Abstract
Diverse membrane fusion reactions in biology involve close contact between two lipid bilayers, followed by the local distortion of the individual bilayers and reformation into a single, merged membrane. We consider the structures and energies of the fusion intermediates identified in experimental and theoretical work on protein-free lipid bilayers. On the basis of this analysis, we then discuss the conserved fusion-through-hemifusion pathway of merger between biological membranes and propose that the entire progression, from the close juxtaposition of membrane bilayers to the expansion of a fusion pore, is controlled by protein-generated membrane stresses.
Cell-to-cell fusion in fertilization, development and carcinogenesis1–3; the membrane-fusion stage of the entry of enveloped viruses4–6; and intracellular fusion reactions in exocytosis, protein trafficking, mitochondrial remodeling and resealing of plasma membranes7–10 are controlled by very different proteins and involve very different membranes (see also Reviews by Wickner and Schekman11, Rizo and Rosenmund12 and Harrison13 in this Special Focus issue). Thus, understanding the molecular mechanisms of specific fusion reactions requires a detailed characterization of the protein structures and protein-lipid interactions that might be as diverse as the proteins and membranes involved. However, some important mechanistic motifs seem to be shared by many disparate fusion reactions. In this Review, we focus on these conserved motifs and attempt to formulate a general description of the job of any fusion protein based on the analysis of the fundamental properties of lipid bilayers that control the propensity of membranes to fuse.
Fusion of protein-free lipid bilayers
The ability of lipids to spontaneously assemble into bilayer structures such as liposomes, black lipid membranes and supported bilayers has been instrumental in modeling the conditions of bilayer fusion and defining the sequences of the intermediate structures formed in the course of bilayer merger.
Intermediate structures in bilayer fusion
Investigations of the fusion pathways for protein-free lipid membranes have identified and characterized two important types of intermediates: hemifusion structures and fusion pores (reviewed in refs. 14,15 and by Jackson and Chapman16 in this Special Focus issue).
Hemifusion structures represent connections between outer leaflets of apposed membranes, while the inner leaflets remain distinct (Fig. 1a). In most cases, hemifusion has been identified operationally as lipid mixing without content mixing or as mixing of the lipids of the contacting (outer) leaflets but not the inner leaflets of the two bilayers. Hemifusion has also been confirmed by electrophysiological measurements17,18. A hemifusion connection is often a transient structure that either dissociates, leaving two separated membranes, or gives rise to a fusion pore15,18.
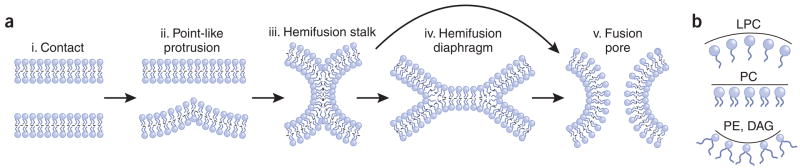
Figure 1.
Fusion-through hemifusion pathway of lipid bilayer fusion. (a) (i) Pre-fusion contact. (ii) A point-like membrane protrusion minimizes the energy of the hydration repulsion between the proximal leaflets of the membranes coming into immediate contact. (iii) A hemifusion stalk with proximal leaflets fused and distal leaflets unfused. (iv) Stalk expansion yields the hemifusion diaphragm. (v) A fusion pore forms either in the hemifusion diaphragm bilayer or directly from the stalk. Dashed lines show the boundaries of the hydrophobic surfaces of monolayers. (b) Different lipids spontaneously form monolayers of different curvatures and, thus, demonstrate different effective molecular shapes. Monolayers formed by inverted cone–shaped lysophosphatidylcholine (LPC) and by cone-shaped phosphatidylethanolamine (PE) and diacylglycerol (DAG) bulge in the direction of the polar heads and in the direction of the hydrocarbon chains, respectively. Cylindrical phosphatidylcholine (PC) forms an almost flat monolayer.
A fusion pore is a connection between merging membranes involving both outer and inner leaflets (Fig. 1a). Formation of a fusion pore establishes an aqueous connection between the volumes initially separated by the apposed membranes. Fusion pore formation and expansion have been studied using electrophysiological approaches and fluorescence assays that monitor mixing between aqueous contents and/or the lipids of the inner leaflets. These studies have established that fusion pores can close18 and that the fusion pore edge is covered with the polar heads of the lipids17.
Conditions of lipid bilayer fusion
Even long-term contacts between protein-free bilayers of compositions that mimic the usual compositions of biological membranes do not result in fusion. However, conditions have been found under which lipid bilayers do fuse in the absence of any proteins.
The propensity of lipid bilayers to hemifuse and develop fusion pores has been found to depend on lipid composition14. The impact of a given lipid on the formation of different fusion intermediates has been shown to correlate with its effective spontaneous curvature—the curvature of a monolayer formed spontaneously by this lipid in the absence of constraints. The spontaneous curvature of a lipid is determined by its molecular structure and by lipid interactions within the monolayer (reviewed in ref. 14). Lipids such as lysophosphatidylcholine (LPC) and polyphosphoinositides tend to self-assemble into curved monolayers whose surfaces bulge in the direction of the polar heads (Fig. 1b). The curvatures of such monolayers and, consequently, the effective spontaneous curvatures of the constituting lipids are defined as positive. The tendency of a lipid to form curved monolayers is also often described by its effective molecular shape, the shape of a constraint-free monolayer element, which contains on average one lipid molecule. The positive spontaneous curvature describes lipid molecules that have the effective shape of an inverted cone. In contrast, such lipids as unsaturated phosphatidylethanolamine and diacylglycerol tend to form monolayers with surfaces bulging in the direction of the hydrocarbon chains. Hence, these lipids can be described as having a negative spontaneous curvature and cone-like effective shape. Finally, lipids such as phosphatidylcholine tend to form almost flat monolayers with a slightly negative curvature and thus can be seen, in first approximation, as having the effective shape of a cylinder and a spontaneous curvature that is close to zero.
Fusion dependence on the effective molecular shapes of lipids is thought to reflect the effects of the spontaneous curvature of membrane monolayers on their propensity to bend into fusion intermediates (reviewed in ref. 14). Lipids of nonzero spontaneous curvature support bending of the lipid monolayer toward a certain curvature and thus, depending on the net curvature of a particular fusion intermediate, either promote or inhibit its formation. The finding that inverted cone-shaped LPC and cone-shaped phosphatidylethanolamine inhibit and promote hemifusion, respectively, when added to the contacting leaflets of the apposed bilayers, indicates that hemifusion involves formation of intermediates of net negative curvatures. On the other hand, LPC facilitates and phosphatidylethanolamine inhibits the formation of a pore in a single lipid bilayer and of a fusion pore if added to the distal leaflets of the fusing membranes. These lipid effects are consistent with the net positive curvature of the pore edge.
Another fusion condition revealed in studies of protein-free lipid bilayers is the establishment of a sufficiently close inter-bilayer contact. Fusion between bilayers, which do not merge spontaneously, can be promoted by a direct dehydration that drives bilayers into very close contact, with a trans-bilayer distance of less than 1 nm (ref. 19).
Experiments with liposomes have also uncovered the dependence of fusion on liposome size, with the smallest liposomes being the most fusogenic20. These studies have emphasized the role of membrane tension in advancing beyond early fusion intermediates and, in particular, in driving the evolution of hemifusion structures toward fusion pore formation and expansion17,21,22. On the other hand, tension generated by osmotic stress was reported to inhibit post-hemifusion stages in polyethylene glycol–induced fusion between liposomes20.
Physical modeling of membrane fusion
Efforts of many groups of physicists and physical chemists have been devoted over the past decades to modeling the process of lipid bilayer fusion. The aim of these theoretical studies has been to reveal which key physical properties the lipid monolayers constituting the membranes must possess, and to which external conditions the bilayers must be subjected, to overcome the intrinsic resistance of the apposed membranes to the drastic structural rearrangements related to their fusion.
This research followed two major strategies. One, and historically the first, strategy, applied since the 1980s, is based on modeling the membranes as macroscopic continuous films that can be described by the methods of classical physics, such as the elastic theory of lipid monolayers20,23–30 and the self-consistent mean field theory of the lipid bilayer interior31–33. We will refer to this strategy as the continuum approach. This approach is used to determine (i) the conditions guaranteeing that the state of fused membranes is energetically more favorable than the initial state of two separate membranes, and thus that the membranes have a tendency to fuse; (ii) the sequence of structural transformations that the two initially separated lipid bilayers undergo upon their merger; (iii) the energy cost of every sequential intermediate structure emerging in the course of these transformations; and (iv) the conditions under which these intermediate structures do not present energy barriers that kinetically restrict the fusion process and, hence, limit fusion feasibility.
The second strategy, which has been undertaken since the beginning of the 1990s, uses computer simulations of the membrane fusion process and will be referred to as the simulation approach. This approach is based on the state-of-the-art computational methods developed in soft matter physics, such as molecular dynamics of coarse-grained34,35 and atomistic-detail36 models of lipids and the aqueous solvent; Monte Carlo simulations of diblock copolymer membranes within a homopolymeric solvent37; brownian-dynamics simulations of simplified coarse-grained models of lipids with no explicit solvent38,39; and dissipative particle-dynamics simulations of a coarse-grained lipid and water model, accounting correctly for the hydrodynamic forces developed in the system40,41. All of these simulations can be regarded as computer experiments, with systems mimicking the lipid-water mixtures with different degrees of accuracy. The propensity of the membranes to fuse and the intermediate structures emerging in the course of bilayer merger are directly ‘observed’ rather then derived by physical analysis.
Each of these approaches has its advantages and drawbacks, in terms of both methodology and reliability of the results.
Methodological differences
Each approach uses certain assumptions about physical properties and organization of the membranes and the surrounding medium. The more sophisticated the model is, the closer to reality, in principle, the determined structure and energy of the fusion intermediates may be. However, sophistication of the model has its price. Increase in model complexity requires the involvement of a growing number of physical parameters, which are inaccessible to direct experimental determination. The current models can be ordered according to the degree of their sophistication.
The most phenomenological and simple approach, relying on a minimal number of assumptions about the detailed structure of the system, is the continuum approach based on the elastic model of lipid membranes (see ref. 25 and references therein). This approach requires, however, certain guesses about the structure of the fusion intermediates, appealing to the researcher’s physical intuition20,23–30,42. At the same time, the energies of the fusion intermediates predicted within this approach are determined by only a few material characteristics of the lipid monolayers, namely the elastic moduli of monolayer bending, stretching and tilt of the hydrocarbon chains and the modulus of the gaussian curvature. These elastic moduli have been directly measured (for review, see ref. 14) or reliably determined on the basis of experimental data43–46.
The next in the sophistication scale is the continuum model using self-consistent field theory of the lipid bilayers and the aqueous solution32,33,47–49. This model requires knowledge of the self-consistent field parameters determining interactions of lipid molecules among themselves and with water. To simplify the model, the lipid molecules are considered as diblock copolymers composed of hydrophobic and hydrophilic homopolymers, and the water molecules are represented by hydrophilic homopolymers32. Because the energy of a specific membrane structure predicted by this model turned out to be lower by a factor of 2.6 than that measured for lipid bilayers, all calculated energies were multiplied by this factor in order to achieve predictions relevant to lipid membranes47. The principal implicit assumptions of the self-consistent field theory model of membrane fusion are that, in spite of considerable differences of molecular structures, the conformations of the fusion intermediates built by the diblock copolymers in a polymer-like solvent are similar to those formed by phospholipids in water, and that the energy rescaling by the same coefficient, 2.6, is valid for all fusion intermediates.
Models developed with a simulation approach require further sophistication, as they use direct-interaction forces between the coarse grains representing groups of atoms50 or the ‘atoms’36 that build up the lipid and solvent molecules. These forces include Lennard-Jones interactions ranging in strength from weak (hydrophobic interactions) to strong (polar interaction)36,50, screened Coulomb interactions and the angle potentials for the forces between the zwitterionic head groups36,50, bond-mimicking interactions between chemically connected sites36,50, and interactions of analogous types37,39,41. The strengths of all these forces are determined by parameters that have been found by fitting the quantitative predictions of the model to the experimentally measured physical characteristics of lipid membranes, such as the bending and stretching moduli, the line tension of pores formed in the lipid bilayers, the rate of lateral diffusion of lipid molecules in the membrane plane, the rate of water permeation through the lipid bilayer matrix, and the temperatures of the lipid transition between the liquid and the crystalline phases and between different mesophases (for example, see refs. 50,51). The time scales captured within the simulation approach vary from tens of nanoseconds for simulations in ‘atomistic detail’36 to submilliseconds for coarse-grained approaches34,41.
One of the main implicit assumptions underlying the use of simulation models for analysis of membrane fusion intermediates is that the sets of parameter values fitted to account for the specific membrane properties mentioned above are also suitable for describing the intramembrane energy changes in the course of the structural rearrangements accompanying the fusion process.
Differences in predictions for fusion pathways
One of the most obvious and important differences between the continuous and the simulation approaches is in the limitations imposed on the possible conformations of the fusion intermediates.
Continuum approach models routinely assume that the fusion intermediates have axially symmetric shapes. Fusion is proposed to start from a point-like membrane protrusion42 (Fig. 1a) that transforms into the hourglass-like connection between the apposed monolayers. This early hemifusion connection is referred to as the fusion stalk24 (Figs. 1a and 2a). Two scenarios for the further evolution of fusion intermediates have been suggested. The first assumes axially symmetric expansion of the stalk into a round hemifusion diaphragm (Fig. 1a). The fusion pore forms either within a hemifusion diaphragm or along its perimeter23–25. Alternatively, it has been proposed that the stalk decays directly into the fusion pore, so that the stage of hemifusion diaphragm formation is cut short29,30.
Figure 2.

The stalk is the key intermediate in most of the theoretical models developed with the continuous and the simulation approaches. (a) Stalk structure computed by analysis of bending, splay and tilt of the lipid molecules in the membrane monolayers with the elastic model (continuous approach)27. (b) Stalk structure computed by the self-consistent field model (continuous approach)48. Light regions indicate the areas of head groups of the bilayer. (c) Stalk structure ‘observed’ by molecular dynamics simulation of the fusion between liposomes composed of dipalmitoyl phosphatidylcholine and palmitic acid using an atomistically detailed model. Water molecules (gray) and head group atoms of the lipids are depicted as spheres; tails are shown as bonds, with gray used to distinguish water molecules originating on different sides of the fusing membranes. The coloring also distinguishes between lipid molecules coming from different leaflets of the bilayers: dipalmitoyl phosphatidylcholine molecules in the inner or outer leaflets (green and purple), and palmitic acid in the inner or outer leaflets (cyan or magenta, respectively).
In contrast to the continuum approach, the simulation approach is essentially free of any assumptions about the character and sequence of the fusion intermediates, as the computational protocol does not impose any constraints on the conformations adopted by the system. However, the character of the membrane conformations emerging in the course of simulation may be ‘model-dependent’; that is, strongly influenced by the features of the specific computational model used to describe the lipid molecules and the solvent and by technical limitations of the simulations, such as short simulation times and the relatively small numbers of lipid molecules constituting the fusing membranes. Indeed, at the current stage, different simulation methods produce different fusion pathways. Most of these pathways seem to at least partially match those suggested by the continuous approach.
Although the existing simulations could not resolve any pre-stalk fusion intermediates, including the hypothetical point-like protrusion42, practically all of them (but see refs. 40,41), including the most recent and sophisticated molecular dynamics simulation in atomic detail36, confirmed the axially symmetric fusion stalk as the first lipidic bridge forming between the contacting monolayers of the apposed membranes34,36,37 (Fig. 2). Hence, the fusion stalk suggested by the continuum approach in the very beginning of the era of membrane fusion modeling24 seems to be the most reliable structure, one whose feasibility has been confirmed experimentally in multilayer lipid systems52.
By contrast, the simulation results on stalk evolution leading, eventually, to fusion pore formation remain somewhat confusing. Atomic-detail molecular dynamics simulations of fusion of membranes in five out of six simulations yielded the stalk–hemifusion diaphragm–pore pathway suggested by the continuum models36. The only difference from the assumptions of the continuous approach was that the simulated hemifusion diaphragm had a banana-like rather than a round shape. In one simulation, one of the monolayers ruptured close to the hemifusion diaphragm rim, leading to trans-membrane lipid mixing. It is unclear whether this relatively rarely observed fusion-through-rupture sequence is a legitimate alternative pathway or an artifactual consequence of the unrealistically small (13.6-nm) diameter of the fusing vesicles. Experimentally, the smallest liposomes have diameters exceeding 20 nm (ref. 53).
In molecular dynamics simulations of fusion of small (15-nm) mixed vesicles using the Marrink-Mark coarse-grained model, the relative prevalence of a stalk–hemifusion diaphragm–fusion pore pathway over a less frequent direct transition from stalk to fusion pore depended on the lipid composition. The latter pathway had a higher probability at lower concentration of phosphatidylethanolamine54. Neither hemifusion diaphragm asymmetry nor membrane rupture was reported in this work.
Other molecular dynamics simulations demonstrated another fusion pathway, one that has never been suggested and analyzed by the continuum approach. The early coarse-grained model of 15-nm vesicles demonstrated that, in addition to the standard stalk–hemifusion diaphragm–fusion pore pathway in which each of the two membranes donates one monolayer to the forming hemifusion diaphragm and no membrane rupture occurs, there is an alternative pathway of hemifusion diaphragm formation34. In this pathway, instead of expanding radially, the stalk elongates and adopts a banana-like shape. Subsequent rupture of one of the membranes yields a hemifusion diaphragm composed of monolayers that came from the same initial bilayer. Such a pathway was observed in 50–70% of simulations, dependent on the lipid composition. Similar pathways with formation of a pore next to the stalk followed by stalk elongation around the pore were also observed in Monte Carlo simulations of fusion of diblock copolymer membranes37 and in brownian-dynamics simulations with a rod-like model of lipids38,39.
Summarizing, although stalk formation appears to be a common result of practically all continuous and simulation models of membrane fusion so far, the predictions concerning the pathway of stalk evolution into a fusion pore depend on the details of the models used and hence remain under debate. At the same time, the fusion-through-hemifusion pathway proposed in the early theoretical models of membrane fusion23,24 seems to be the one observed, at least under certain conditions and with some variations, in most of the simulations. Thus, we expect that resolving the current technical constraints of simulation techniques will validate this pathway as the prevalent pathway of lipid bilayer fusion.
Analysis of fusogenic conditions
The continuum approach seems to be more suitable than the present simulation models for determining fusogenic conditions and, hence, the job descriptions of fusion proteins. Continuum models have been used to analyze the dependence of the energies of the pre-fusion and post-fusion states and different fusion intermediates on such externally controlled factors as the monolayer lipid composition17,55, the thickness of the water layer separating the apposed membranes and the corresponding membrane interactions26, the membrane lateral tension and the curvature of the fusing membranes29,56–58. Continuum models yield direct predictions of fusion probability and kinetics as functions of these physical factors, and they make possible direct estimations of the parameter values necessary to drive fusion at biologically feasible rates. The present simulation models are more suitable for verification of these predictions by computer experiments under relevant conditions, provided that these conditions can be captured by the numerical procedures.
Over many years, the continuum models have predicted the above-mentioned dependence of fusion probability and rate on the spontaneous curvatures of the membrane monolayers (for review, see ref. 14). This prediction was verified by various simulations (see, for example, ref. 54) and confirmed experimentally (for review, see ref. 59). Notably, continuum models have been used to demonstrate that membrane stresses that are accumulated in the fusion site and released upon stalk formation and evolution promote fusion initiation. These studies proposed and analyzed two schemes for the generation of such stresses. The first one suggests the formation of strongly curved membrane patches accumulating the bending energy, which is released in the course of fusion49,56,57. The second scheme involves bringing together membrane patches within 1 nm, leading to accumulation of a large amount of energy from intermembrane repulsion, which is relaxed under hemifusion26. The effects of membrane curvatures have been verified in simulations54 and used to explain synaptotagmin-mediated fusion57. Promotion of fusion by creation of close intermembrane contact has never been an explicit goal of simulations, but, de facto, stalk formation observed in simulations of membranes ‘preset’ at very small distances between them34,36 may be seen as such verification. Stalk formation upon very close membrane contact has been observed experimentally52. Finally, both promotion of the transition from stalk to hemifusion diaphragm by the lateral tension in the external monolayers and fusion pore expansion by tension existing in the whole bilayer follow directly from the continuum stalk-pore model of membrane fusion23. Fusion promotion by tension was also observed in numerical simulations37,41 and in experimental studies21.
Estimates based on the continuum approach and verified by simulations will help elucidate the specific organization and function of the protein fusion machinery. However, the development of such protein-bilayer models is still in its initial stage.
Prospects
Up to now, the main theoretical models of membrane fusion have focused on the structural rearrangements of protein-free lipid bilayers. Attempts to account explicitly for the mechanisms of action of fusion proteins were undertaken in only a very few studies56,57. Extensive theoretical modeling of involvement in the membrane fusion reaction of the different regions of fusion proteins, including fusion peptides and transmembrane domains, is necessary for elucidation of the fusogenic action of the diverse fusion proteins characterized so far. This will require a substantial technical advancement in numerical methods that will considerably extend the available time and space scales. Models based on the continuous approach will have to take into account membrane strains and stresses generated by protein domain insertion into the membrane matrix and by protein scaffolds of different configurations sculpting the membrane into stressed shapes. The first steps in this direction have been recently undertaken60–62.
Pathway and mechanisms of biological membrane fusion
Hemifusion intermediates
Different hallmarks of the hemifusion stalk–lipidic pore sequence described above for lipid bilayers have been documented for diverse biological fusion reactions. Viral, intra-cellular and developmental fusion proteins mediate hemifusion, detected as lipid mixing in the absence of content mixing (reviewed in refs. 2,63). This operational definition of hemifusion has several important limitations. In most of the reports, some of the membrane contacts recognized as hemifusion might have a pore that is too small (<1 nm diameter) to pass the conventional content probes used64,65. On the other hand, hemifusion can also be underreported because, in some cases, lipid mixing between hemifused membranes seems to be effectively inhibited by fusion protein assemblies, yielding a ‘restricted hemifusion’ phenotype64,66–69. In addition, hemifusion connections, like early fusion pores70, are reversible structures, as demonstrated by (i) partial lipid mixing between fusing membranes69,71, (ii) inefficiency of treatments that transform hemifusion into full fusion after inactivation or proteolysis of viral fusion proteins66, and (iii) separation between membranes after they have undergone lipid mixing72.
Although both experimental data and theoretical analysis indicate that hemifusion structures need energy input to be formed and maintained, these structures can in principle be stabilized by protein scaffolds. It has been hypothesized that exocytotic vesicles might be held at the plasma membrane in a ready-to-go hemifused state to accelerate the completion of fusion upon a triggering event73,74. Indeed, a recent conical electron tomography study suggests that, before calcium stimulation, the synaptic vesicle–plasma membrane docking zone contains a stable but very small hemifused region (~6 nm, or ~0.5% of the vesicle surface)75. Stable hemifusion connections have been also proposed as possible intermediates of fertilization envelope formation in the sea urchin egg on the basis of fluorescence microscopy analysis of the rates of lipid transfer between docked exocytotic vesicles and plasma membrane76. Recent work77 suggests an interesting molecular biological context for the hypothesis that stable hemifusion represents a primed pre-fusion state in SNARE-dependent intracellular fusion. In the proposed mechanism, a hemifusion intermediate formed by the assembly of a SNARE complex is stabilized by complex-associated complexin until the calcium sensor synaptotagmin, in the presence of calcium, relieves the block and permits a fast transition from hemifusion to complete fusion77.
Although the ability of protein fusogens to mediate hemifusion is consistent with the hypothesis that fusion proceeds through hemifusion (either by the stalk–hemifusion diaphragm–pore pathway25,63 or by the direct transition from a hemifusion stalk to a pore31,78), it does not prove this hypothesis. In a large contact zone, as is characteristic of cell-cell fusion, formation of hemifusion intermediates and fusion pores might proceed independently rather than be part of the same pathway. Two experimental approaches have been instrumental in verifying that hemifusion is an intermediate in the formation of an expanding fusion pore rather than a branch-off from this pathway64. First, as in the case of protein-free bilayers, adding the hemifusion-inhibiting lipid LPC to contacting leaflets of biological membranes inhibits fusion pore formation in disparate fusion reactions mediated by viral, intracellular and developmental fusogens2. The reversibility of this inhibition and its observation at sub-lytic (~5 molar percent) membrane concentrations of LPC indicates that this inhibition involves neither solubilization nor irreversible denaturation of membrane proteins79,80.
Second, recently developed, elegant approaches have permitted imaging of individual fusion events for small (~100-nm diameter) vesicles (viral particles81,82 and SNARE proteoliposomes69,83). The lipid-mixing-before-content-mixing sequence detected with these approaches in small contact zones between fusing membranes very much decreases the likelihood of the possibility of a fusion pore opening that is mechanistically independent of a hemifusion intermediate already formed within the same contact zone.
Importantly, because the two leaflets of each of the fusing membranes in fusion-through-hemifusion pathway are breached one after the other rather than simultaneously, this pathway allows fusion to proceed without breaking the barrier function of the membranes. However, under some conditions, protein-mediated fusion is accompanied by a leakage84. It remains to be clarified whether this leakage is important for fusion—for instance, representing the fusion-through-rupture sequence observed in some molecular dynamics simulations37—or reflects a membrane destabilization by activated fusion proteins that is mechanistically irrelevant for fusion.
Lining of the fusion pore
Fusion reactions mediated by viral, intracellular and developmental fusogens are thought to involve multi-protein machinery that assembles at the future fusion site and surrounds the early fusion intermediates with a protein ring (reviewed in refs. 2,14). This ring either serves as a key structural component of the earliest fusion intermediates or primes the enclosed membrane bilayers for fusion by catalyzing lipid-involving intermediates characteristic of the fusion of protein-free bilayers (see above). These two models suggest different structures of nascent fusion pores. Within the first class of models85,86, it has been proposed that the nascent fusion pore is an entirely proteinaceous channel-like structure walled by the transmembrane domains (TMD) of fusion proteins. This hypothesis has been substantiated by a systematic characterization of the conductance of the fusion pores for different mutants of the t-SNARE protein syntaxin, which is essential in exocytosis. Mutations in the amino acid residues along one face of an α-helical structure of the syntaxin TMD alter the conductance of the fusion pore in a way that correlates with the sizes of the side chains of these residues (reviewed in ref. 87). These very interesting findings have been interpreted as evidence that TMDs of several syntaxin molecules assemble to form the lining of the fusion pore. In the proposed model, a protein-lined fusion pore that spans both membranes is opened by the joining of two hemipores, one in each of the membranes, and then a separation between protein subunits permits the formation of lipidic connections between the membranes. The protein pore hypothesis explains the dependence of fusion on the TMDs and also explains earlier reports that opening of the smallest initial pores precedes detectable lipid mixing64,65. However, it seems that each of these two lines of evidence might have alternative interpretations.
Modification or replacement of TMD regions of protein fusogens is likely to affect protein expression, localization and oligomerization. Thus, although the evidence that TMDs of different fusion proteins are important for fusion and modifications of TMD might inhibit fusion and especially fusion pore opening is very strong88–92, a direct structural role of the TMD as the lining of a nascent fusion pore is much more difficult to establish. Because TMDs of the channel-forming proteins provide both a polar interface with the water filling the channel lumen and a hydrophobic interface with a bilayer interior, their sequence is critical for channel function. However, the wide range of TMD sequences of hemagglutinin support the formation of expanding fusion pores93, and both hemagglutinin94,95 and SNAREs96,97 with TMDs replaced by lipid anchors do fuse membranes. In addition, in several experimental systems, lipid mixing precedes content mixing (for instance, refs. 69,81,98). Moreover, as restricted hemifusion intermediates (see above) do not allow lipid flow between the membranes, the lack of lipid transfer before fusion pore opening does not necessarily indicate the lack of a lipidic connection.
It is difficult to reconcile the protein pore hypothesis with the marked similarities between lipid dependences of biological fusion mediated by viral80,99, intracellular80,100–104 and developmental fusogens105 and those of fusion between protein-free bilayers. For instance, like fusion between protein-free bilayers, and as expected for a pore lined by lipids, fusion pore formation in viral fusion and in exocytosis is promoted by inverted cone–shaped lipids in the distal leaflets of the fusing membranes64,78,102. This dependence on the effective shape of the lipids suggests that a fusion pore forms by lipid monolayer bending into a micelle-like curvature of the pore edge, and thus, although proteins can be present at or near the pore edge, they do not serve as critical structural components of the initial pore.
To bring together the proteinaceous fusion pore model with the evidence for hemifusion, it has recently been proposed that protein regions that bridge the transmembrane gap outside the protein-lined pore might be covered by lipids establishing a lipidic connection between the contacting leaflets of the membranes86,87. In contrast to hemifusion intermediates for protein-free bilayers, the curvature and the energy of such supported hemifusion connection would be determined by the shape of the protein scaffold and by interactions between lipid tails and the protein surface, respectively63. Thus, we believe it is unlikely that the combination of scaffold-supported hemifusion and a proteinaceous pore would explain why hemifusion of biological membranes and hemi-fusion of protein-free bilayers depend similarly on the lipid composition of contacting leaflets of the membranes.
Mechanisms of protein-mediated fusion
The specific mechanisms by which proteins promote hemifusion and fusion pore development remain elusive. Biological fusion processes start with two membranes separated by at least a 10–20 nm gap filled with membrane proteins, including proteins that are responsible for membrane binding. At the next stage(s), opposing protein-depleted patches of the membranes must be brought together to form a much closer contact between membrane bilayers. Local displacement of the proteins from the future fusion site requires protein mobility. Subsequent remodeling of these bilayer patches, for many, if not all, biological fusion events, apparently involves the hemifusion–lipidic pore pathway. Fusion pore expansion joins the membranes fully and thus completes the fusion reaction. Each of these stages can be, and probably is, controlled by proteins63.
Recent work on the identification and characterization of fusion proteins has emphasized the diversity of the basic designs of the fusion machinery (reviewed in ref. 2). In viral fusion, protein fusogens are located at one of the fusing membranes. In SNARE-dependent intracellular fusion, two fusing membranes carry different but complementary sets of protein fusogens. Finally, in developmental cell fusion in Caenorhabditis elegans, the same protein fusogens must be present on both fusing cell membranes. Structural analysis of fusogens of several enveloped viruses has also revealed major differences among the pre-fusion conformations of these proteins. However, the final, post-fusion conformations of the proteins are found to have a very similar hairpin fold in which fusion peptides—conserved membrane-interacting amphiphilic peptide regions—are positioned at the same end of rod-like molecules as the TMDs. The conserved structure of diverse fusogens suggests a conserved mechanism of coupling between protein rearrangements and membrane rearrangements4,5.
Fusion proteins may drive membrane merger by generating local changes in lipid composition, producing a composition promoting the lipid monolayer bending necessary for the formation of fusion intermediates. Indeed, some of the intracellular compartments involved in an ongoing remodeling have high local concentrations of cone-shaped lipids106,107, and some fusion reactions seem to be regulated by phospholipase activity74,107,108. For instance, in nuclear envelope assembly in sea urchin eggs, phospholipase C generates the cone-shaped fusogenic lipid diacylglycerol107. However, as several fusion reactions mediated by viral and intracellular fusogens have been reconstituted in proteoliposomes lacking significant concentrations of fusogenic lipids, it seems that alteration of local composition is not the only and not the most general way in which proteins promote fusion.
Accumulating evidence suggests that the main role of the fusion proteins is to generate and control the membrane elastic stresses that, in analogy to lipid bilayer fusion, seem to be key in biological fusion. It has been proposed that the critical step in the initiation of lipid rearrangements by the fusion proteins consists in local bending of membrane bilayers into ‘dimples’56 (also referred to as a ‘nipples’29) pointing toward the adjacent membrane (Fig. 3). Such membrane bending brings the membrane bilayers into close contact56,57 and primes the protein-depleted, stressed tops of the bilayer bulges for fusion by lowering the energy barriers for hemifusion and pore opening29,56,57. How do the proteins do this job? Several models suggest that fusion is driven by the energy released in the course of formation of the hairpin conformation and transmitted to the membranes through TMDs and fusion peptides anchoring the fusogens to the membrane matrix. However, although this refolding of fusion protein ectodomains is most likely important in fusion, the diverse fusion peptides of different proteins are clearly not just membrane anchors. Mutations in the fusion peptide of influenza hemagglutinin, including those that do not change hydrophobicity but do disturb the fusion peptide’s boomerang-like structure, have pronounced effects on the fusogenic activity of hemagglutinin109,110. The importance of peptide–membrane interactions in fusion is also emphasized by the ability of fusion-associated small transmembrane (FAST) proteins of non-enveloped viruses, which do not form rigid hairpin structures, to fuse infected and uninfected cells111. Finally, it seems that fusion loops of the fusogen of vesicular stomatitis virus112 are too short to serve as ‘anchors’ to transfer significant energy to the membrane, suggesting that their most important function may be disrupting the structure of the bilayers rather than anchoring the ectodomain of the protein to the target membrane.
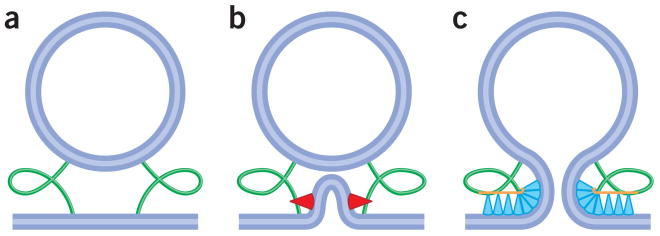
Figure 3.
Hypothetical pathway of biological fusion powered by protein-generated membrane stresses. (a) In the initial state, apposing membrane bilayers are separated by at least a 10–20 nm gap. The contact might involve protein fusogens themselves or be mediated by specialized tethering molecules (green shapes). (b) Fusion proteins induce local bending of membrane bilayer(s) and establish very close contact between the membranes. Generation of large membrane curvature might involve shallow insertion of amphiphilic protein domains (red shapes) into the membrane10,62. The highly stressed and protein-depleted tops of the bilayer bulges are primed for hemifusion and pore opening10,29,56,57. (c) Activated fusion proteins (blue shapes) might drive fusion pore expansion by assembling into an interconnected protein coat surrounding the fusion site119. This membrane-associated fusion coat has an intrinsic curvature opposite to that of the budding and fission coats. The coat, bending toward its preferred curvature, deforms the underlying membrane and produces tension that drives fusion and expands the fusion pore.
An emerging mechanism by which fusion peptides may promote the early fusion stages is based on their potential ability to generate large membrane curvatures. The fusion peptides are amphipathic, and therefore, their embedding into the membrane matrix must be shallow, so that the hydrophobic side of the peptide faces the hydrocarbon moiety of the lipid monolayer while the hydrophilic groups remain close to the lipid-water interface. Such a mode of embedding is similar to that of the amphipathic α-helices of small G proteins113 and of the N-BAR domains114 or hydrophobic loops of C2 domains of synaptotagmin115, whose insertion depths equals approximately one-third of the monolayer thickness57. Both the amphipathic α-helices and the hydrophobic loops of the C2 domains, added to flat lipid membranes, have been shown to generate narrow tubules of 15 to 20 nm in diameter57,114,116. A theoretical analysis taking into account the intra-monolayer stresses generated by the shallow insertion of protein domains into the membrane monolayer and partial relaxation of these stresses as a result of membrane bending validates and quantifies these effects62. The end caps of these tubules were found to be highly fusogenic, suggesting that synaptotagmin promotes SNARE-mediated fusion by producing strongly curved membrane dimples analogous to tubule end-caps57. These dimples (Fig. 3b) play a dual role: they facilitate establishment of a close intermembrane contact, and, by releasing the membrane bending energy preaccumulated within the strongly curved dimple monolayers, they drive formation of the fusion stalk, followed by formation of a nascent fusion pore. Note that, in this hypothesis, synaptotagmin stabilizes a dimple by forming a cylindrical belt around a protein-free end cap. This is an important distinction from the effects of lipids (such as LPC and phosphatidylethanolamine) that enter into the fusion intermediates and affect fusion by changing the spontaneous curvature of the monolayer in these locations.
This mechanism of promotion of the early fusion stages by bending membrane(s) into stressed dimples might be general and underlie, in addition to the role of synaptotagmin, the action of membrane-interacting regions of diverse fusion proteins. Ongoing analysis of the structures of membrane-associated fusion peptides and their abilities to bend lipid bilayers, along with theoretical analyses, will validate or disprove this hypothesis.
Expansion of the nascent fusion pore represents the most energy-demanding fusion stage and requires further driving forces14,91,117,118. For lipid bilayers, fusion is driven by surface tension of black lipid membranes, by the lateral tension generated by osmotic processes or adhesion and, in the case of very small vesicles, by bending stresses. In biological fusion, a persistent energy input that drives fusion from early reversible intermediates to an expanding fusion pore can be provided by the components of the fusion machinery119. For instance, pore expansion may be driven by lateral assembly of activated fusogens into a membrane-associated protein coat (Fig. 3c), acting analogously to the coats driving membrane fission but deforming the membrane in an opposite direction119. This hypothesis is supported by indirect experimental data in the case of fusion driven by hemagglutinin120 but can be applicable only to fusion reactions that involve multiple fusion proteins. The relatively slow fusion of HIV apparently requires just a single HIV env trimer121 and, thus, might rely on HIV env–independent mechanisms for fusion completion.
Concluding remarks
The fusion-through-hemifusion pathway, which is intrinsic for membrane bilayers and shared by disparate biological fusion reactions, involves local membrane deformations and, therefore, is driven by membrane stresses. Even for the best-characterized fusion processes, we still do not know how proteins generate the required stresses and bring the stressed membrane patches into close contact. One of the mechanisms proposed to account for the generation of the bending moments necessary for hemifusion and fusion pore opening involves a local shallow insertion of the amphiphilic regions of the fusion proteins into the membrane matrix10,57. In addition, the bending moments may be generated by the TMDs of the fusion proteins if these domains are subjected to a tilting force coming from a refolding of the ectodomains or their complexes. Effective force transmission from the ectodomains to the TMD requires, however, a sufficiently rigid linker region122. Mechanisms that underlie fusion pore expansion are even less well understood. They may involve assembly of a fusion protein coat (see above), cytoskeleton components and trans-membrane osmotic pressure. Pore expansion might also be driven by a negative line tension of the pore rim (energy per unit length of the rim) induced by local accumulation of cytosolic membrane proteins (Chen, A. et al., unpublished data).
Although research on the mechanics of membrane fusion has clarified some of the job requirements for fusion proteins, the specific ways in which protein fusogens generate the membrane stresses and tensions required at different stages of the fusion reaction are likely to involve both already-identified and yet-to-be-discovered mechanistic motifs. Understanding of these general motifs will help to elucidate the all-important molecular details of mechanisms of diverse fusion reactions, and it will bring about new ways of controlling the ubiquitous phenomenon of membrane fusion.
Acknowledgments
We apologize for not citing many important papers because of space limitations. Figure 2b,c were kindly provided by M. Schick and S.J. Marrink, respectively. The work of L.V.C. is supported by the Intramural Research Program of the US National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development. The work of M.M.K. is supported by the Israel Science Foundation and Marie Curie Network ‘Flippases’.
References
- 1.Duelli D, Lazebnik Y. Cell-to-cell fusion as a link between viruses and cancer. Nat Rev Cancer. 2007;7:968–976. doi: 10.1038/nrc2272. [DOI] [PubMed] [Google Scholar]
- 2.Sapir A, Avinoam O, Podbilewicz B, Chernomordik LV. Viral and developmental cell fusion mechanisms: conservation and divergence. Dev Cell. 2008;14:11–21. doi: 10.1016/j.devcel.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–2193. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Kielian M, Rey FA. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat Rev Microbiol. 2006;4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weissenhorn W, Hinz A, Gaudin Y. Virus membrane fusion. FEBS Lett. 2007;581:2150–2155. doi: 10.1016/j.febslet.2007.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earp LJ, Delos SE, Park HE, White JM. The many mechanisms of viral membrane fusion proteins. Curr Top Microbiol Immunol. 2005;285:25–66. doi: 10.1007/3-540-26764-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 8.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 9.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 10.Martens S, McMahon HT. Disparate and common players in cellular membrane fusion events. Nat Rev Mol Cell Biol. 21 May 2008; doi: 10.1038/nrm2417. advance online publication. [DOI] [PubMed] [Google Scholar]
- 11.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 15.Lentz BR, Malinin V, Haque ME, Evans K. Protein machines and lipid assemblies: current views of cell membrane fusion. Curr Opin Struct Biol. 2000;10:607–615. doi: 10.1016/s0959-440x(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 16.Jackson MB, Chapman ER. The fusion pores of Ca2+-triggered exocytosis. Nat Struct Mol Biol. 2008;15:684–689. doi: 10.1038/nsmb.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chernomordik LV, Melikyan GB, Chizmadzhev YA. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta. 1987;906:309–352. doi: 10.1016/0304-4157(87)90016-5. [DOI] [PubMed] [Google Scholar]
- 18.Chanturiya A, Chernomordik LV, Zimmerberg J. Flickering fusion pores comparable with initial exocytotic pores occur in protein-free phospholipid bilayers. Proc Natl Acad Sci USA. 1997;94:14423–14428. doi: 10.1073/pnas.94.26.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Huang HW. A rhombohedral phase of lipid containing a membrane fusion intermediate structure. Biophys J. 2003;84:1808–1817. doi: 10.1016/S0006-3495(03)74988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malinin VS, Frederik P, Lentz BR. Osmotic and curvature stress affect PEG-induced fusion of lipid vesicles but not mixing of their lipids. Biophys J. 2002;82:2090–2100. doi: 10.1016/S0006-3495(02)75556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen FS, Zimmerberg J, Finkelstein A. Fusion of phospholipid vesicles with planar phospholipid bilayer membranes. II Incorporation of a vesicular membrane marker into the planar membrane. J Gen Physiol. 1980;75:251–270. doi: 10.1085/jgp.75.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohki S. Surface tension, hydration energy and membrane fusion. In: Ohki S, Doyle D, Flanagan TD, Hui SW, Mayhew E, editors. Molecular Mechanisms of Membrane Fusion. Plenum; New York: 1988. pp. 123–139. [Google Scholar]
- 23.Kozlov MM, Leikin SL, Chernomordik LV, Markin VS, Chizmadzhev YA. Stalk mechanism of vesicle fusion. Intermixing of aqueous contents. Eur Biophys J. 1989;17:121–129. doi: 10.1007/BF00254765. [DOI] [PubMed] [Google Scholar]
- 24.Kozlov MM, Markin VS. Possible mechanism of membrane fusion. Biophysics. 1983;28:255–261. The work was the first to propose the stalk intermediate of membrane fusion and presents calculations of the stalk energy as a function of the spontaneous curvature of the membrane monolayers. [PubMed] [Google Scholar]
- 25.Kozlovsky Y, Chernomordik L, Kozlov MM. Lipid intermediates in membrane fusion: formation, structure, and decay of hemifusion diaphragm. Biophys J. 2002;83:2634–2651. doi: 10.1016/S0006-3495(02)75274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozlovsky Y, Efrat A, Siegel DP, Kozlov MM. Stalk phase formation: effects of dehydration and saddle splay modulus. Biophys J. 2004;87:2508–2521. doi: 10.1529/biophysj.103.038075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozlovsky Y, Kozlov MM. Stalk model of membrane fusion: solution of energy crisis. Biophys J. 2002;82:882–895. doi: 10.1016/S0006-3495(02)75450-7. Energy of fusion stalk is computed using the tilt-splay model for membrane mechanics. The analysis predicts a biologically feasible energy barrier of stalk formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markin V, Albanesi J. Membrane fusion: stalk model revisited. Biophys J. 2002;82:693–712. doi: 10.1016/S0006-3495(02)75432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuzmin PI, Zimmerberg J, Chizmadzhev YA, Cohen FS. A quantitative model for membrane fusion based on low-energy intermediates. Proc Natl Acad Sci USA. 2001;98:7235–7240. doi: 10.1073/pnas.121191898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel DP. Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate mechanisms. Biophys J. 1993;65:2124–2140. doi: 10.1016/S0006-3495(93)81256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May S. Structure and energy of fusion stalks: The role of membrane edges. Biophys J. 2002;83:2969–2980. doi: 10.1016/S0006-3495(02)75303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsov K, Muller M, Schick M. Field theoretic study of bilayer membrane fusion. I Hemifusion mechanism. Biophys J. 2004;87:3277–3290. doi: 10.1529/biophysj.103.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsov K, Muller M, Schick M. Field theoretic study of bilayer membrane fusion: II Mechanism of a stalk-hole complex. Biophys J. 2006;90:915–926. doi: 10.1529/biophysj.105.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrink SJ, Mark AE. The Mechanism of Vesicle Fusion as Revealed by Molecular Dynamics Simulations. J Am Chem Soc. 2003;125:11144–11145. doi: 10.1021/ja036138+. [DOI] [PubMed] [Google Scholar]
- 35.Marrink SJ, Tieleman DP. Molecular dynamics simulation of spontaneous membrane fusion during a cubic-hexagonal phase transition. Biophys J. 2002;83:2386–2392. doi: 10.1016/s0006-3495(02)75252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knecht V, Marrink SJ. Molecular dynamics simulations of lipid vesicle fusion in atomic detail. Biophys J. 2007;92:4254–4261. doi: 10.1529/biophysj.106.103572. The work presents the molecular dynamic simulation of membrane fusion in atomic detail, largely confirms the fusion pathway through a hemifusion diaphragm and provides a computational basis for more detailed investigation of the fusion intermediates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller M, Katsov K, Schick M. A new mechanism of model membrane fusion determined from Monte Carlo simulation. Biophys J. 2003;85:1611–1623. doi: 10.1016/S0006-3495(03)74592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noguchi H, Takasu M. Self-assembly of amphiphiles into vesicles: A Brownian dynamics simulation. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 2001;64:041913. doi: 10.1103/PhysRevE.64.041913. [DOI] [PubMed] [Google Scholar]
- 39.Noguchi H, Takasu M. Fusion pathways of vesicles: a Brownian dynamics simulation. J Chem Phys. 2001;115:9547–9551. [Google Scholar]
- 40.Grafmuller A, Shillcock J, Lipowsky R. Pathway of membrane fusion with two tension-dependent energy barriers. Phys Rev Lett. 2007;98:218101. doi: 10.1103/PhysRevLett.98.218101. [DOI] [PubMed] [Google Scholar]
- 41.Shillcock JC, Lipowsky R. Tension-induced fusion of bilayer membranes and vesicles. Nat Mater. 2005;4:225–228. doi: 10.1038/nmat1333. [DOI] [PubMed] [Google Scholar]
- 42.Efrat A, Chernomordik LV, Kozlov MM. Point-like protrusion as a prestalk intermediate in membrane fusion pathway. Biophys J. 2007;92:L61–L63. doi: 10.1529/biophysj.106.103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamm M, Kozlov MM. Tilt model of inverted amphiphilic mesophases. Eur Phys J B. 1998;6:519–528. [Google Scholar]
- 44.Hamm M, Kozlov MM. Elastic energy of tilt and bending of fluid membranes. Eur Phys J E. 2000;3:323–335. [Google Scholar]
- 45.May S, Kozlovsky Y, Ben-Shaul A, Kozlov MM. Tilt modulus of a lipid monolayer. Eur Phys J E. 2004;14:299–308. doi: 10.1140/epje/i2004-10019-y. [DOI] [PubMed] [Google Scholar]
- 46.Siegel DP, Kozlov MM. The gaussian curvature elastic modulus of N-monomethylated dioleoylphosphatidylethanolamine: relevance to membrane fusion and lipid phase behavior. Biophys J. 2004;87:366–374. doi: 10.1529/biophysj.104.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JY, Schick M. Dependence of the energies of fusion on the intermembrane separation: optimal and constrained. J Chem Phys. 2007;127:075102. doi: 10.1063/1.2766945. [DOI] [PubMed] [Google Scholar]
- 48.Lee JY, Schick M. Field theoretic study of bilayer membrane fusion III: membranes with leaves of different composition. Biophys J. 2007;92:3938–3948. doi: 10.1529/biophysj.106.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JY, Schick M. Calculation of free energy barriers to the fusion of small vesicles. Biophys J. 2008;94:1699–1706. doi: 10.1529/biophysj.107.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marrink SJ, de Vries AH, Mark AE. Coarse grained model for semiquantitative lipid simulations. J Phys Chem B. 2004;108:750–760. [Google Scholar]
- 51.Lindahl E, Edholm O. Mesoscopic undulations and thickness fluctuations in lipid bilayers from molecular dynamics simulations. Biophys J. 2000;79:426–433. doi: 10.1016/S0006-3495(00)76304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang L, Huang HW. Observation of a membrane fusion intermediate structure. Science. 2002;297:1877–1879. doi: 10.1126/science.1074354. [DOI] [PubMed] [Google Scholar]
- 53.Cornell BA, Fletcher GC, Middlehurst J, Separovic F. The lower limit to the size of small sonicated phospholipid vesicles. Biochim Biophys Acta. 1982;690:15–19. doi: 10.1016/0005-2736(82)90233-4. [DOI] [PubMed] [Google Scholar]
- 54.Kasson PM, Pande VS. Control of membrane fusion mechanism by lipid composition: predictions from ensemble molecular dynamics. PLoS Comput Biol. 2007;3:e220. doi: 10.1371/journal.pcbi.0030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chernomordik LV, Melikyan GB, Abidor IG, Markin VS, Chizmadzhev YA. The shape of lipid molecules and monolayer membrane fusion. Biochim Biophys Acta. 1985;812:643–655. [Google Scholar]
- 56.Kozlov MM, Chernomordik LV. A mechanism of protein-mediated fusion: coupling between refolding of the influenza hemagglutinin and lipid rearrangements. Biophys J. 1998;75:1384–1396. doi: 10.1016/S0006-3495(98)74056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. The work demonstrates the ability of synaptotagmin C2 to bend membranes into 17-nm-diameter tubes with fusogenic end caps and suggests a new model of protein mediated membrane fusion based on calcium-dependent insertion of the synaptotagmin C2 domain into the membrane matrix. [DOI] [PubMed] [Google Scholar]
- 58.Hed G, Safran SA. Initiation and dynamics of hemifusion in lipid bilayers. Biophys J. 2003;85:381–389. doi: 10.1016/S0006-3495(03)74482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chernomordik L. Non-bilayer lipids and biological fusion intermediates. Chem Phys Lipids. 1996;81:203–213. doi: 10.1016/0009-3084(96)02583-2. [DOI] [PubMed] [Google Scholar]
- 60.Blood PD, Voth GA. Direct observation of Bin/amphiphysin/Rvs (BAR) domain-induced membrane curvature by means of molecular dynamics simulations. Proc Natl Acad Sci USA. 2006;103:15068–15072. doi: 10.1073/pnas.0603917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayton GS, Blood PD, Voth GA. Membrane remodeling from N-BAR domain interactions: insights from multi-scale simulation. Biophys J. 2007;92:3595–3602. doi: 10.1529/biophysj.106.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campelo F, McMahon HT, Kozlov MM. The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys J. 2008 doi: 10.1529/biophysj.108.133173. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chernomordik LV, Zimmerberg J, Kozlov MM. Membranes of the world unite! J Cell Biol. 2006;175:201–207. doi: 10.1083/jcb.200607083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chernomordik LV, Frolov VA, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemi-fusion, and lipidic fusion pore formation. J Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. The first work (i) describing hemifusion mediated by wild-type protein fusogen, (ii) identifying restricted hemifusion intermediates and (iii) reporting the dependence of fusion pore formation on the lipid composition of the distal membrane leaflets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmerberg J, Blumenthal R, Sarkar DP, Curran M, Morris SJ. Restricted movement of lipid and aqueous dyes through pores formed by influenza hemagglutinin during cell fusion. J Cell Biol. 1994;127:1885–1894. doi: 10.1083/jcb.127.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leikina E, Chernomordik LV. Reversible merger of membranes at the early stage of influenza hemagglutinin-mediated fusion. Mol Biol Cell. 2000;11:2359–2371. doi: 10.1091/mbc.11.7.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markosyan RM, Bates P, Cohen FS, Melikyan GB. A study of low pH-induced refolding of Env of avian sarcoma and leukosis virus into a six-helix bundle. Biophys J. 2004;87:3291–3298. doi: 10.1529/biophysj.104.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaitseva E, Mittal A, Griffin DE, Chernomordik LV. Class II fusion protein of alphaviruses drives membrane fusion through the same pathway as class I proteins. J Cell Biol. 2005;169:167–177. doi: 10.1083/jcb.200412059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoon TY, Okumus B, Zhang F, Shin YK, Ha T. Multiple intermediates in SNARE-induced membrane fusion. Proc Natl Acad Sci USA. 2006;103:19731–19736. doi: 10.1073/pnas.0606032103. By imaging real-time dynamics of single fusion events between the SNARE-carrying liposomes, this work identifies fusion intermediates with distinct extents of lipid mixing and characterizes the dwell times of docked and hemifused states and the lifetime of early fusion pores. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Melikyan GB, Niles WD, Cohen FS. Influenza virus hemagglutinin-induced cell-planar bilayer fusion: quantitative dissection of fusion pore kinetics into stages. J Gen Physiol. 1993;102:1151–1170. doi: 10.1085/jgp.102.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mittal A, Leikina E, Chernomordik LV, Bentz J. Kinetically differentiating influenza hemagglutinin fusion and hemifusion machines. Biophys J. 2003;85:1713–1724. doi: 10.1016/S0006-3495(03)74601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giraudo CG, et al. SNAREs can promote complete fusion and hemifusion as alternative outcomes. J Cell Biol. 2005;170:249–260. doi: 10.1083/jcb.200501093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 74.Zimmerberg J, Chernomordik LV. Neuroscience. Synaptic membranes bend to the will of a neurotoxin. Science. 2005;310:1626–1627. doi: 10.1126/science.1122439. [DOI] [PubMed] [Google Scholar]
- 75.Zampighi GA, et al. Conical electron tomography of a chemical synapse: vesicles docked to the active zone are hemi-fused. Biophys J. 2006;91:2910–2918. doi: 10.1529/biophysj.106.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong JL, Koppel DE, Cowan AE, Wessel GM. Membrane hemifusion is a stable intermediate of exocytosis. Dev Cell. 2007;12:653–659. doi: 10.1016/j.devcel.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schaub JR, Lu X, Doneske B, Shin YK, McNew JA. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat Struct Mol Biol. 2006;13:748–750. doi: 10.1038/nsmb1124. [DOI] [PubMed] [Google Scholar]
- 78.Razinkov VI, Melikyan GB, Epand RM, Epand RF, Cohen FS. Effects of spontaneous bilayer curvature on influenza virus-mediated fusion pores. J Gen Physiol. 1998;112:409–422. doi: 10.1085/jgp.112.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chernomordik LV, Leikina E, Frolov V, Bronk P, Zimmerberg J. An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids. J Cell Biol. 1997;136:81–94. doi: 10.1083/jcb.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chernomordik LV, et al. Lysolipids reversibly inhibit Ca2+-, GTP- and pH-dependent fusion of biological membranes. FEBS Lett. 1993;318:71–76. doi: 10.1016/0014-5793(93)81330-3. This paper demonstrates that lipids known to inhibit hemifusion stage of fusion between protein-free bilayers also inhibit disparate biological fusion reactions, suggesting that these reactions proceed through a common hemifusion intermediate. [DOI] [PubMed] [Google Scholar]
- 81.Melikyan GB, Barnard RJ, Abrahamyan LG, Mothes W, Young JA. Imaging individual retroviral fusion events: from hemifusion to pore formation and growth. Proc Natl Acad Sci USA. 2005;102:8728–8733. doi: 10.1073/pnas.0501864102. Different stages of virus–cell fusion mediated by avian sarcoma and leukosis virus envelope glycoproteins were dissected by imaging single virions. The findings suggest that fusion involves a direct transition from hemifusion into a small and then growing pore within a small virus–cell contact zone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Markosyan RM, Cohen FS, Melikyan GB. Time-resolved imaging of HIV-1 Envmediated lipid and content mixing between a single virion and cell membrane. Mol Biol Cell. 2005;16:5502–5513. doi: 10.1091/mbc.E05-06-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu T, Wang T, Chapman ER, Weisshaar JC. Productive hemifusion intermediates in fast vesicle fusion driven by neuronal SNAREs. Biophys J. 2008;94:1303–1314. doi: 10.1529/biophysj.107.107896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frolov VA, Dunina-Barkovskaya AY, Samsonov AV, Zimmerberg J. Membrane permeability changes at early stages of influenza hemagglutinin-mediated fusion. Biophys J. 2003;85:1725–1733. doi: 10.1016/S0006-3495(03)74602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindau M, Almers W. Structure and function of fusion pores in exocytosis and ectoplasmic membrane fusion. Curr Opin Cell Biol. 1995;7:509–517. doi: 10.1016/0955-0674(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 86.Jackson MB, Chapman ER. Fusion pores and fusion machines in Ca2+-triggered exocytosis. Annu Rev Biophys Biomol Struct. 2006;35:135–160. doi: 10.1146/annurev.biophys.35.040405.101958. [DOI] [PubMed] [Google Scholar]
- 87.Jackson MB. In search of the fusion pore of exocytosis. Biophys Chem. 2007;126:201–208. doi: 10.1016/j.bpc.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kemble GW, Danieli T, White JM. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. Replacing transmembrane domain of influenza hemagglutinin with a lipid anchor yielded the first direct demonstration that protein fusogens can induce hemifusion and emphasized the functional importance of transmembrane domain of the fusogen. [DOI] [PubMed] [Google Scholar]
- 89.Cleverley DZ, Lenard J. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc Natl Acad Sci USA. 1998;95:3425–3430. doi: 10.1073/pnas.95.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grote E, Baba M, Ohsumi Y, Novick PJ. Geranylgeranylated SNAREs are dominant inhibitors of membrane fusion. J Cell Biol. 2000;151:453–466. doi: 10.1083/jcb.151.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu Y, Zhang F, Su Z, McNew JA, Shin YK. Hemifusion in SNARE-mediated membrane fusion. Nat Struct Mol Biol. 2005;12:417–422. doi: 10.1038/nsmb921. This study on fusion between SNARE-proteoliposomes was the first direct demonstration that intracellular fusogens can mediate hemifusion. [DOI] [PubMed] [Google Scholar]
- 92.Langosch D, Hofmann M, Ungermann C. The role of transmembrane domains in membrane fusion. Cell Mol Life Sci. 2007;64:850–864. doi: 10.1007/s00018-007-6439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Melikyan GB, Lin S, Roth MG, Cohen FS. Amino acid sequence requirements of the transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mol Biol Cell. 1999;10:1821–1836. doi: 10.1091/mbc.10.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frolov V, Cho MS, Bronk P, Reese T, Zimmerberg J. Multiple local contact sites are induced by GPI-linked influenza hemagglutinin during hemifusion and flickering pore formation. Traffic. 2000;1:622–630. doi: 10.1034/j.1600-0854.2000.010806.x. [DOI] [PubMed] [Google Scholar]
- 95.Markosyan RM, Cohen FS, Melikyan GB. The lipid-anchored ectodomain of influenza virus hemagglutinin (GPI-HA) is capable of inducing nonenlarging fusion pores. Mol Biol Cell. 2000;11:1143–1152. doi: 10.1091/mbc.11.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McNew JA, et al. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J Cell Biol. 2000;150:105–117. doi: 10.1083/jcb.150.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jun Y, Xu H, Thorngren N, Wickner W. Sec18p and Vam7p remodel trans-SNARE complexes to permit a lipid-anchored R-SNARE to support yeast vacuole fusion. EMBO J. 2007;26:4935–4945. doi: 10.1038/sj.emboj.7601915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jun Y, Wickner W. Assays of vacuole fusion resolve the stages of docking, lipid mixing, and content mixing. Proc Natl Acad Sci USA. 2007;104:13010–13015. doi: 10.1073/pnas.0700970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gaudin Y. Rabies virus-induced membrane fusion pathway. J Cell Biol. 2000;150:601–612. doi: 10.1083/jcb.150.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vogel SS, Leikina EA, Chernomordik LV. Lysophosphatidylcholine reversibly arrests exocytosis and viral fusion at a stage between triggering and membrane merger. J Biol Chem. 1993;268:25764–25768. [PubMed] [Google Scholar]
- 101.Reese C, Heise F, Mayer A. Trans-SNARE pairing can precede a hemifusion intermediate in intracellular membrane fusion. Nature. 2005;436:410–414. doi: 10.1038/nature03722. [DOI] [PubMed] [Google Scholar]
- 102.Amatore C, et al. Regulation of exocytosis in chromaffin cells by trans-insertion of lysophosphatidylcholine and arachidonic acid into the outer leaflet of the cell membrane. ChemBioChem. 2006;7:1998–2003. doi: 10.1002/cbic.200600194. [DOI] [PubMed] [Google Scholar]
- 103.Churchward MA, et al. Specific lipids supply critical negative spontaneous curvature–an essential component of native Ca2+-triggered membrane fusion. Biophys J. 2008;94:3976–3986. doi: 10.1529/biophysj.107.123984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramos C, Rafikova ER, Melikov K, Chernomordik LV. Transmembrane proteins are not required for early stages of nuclear envelope assembly. Biochem J. 2006;400:393–400. doi: 10.1042/BJ20061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Podbilewicz B, et al. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev Cell. 2006;11:471–481. doi: 10.1016/j.devcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 106.Kobayashi T, et al. Separation and characterization of late endosomal membrane domains. J Biol Chem. 2002;277:32157–32164. doi: 10.1074/jbc.M202838200. [DOI] [PubMed] [Google Scholar]
- 107.Byrne RD, et al. PLCγ is enriched on poly-phosphoinositide-rich vesicles to control nuclear envelope assembly. Cell Signal. 2006;19:913–922. doi: 10.1016/j.cellsig.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 108.Rigoni M, et al. Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures. Science. 2005;310:1678–1680. doi: 10.1126/science.1120640. [DOI] [PubMed] [Google Scholar]
- 109.Blumenthal R, Clague MJ, Durell SR, Epand RM. Membrane fusion. Chem Rev. 2003;103:53–69. doi: 10.1021/cr000036+. [DOI] [PubMed] [Google Scholar]
- 110.Lai AL, Park H, White JM, Tamm LK. Fusion peptide of influenza hemagglutinin requires a fixed angle boomerang structure for activity. J Biol Chem. 2006;281:5760–5770. doi: 10.1074/jbc.M512280200. [DOI] [PubMed] [Google Scholar]
- 111.Shmulevitz M, Duncan R. A new class of fusion-associated small transmembrane (FAST) proteins encoded by the non-enveloped fusogenic reoviruses. EMBO J. 2000;19:902–912. doi: 10.1093/emboj/19.5.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roche S, Bressanelli S, Rey FA, Gaudin Y. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science. 2006;313:187–191. doi: 10.1126/science.1127683. This high resolution x-ray study, along with an earlier work of this group, characterizes a new class of viral fusion proteins (class III) that uses a bipartite fusion domain consisting of two short, noncontiguous hydrophobic loops. [DOI] [PubMed] [Google Scholar]
- 113.Antonny B. Membrane deformation by protein coats. Curr Opin Cell Biol. 2006;18:386–394. doi: 10.1016/j.ceb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 114.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 115.Chapman ER, Davis AF. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J Biol Chem. 1998;273:13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- 116.Ford MG, et al. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 117.Cohen FS, Melikyan GB. The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement. J Membr Biol. 2004;199:1–14. doi: 10.1007/s00232-004-0669-8. [DOI] [PubMed] [Google Scholar]
- 118.Zimmerberg J, Akimov SA, Frolov V. Synaptotagmin: fusogenic role for calcium sensor? Nat Struct Mol Biol. 2006;13:301–303. doi: 10.1038/nsmb0406-301. [DOI] [PubMed] [Google Scholar]
- 119.Kozlov MM, Chernomordik LV. The protein coat in membrane fusion: lessons from fission. Traffic. 2002;3:256–267. doi: 10.1034/j.1600-0854.2002.030403.x. [DOI] [PubMed] [Google Scholar]
- 120.Leikina E, et al. Influenza hemagglutinins outside of the contact zone are necessary for fusion pore expansion. J Biol Chem. 2004;279:26526–26532. doi: 10.1074/jbc.M401883200. [DOI] [PubMed] [Google Scholar]
- 121.Yang X, Kurteva S, Ren X, Lee S, Sodroski J. Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus type 1. J Virol. 2005;79:12132–12147. doi: 10.1128/JVI.79.19.12132-12147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Knecht V, Grubmuller H. Mechanical coupling via the membrane fusion SNARE protein syntaxin 1A: a molecular dynamics study. Biophys J. 2003;84:1527–1547. doi: 10.1016/S0006-3495(03)74965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]