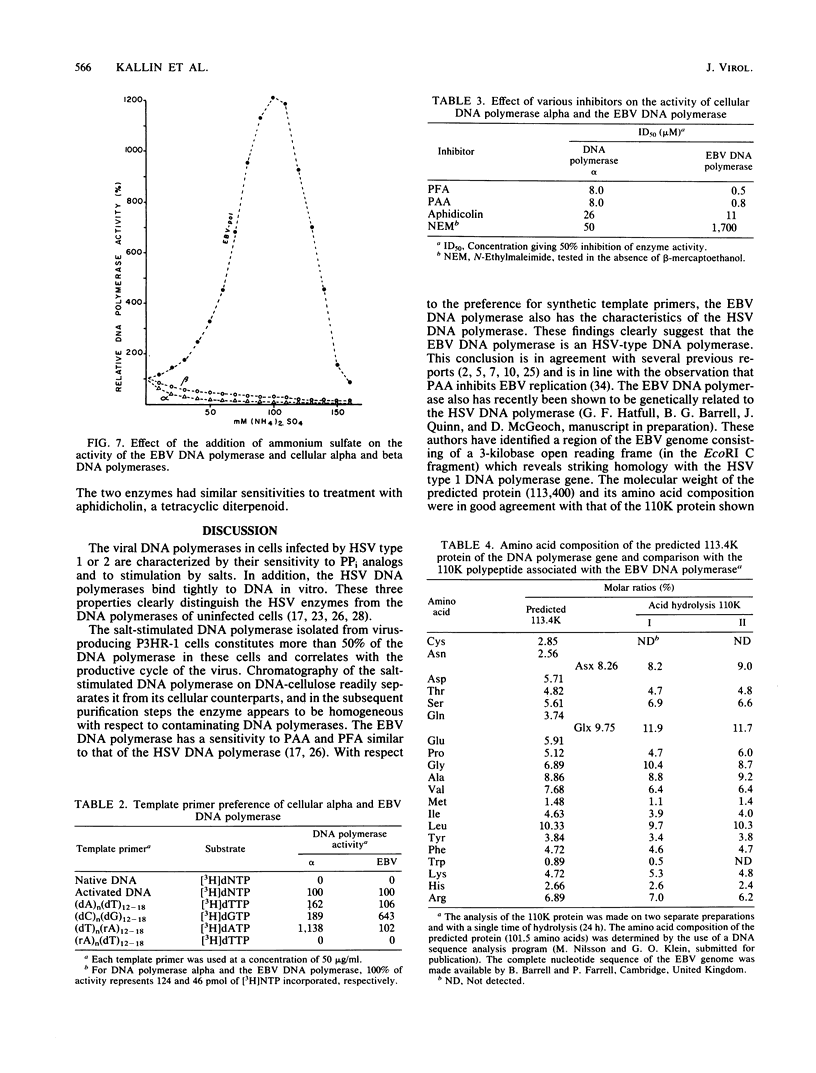
The Epstein-Barr virus DNA polymerase was purified from extracts of P3HR-1 cells treated with n-butyrate for induction of the viral cycle. Sequential chromatography on DNA cellulose, phosphocellulose, and blue Sepharose yielded an enzyme preparation purified more than 1,300-fold. The purified enzyme was distinct from cellular enzymes but resembled the viral DNA polymerase in cells infected with herpes simplex virus type 1 or 2. The active enzyme had an apparent molecular weight of 185,000 as estimated by gel filtration on Sephacryl S-300. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed a major polypeptide corresponding to a molecular weight of ca. 110,000. This polypeptide correlated with the catalytic function of the purified enzyme, whereas the other, less abundant polypeptides did not. By immunoblotting, the 110,000-molecular-weight polypeptide could be identified as a viral polypeptide. It could not be determined whether the native enzyme was composed of more than one polypeptide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Bertino J. R. Isolation of a herpesvirus-specific DNA polymerase from tissues of an American patient with Burkitt lymphoma. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4504–4508. doi: 10.1073/pnas.75.9.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaudeen H. S., Rani G. Cellular and Epstein-Barr virus specific DNA polymerases in virus-producing Burkitt's lymphoma cell lines. Nucleic Acids Res. 1982 Apr 10;10(7):2453–2465. doi: 10.1093/nar/10.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. P., O'Callaghan D. J., Randall C. C. Purification and characterization of equine herpesvirus-induced DNA. Virology. 1977 Jan;76(1):395–408. doi: 10.1016/0042-6822(77)90311-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Chen J. Y., Hoffmann P. J., Glaser R. Studies on the activity of DNase associated with the replication of the Epstein-Barr virus. Virology. 1980 Jan 30;100(2):334–338. doi: 10.1016/0042-6822(80)90524-3. [DOI] [PubMed] [Google Scholar]
- Clough W. Deoxyribonuclease activity found in Epstein--Barr virus producing lymphoblastoid cells. Biochemistry. 1979 Oct 16;18(21):4517–4521. doi: 10.1021/bi00588a009. [DOI] [PubMed] [Google Scholar]
- Datta A. K., Feighny R. J., Pagano J. S. Induction of Epstein-Barr virus-associated DNA polymerase by 12-O-tetradecanoylphorbol-13-acetate. Purification and characterization. J Biol Chem. 1980 Jun 10;255(11):5120–5125. [PubMed] [Google Scholar]
- Dillner J., Sternås L., Kallin B., Alexander H., Ehlin-Henriksson B., Jörnvall H., Klein G., Lerner R. Antibodies against a synthetic peptide identify the Epstein-Barr virus-determined nuclear antigen. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4652–4656. doi: 10.1073/pnas.81.15.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson B., Larsson A., Helgstrand E., Johansson N. G., Oberg B. Pyrophosphate analogues as inhibitors of herpes simplex virus type 1 DNA polymerase. Biochim Biophys Acta. 1980 Mar 28;607(1):53–64. doi: 10.1016/0005-2787(80)90220-8. [DOI] [PubMed] [Google Scholar]
- Feighny R. J., Henry B. E., 2nd, Datta A. K., Pagano J. S. Induction of DNA polymerase activity after superinfection of Raji cells with Epstein-Barr virus. Virology. 1980 Dec;107(2):415–423. doi: 10.1016/0042-6822(80)90308-6. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Prezyna C., Benz W. C. Two Epstein-Barr virus-associated DNA polymerase activities. J Biol Chem. 1978 Dec 10;253(23):8617–8628. [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. The deoxyribonuclease induced after infection of KB cells by herpes simplex virus type 1 or type 2. I. Purification and characterization of the enzyme. J Biol Chem. 1978 May 25;253(10):3557–3562. [PubMed] [Google Scholar]
- Hummel M., Kieff E. Mapping of polypeptides encoded by the Epstein-Barr virus genome in productive infection. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5698–5702. doi: 10.1073/pnas.79.18.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U., Spanos A., Albert W., Grummt F., Banks G. R. Evidence that a high molecular weight replicative DNA polymerase is conserved during evolution. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6771–6775. doi: 10.1073/pnas.78.11.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallin B., Klein G. Epstein-Barr virus carried by Raji cells: a mutant in early functions? Intervirology. 1983;19(1):47–51. doi: 10.1159/000149336. [DOI] [PubMed] [Google Scholar]
- Kallin B., Luka J., Klein G. Immunochemical characterization of Epstein-Barr virus-associated early and late antigens in n-butyrate-treated P3HR-1 cells. J Virol. 1979 Dec;32(3):710–716. doi: 10.1128/jvi.32.3.710-716.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf K. W. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur J Biochem. 1979 Jul;98(1):231–244. doi: 10.1111/j.1432-1033.1979.tb13181.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litman R. M. A deoxyribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus) isolated on deoxyribonucleic acid-cellulose. J Biol Chem. 1968 Dec 10;243(23):6222–6233. [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Luka J., Sternås L., Jörnvall H., Klein G., Lerner R. Antibodies of predetermined specificity for the NH2 terminus of a cellular protein p53 react with the native molecule: evidence for the presence of different p53s. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1199–1203. doi: 10.1073/pnas.80.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar E. C., Huang E. S. Comparative study of herpes group virus-induced DNA polymerases. Intervirology. 1979;12(2):73–83. doi: 10.1159/000149071. [DOI] [PubMed] [Google Scholar]
- Morrison J. M., Keir H. M. A new DNA-exonuclease in cells infected with herpes virus: partial purification and properties of the enzyme. J Gen Virol. 1968 Dec;3(3):337–347. doi: 10.1099/0022-1317-3-3-337. [DOI] [PubMed] [Google Scholar]
- Ooka T., Lenoir G., Daillie J. Characterization of an Epstein-Barr virus-induced DNA polymerase. J Virol. 1979 Jan;29(1):1–10. doi: 10.1128/jvi.29.1.1-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander M., Cheng Y. C. Properties of herpes simplex virus type 1 and type 2 DNA polymerase. Biochim Biophys Acta. 1980 Sep 19;609(2):232–245. doi: 10.1016/0005-2787(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. Nonstructural proteins of herpes simplex virus. I. Purification of the induced DNA polymerase. J Virol. 1977 Nov;24(2):618–626. doi: 10.1128/jvi.24.2.618-626.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubal J., Kallin B., Luka J., Klein G. Early DNA-binding polypeptides of Epstein-Barr virus. Virology. 1981 Aug;113(1):285–292. doi: 10.1016/0042-6822(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Spanos A., Sedgwick S. G., Yarranton G. T., Hübscher U., Banks G. R. Detection of the catalytic activities of DNA polymerases and their associated exonucleases following SDS-polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Apr 24;9(8):1825–1839. doi: 10.1093/nar/9.8.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternås L., Luka J., Kallin B., Vestergaard B. F., Klein G. Detection and characterization of EBV antigens by micro-ELISA and chromatofocusing. J Virol Methods. 1982 May;4(4-5):229–240. doi: 10.1016/0166-0934(82)90069-6. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Schuster T. C., Hopkins R. F., 3rd, Neubauer R. H., Rabin H. Identification of an Epstein-Barr virus nuclear antigen by fluoroimmunoelectrophoresis and radioimmunoelectrophoresis. J Virol. 1981 Jun;38(3):996–1004. doi: 10.1128/jvi.38.3.996-1004.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]