Abstract
Rat cortical astrocytes regulate their cell volume in response to hypotonic challenge. This regulation is believed to depend largely on the release of chloride or organic osmolytes through anion channels. Using whole-cell recordings, we identified weakly outwardly rectifying chloride currents that could be activated in response to hypotonic challenge. These currents exhibited the following permeability sequence upon replacement of chloride in the bathing solution with various anions: I->NO3->Cl->Gluc- ≥MeS->Ise-. Interestingly, extracellular I-, albeit showing the greatest permeability, blocked the currents with an IC50 of ≈50 mM. Currents were almost completely inhibited by 123 μM NPPB and partially inhibited by 200 μM niflumic acid or 200 μM DIDS. Additionally, the total number of Cl- ions effluxed through the hypotonically activated channels was markedly similar to the total solute efflux during volume regulation. We therefore propose the hypotonically activated chloride channel as a major contributor to volume regulation of astrocytes. To examine potential candidate chloride channel genes expressed by astrocytes, we employed RTPCR to demonstrate the presence of transcripts for ClC-2, 3, 4, 5, and 7, as well as for VDAC and CFTR in cultured astrocytes. Moreover, we performed immunostaining with antibodies against each of these channels and showed the strongest expression of ClC-2 and ClC-3, strong expression of ClC-5 and VDAC, weak expression of ClC-7 and very weak expression of ClC-4 and CFTR. Intriguingly, although we found at least seven Cl- channel proteins from three different gene families in astrocytes, none appeared to be active in resting cells.
Keywords: anion channels, glia, volume regulation
INTRODUCTION
Swelling-activated chloride (Cl-) currents have been described ubiquitously in mammalian cells and have been implicated in a number of physiological roles (for review, see Nilius et al., 1996, 1997), including volume regulation or regulatory volume decrease (Sarkadi and Parker, 1991; Hoffmann and Dunham, 1995; Nilius et al., 1995; Bond et al., 1998; Hermoso et al., 2002) and cell proliferation (Voets et al., 1995, 1997; Schlichter et al., 1996). Shen et al., 2000; Wondergem et al., 2001). In different cell types, these currents have been given several names, including volume-activated Cl- currents (ICl,vol) (Nilius et al., 1994, 1996), swelling-activated Cl- currents (ICl,swell) (Ackerman et al., 1994a, b; Krapivinsky et al., 1994), or volume-regulated anion currents (VRAC) (Hubert et al., 2000; Levitan et al., 2000). However, all these currents typically share a set of common characteristics, including outward rectification with time-dependent inactivation at positive potentials, an iodide I-> Cl- permeability sequence, and sensitivity to a variety of Cl- channel blockers such as 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), 4-4′-diisothiocyantostilbene-2-2′-disulfonic acid (DIDS), niflumic acid, and tamoxifen (for review, see Nilius et al., 1996). Unfortunately, the molecular identity of the ubiquitous swelling-activated Cl- currents remains unknown. In fact, it is even unclear as to whether these currents are the product of a single molecular species or represent a family of swelling-activated channels.
Studies have suggested that cultured astrocytes like-wise exhibit Cl- currents that are activated by cell swelling or morphological perturbation and appear to be somewhat similar to ICl,swell. Bevan et al., (1985) and Gray and Ritchie (1986) originally described these whole-cell Cl- currents in rat cortical astrocytes. The currents were outwardly rectifying, exhibited time-dependent inactivation at positive potentials, and were sensitive to DIDS and 4-acetamido-4′-isothiocyanato-2,2′-stilbenedisulfonic acid (SITS). Although it appeared that these currents were normally present under control conditions, Lascola and Kraig (1996) suggested that activation of these currents required exposure to hypotonic challenge or induction of cell morphological changes. Interestingly, these investigators pointed out that in the earlier studies, the researchers performed their control recordings following brief trypsinization, a technique that induces morphological changes in cells. Further, Lascola and colleagues suggested a linkage of these channels to the actin cytoskeleton and postulated a role for actin cytoskeletal disruption in the activation of these channels (Lascola and Krarg, 1996; Lascola et al., 1998). Crepel et al., (1998) also examined the characteristics of swelling-activated Cl- channels in rat cortical astrocytes. In addition to showing a sensitivity of these channels to NPPB and SITS, they also showed a requirement for tyrosine and mitogen-activated protein (MAP) kinases in their activation.
In the present report, we set out to determine whether swelling-activated Cl- currents in rat cortical astrocytes are the major contributor to volume regulation in these cells, as we previously suggested that Cl- efflux through channels is essential in volume regulation of cultured rat cortical astrocytes (Parkerson and Sontheimer, 2003). Our goal was to elicit swelling-activated Cl- currents using hypotonic challenge, and to evaluate the currents using a set of anion substitutions and Cl- channel blockers similar to our previous study on volume regulation. Additionally, we wanted to determine whether the magnitude of Cl- efflux through these weakly outwardly rectifying channels could account for that required during normal volume regulation in astrocytes. Overall, our results suggest strong compatibility between the properties of hypotonically activated Cl- currents in rat cortical astrocytes and Cl- currents involved in volume regulation. Further, reverse transcription-polymerase chain reaction (RT-PCR) and immunocytochemical experiments showed the expression of several previously cloned Cl- channels in our cultured astrocytes. We were surprised to find the presence of at least seven different Cl- channels from three gene families in astrocytes.
MATERIALS AND METHODS
Cell Culture
Primary astrocyte cultures were established from Sprague-Dawley rats at postnatal day 0-2 by modification of the technique described by McCarthy and deVellis (1980). The protocol was reviewed and approved by the Institutional Animal Care and Use Committee. The pups were placed on ice and decapitated. The brain tissue was dissected in ice-cold Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Grand Island, NY). The meninges and associated blood vessels were removed, and the cortex was separated from surrounding regions. We then microscopically reexamined the tissue and removed any remaining blood vessels until the tissue was essentially vessel-free. The tissue pieces were further minced, washed with DMEM, and incubated in enzyme solution (warmed to 37°C and bubbled with 100% O2 for 10 min prior) for 20 min at 37°C. The enzyme solution consisted of DMEM supplemented with 20 U/ml papain, 1 mmol L-cysteine, and 0.5 mmol EDTA, and 706 U/ml deoxyribonuclease (Worthington, Freehold, NJ). The cells were pelleted by centrifugation for 2 min. The supernatant was aspirated and the remaining pellet was dissociated by trituration (10 −15×) with a fire-polished Pasteur pipette into astrocyte media supplemented with 10 U/ml penicillin, 10 μg/ml streptomycin, and 0.025 μg/ml amphotericin B (Life Technologies). Astrocyte media consisted of DMEM and 7% heat-inactivated fetal calf serum (FCS) (HyClone, Logan, UT). The cells were plated onto poly-ornithine (Sigma) and laminin (Sigma)-coated coverslips or tissue culture-treated dishes (Fisher) and then kept at 37°C in a 95% O2/5% CO2 atmosphere. The medium was replaced every 3 days.
Electrophysiology
The whole-cell patch-clamp technique (Hamill et al., 1981) was used to obtain current recordings from primary astrocytes maintained in culture for 5–12 days. The patch pipettes of thin-walled borosilicate glass (TW150F-4; World Precision, Sarasota, FL) were pulled to micron diameter tips (PP-83; Narishige, Japan) with typical resistances of 2–4 MΩ when filled with KCl pipette solution. The pipette and bath electrodes were silver wire (AGW1010/2030; World Precision), and the bath electrode contacted the bath solution via an agar bridge backfilled with pipette solution to minimize junction potential effects. An inverted Nikon Diaphot 200 equipped with Hoffman Modulation Contrast was used for visualization. The cells were continuously perfused in a Series-20 chamber (Warner Instruments, Hamden, CT) at room temperature with control extracellular solution. In addition, a single barreled microperfusion device was used to apply test solutions in superfusate that allowed for fast exchange of the bath. An Axopatch 200-B (Axon Instruments, Foster City, CA) amplified the current recordings and low-pass filtered the signals at 2 kHz. The preamplifier headstage and pipette holder were mounted onto a three-axis micromanipulator (Newport, Irvine, CA). A Digidata 1320 (Axon Instruments) interfaced to a PC-compatible computer (Dell, Austin, TX) digitized the signal on-line at 10 –100 kHz. The p Clamp8 (Axon Instruments) software package was used for data acquisition. Cell capacitance (Cp) and series resistance (Rs) compensation minimized voltage errors. The amplifier reading of Cp was used as the value of the whole-cell membrane capacitance; this value closely matched that calculated by an uncompensated membrane test using a —5-mV pulse in Clampex.
Solutions for Electrophysiology
The bath and pipette solution contents for electro-physiological recordings are listed in Table 1. ChCl bath and pipette solutions were typically used to isolate chloride currents, although NaCl bath without potassium was used as the starting bath solution during anion substitution experiments in order to minimize junction potentials. Solution osmolarities were tested with a vapor pressure osmometer (Wescor 5500; Wescor, Logan, UT). The osmolarities of our isotonic bath solutions were 310 ± 5 mOsm and of our pipette solutions were 300 ± 5 mOsm. The pH value of our bath solutions was adjusted to 7.4 with NaOH; the pH value of our pipette solutions was adjusted to 7.3 with Tris-base. Bath solutions were made hypoosmotic by addition of water. Drugs were added directly to the bath solution from stock solutions. NPPB, niflumic acid and tamoxifen (tmx) were dissolved at 1,000× final concentration in dimethylsulfoxide (DMSO), cadmium (Cd2+) was dissolved at 1000× final concentration in ddH2O, and DIDS was dissolved at 250× final concentration in 0.1 M KHCO3. DMSO and KHCO3 did not affect currents at their final concentrations.
TABLE 1.
Solution Contents (in mM)
| Bath Solutions |
Pipette Solutions |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | NaCl | ChCl | NaCl (0 K+) |
NaI | NaNO3 | NaGluc | Nalse | NaMeS | 10Nal | 30Nal | KCl | ChCl |
| Na+ | 140.0 | 0 | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 | 10.0 | 10.0 |
| K+ | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 140.0 | 0 |
| Ch+ | 0 | 140.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 135.0 |
| Ca2+ | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.2 | 0.2 |
| Mg2+ | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.0 | 1.0 |
| Cl- | 146.9 | 144.4 | 144.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 134.4 | 114.4 | 142.4 | 137.4 |
| Br- | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| I- | 0 | 0 | 0 | 140.0 | 0 | 0 | 0 | 0 | 10.0 | 30.0 | 0 | 0 |
| NO3- | 0 | 0 | 0 | 0 | 140.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gluc- | 0 | 0 | 0 | 0 | 0 | 140.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ise- | 0 | 0 | 0 | 0 | 0 | 0 | 140.0 | 0 | 0 | 0 | 0 | 0 |
| MeS- | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 140.0 | 0 | 0 | 0 | 0 |
| HEPES- | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 10.0 | 10.0 |
| EGTA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10.0 | 10.0 |
| Glucose | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 0 | 0 |
Data Analysis for Electrophysiology
Electrophysiology data were collected from voltage-step, ramp, or gap-free protocols in Clampex, and traces were analyzed using Clampfit. Data were plotted with Origin 6.0 (MicroCal, Northampton, MA). All data were reported as means ± standard error of the mean (SEM) with number of experiments performed (n). Significance (P) was determined by Student’s t-test. Current densities (pA/pF) were calculated as current amplitude divided by Cp. The relative permeabilities of different anions to Cl- (Panion/PCl-) in whole-cell currents were calculated from shifts in the reversal potential, using the GHK equation (Hille, 2001):
with ΔErev as the reversal potential after switching into solution with the test anion minus the reversal potential in the initial NaCl bath, z as the valence of the ion, T as the absolute temperature, F as Faraday’s constant, R as the gas constant, [Cl-]o as the chloride concentration in the initial solution, and [anion]o as the concentration of anion substitute in the test solution. zF/RT was equal to -0.039 in our conditions. Dose-response curves were plotted as percentage inhibition versus drug concentration. Data were fit to a binding isotherm of the form:
with Kd the half-maximal inhibitory concentration.
Volume Regulation
Cell volume measurements were performed by electronic sizing using a Coulter Counter Multisizer 3 (Beckman-Coulter, Miami, FL). Cells were washed in phosphate-buffered saline (PBS) and lifted by a 3—5-min incubation with 0.05% trypsin and 0.53 mM EDTA. Trypsin was inactivated by the addition of equal volume of astrocyte media, and cells were pelleted by brief centrifugation. Cells were resuspended in NaCl bath solution (Table 1) and passed through a 40-μm nylon cell strainer. A single cell suspension was verified by microscopic visualization of a sample aliquot. Cells were equilibrated for approximately 10 min before the first reading. Cell volume measurements were obtained every minute, and each measurement was an average of 15,000 cells. Bath solutions were made hypoosmotic by the addition of water and hypotonic solution was applied after baseline readings. Coulter counter data were collected with Multisizer 3 software, and size listings were exported to EXCEL. Mean cell volumes were normalized to baseline value. Data were plotted in Origin 6.0 (MicroCal) as means ±SEM with number of experiments performed (n).
Reverse Transcription Polymerase Chain Reaction
RNA was obtained from cells that were grown to confluency in 100-mm dishes. The RNAqueous kit from Ambion (Austin, TX) was used to initially isolate the RNA and further treatment with the DNA-free kit from Ambion was used to remove contaminating DNA. Samples were stored at -20°C until used in RT-PCR.
RT-PCR was performed using the Qiagen One-Step RT-PCR kit (Valencia, CA). Oligonucleotide primers specific for ClC-1 through ClC-7, ClC-K1, ClC-K2, CFTR, and VDAC-1 were custom-made by Life Technologies. Primers for ClC-1, ClC-5, ClC-6, and ClC-7 were identical to those used by Kulka et al., (2002). Primers for ClC-2, ClC-3, ClC-4, ClC-K1, and ClC-K2 were identical to those used by Enz et al., (1999). Primers for CFTR were identical to those used by Huber et al. (1998). Primers for VDAC-1 were identical to those used by Bres et al. (2000). Primers were used at a final concentration of 0.6 μM. Either 1 μl of astrocyte RNA sample or 0.5 μl of rat brain cDNA (Clontech, Palo Alto, CA) was added for each reaction; 10 U of SUPERase-In (Ambion, Austin, TX) was also added to each reaction. Other reaction components were added according to the Qiagen protocol, and the final volume of each reaction mixture was 50 μl.
Reverse transcription and amplification was performed using a thermocycler that was programmed with the following parameters: (1) reverse transcription at 50°C for 30 min; (2) PCR activation at 95°C for 15 min; (3) 35 cycles of denaturation at 94°C for 1 min, annealing at 62°C for 1 min, and extension at 72°C for 1 min; and (4) final extension at 72°C for 10 min; 10 min; 10 μl of each PCR product was separated on a 1% agarose gel. The PCR products were stained with ethidium bromide, visualized under ultraviolet (uv) light, and compared with the expected number of base pairs for each reaction. Controls for DNA contamination were performed with the same thermocycler parameters, but without reverse transcriptase in the reaction mixture (PCR supermix, Life Technologies).
Immunocytochemistry
Cells were grown on coverslips to approximately 80% confluency and were washed with phosphate-buffered saline (PBS). Cells were fixed with 4% paraformalde-hyde for 20 min at room temperature and washed with PBS (1 × 10 min). Cells were blocked with blocking buffer (PBS containing 3% normal goat serum [NGS]) for 30 min at room temperature and then incubated overnight at room temperature in blocking buffer diluted one-half with PBS plus 0.01% sodium azide and primary antibody diluted 1:200. Primary antibodies were rabbit anti-CLC-2, 3, 4, 5, or 7 from Alpha Diagnostics (San Antonio, TX), mouse anti-CFTR from Neo-markers (Fremont, CA), or rabbit anti-VDAC from Oncogene (San Diego, CA). The next morning, cells were washed in PBS (3 × 5 min) and incubated in the dark for 1 h at room temperature in blocking buffer plus secondary antibody diluted 1:500. Secondary antibody was Alexa 488 anti-rabbit IgG or Alexa 488 anti-mouse IgG1. Cells were washed in PBS (3 × 5 min) and during the second wash were counterstained with DAPI. Coverslips were mounted on slides with Gel/Mount (Biomeda; Foster City, CA). Control staining was performed without primary antibody. Fluorescence was visualized using a Zeiss (Thornwood, NY) Axiovert 200M microscope at 100×. Images were captured with an Axiocam (Zeiss) and processed in Axiovision 3.1 (Zeiss).
RESULTS
Astrocytes Lack Whole-Cell Chloride Currents Under Isotonic Conditions
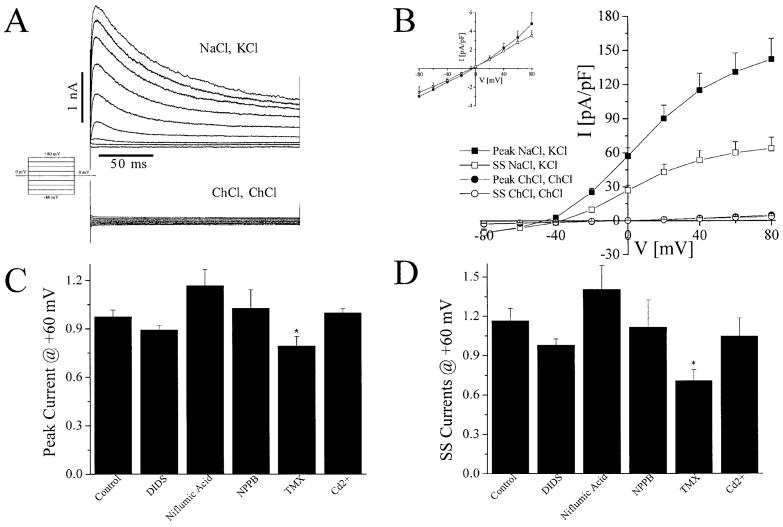
Because we were interested in isolating and characterizing only the hypotonically activated Cl- currents in our cells, we wanted to rule out any potentially confounding effects from other Cl- currents in our cells. The top trace in Figure 1A displays a representative example of recordings using standard NaCl bath and KCl pipette solutions (Table 1). We applied a family of voltage steps ranging from -80 to +80 in +20-mV increments, to demonstrate the overall profile of ionic currents. The currents were outwardly rectifying and displayed both voltage and time dependence.
Fig. 1.
Astrocytes lack whole-cell chloride currents under isotonic conditions. A: Representative whole-cell recordings of voltage steps from -80 to +80 in 20 mV increments for 200 ms. The upper trace was recorded in NaCl bath solution and KCl pipette solution from a holding potential of -80 mV. The lower trace was recorded in ChCl bath solution and ChCl pipette solution from a holding potential of 0 mV. B: Mean I-V plots of cells recorded in NaCl bath solution and KCl pipette solution compared to those recorded in ChCl bath and pipette solutions (filled squares, peak NaCl, KCl; open squares, ss NaCl, KCl; filled circles, peak ChCl, ChCl; open circles, ss ChCl, ChCl). Inset shows an amplified version of the mean I-V plot for ChCl cells only. C,D: Mean normalized currents at peak (C) and ss (D) of cells exposed to chloride channel blockers for 2 min in NaCl bath solution and KCl pipette solution. Values were obtained from currents amplitudes at +60 mV in a series of voltage steps after 2-min exposure to chloride channel blockers and normalized to currents recorded before drug application.
To determine any contribution of Cl- currents to this profile, we substituted the large impermeant cations choline+ (Ch+) or N-methyl-D-glucamine+ (NMDG+) for both Na+ and K+ in the bath and pipette solutions (see ChCl bath, ChCl pipette in Table 1). The bottom trace in Figure 1A shows a representative example of currents recorded with Ch+ in response to voltage steps described above. Our recordings in either Ch+ or NMDG+ revealed an almost complete absence of currents. The mean I-V plots at peak and steady state (ss) of cells recorded in NaCl bath and KCl pipette solutions compared to those recorded in ChCl bath and pipette solutions are shown in Figure 1B; a magnification of the mean I-V plot of cells recorded in ChCl bath and pipette solutions is displayed in the inset. As shown, only a very small residual current was present in ChCl bath and pipette solutions.
To verify further an absence of Cl- currents under isotonic conditions, we also tested known Cl- channel blockers for inhibition of the outwardly rectifying currents in NaCl bath and KCl pipette solutions. Currents were recorded in response to voltage steps after a 2-min application of DIDS (200 μM), niflumic acid (200 μM), NPPB (123 μM), tmx (10 μM), or Cd2+ (500 μM) and normalized to those before drug application. The results for peak and ss currents at +60 mV are shown in Figure 1C,D. No significant difference in current magnitude was observed with application of any drug, except tmx, which we have previously shown to also inhibit cation currents (Smitherman and Sontheimer, 2001). We concluded that cultured rat cortical astrocytes do not express Cl- currents under isotonic conditions. This finding agrees with previous studies on cortical astrocytes by Lascola and Kraig (1996).
Hypotonic Challenge Activates Whole-Cell Chloride Currents in Astrocytes
Next, we were interested in isolating the whole-cell Cl- currents that could be activated by hypotonic challenge, as we hypothesized that the Cl- channels activated by cell swelling play a major role in the volume regulation of astrocytes. To elicit hypotonically activated Cl- currents in our cells, we exposed them to a superfusate of 35% hypotonic ChCl bath while recording in normal ChCl bath and pipette solutions. A series of voltage steps was applied to the cells before (Fig. 2A) and after a 2-min application of hypotonic solution (Fig. 2B). Figure 2C shows a subtraction of the currents before and after 2-min hypotonic challenge in order to isolate hypotonically activated currents. The hypotonically activated currents were weakly outwardly rectifying and showed time-dependent inactivation above +60 mV. The mean I-V plot at peak and ss for these hypotonically activated currents (n = 4) is shown in Figure 2D. Currents were also monitored every 10 s by voltage ramps ranging from -120 mV to +100 mV from a holding potential of 0 mV in response to a 2-min hypotonic challenge. Currents began to activate at ≈1 min after the application of hypotonic challenge and increased in magnitude during the next minutes (Fig. 2E). If returned to an isosmotic bath solution following a short pulse of hypotonic challenge (≈2 min or less), we first observed a transient increase in outward current and a negative shift in reversal of the currents (data not shown), consistent with the change in extra-cellular Cl- concentration from hypotonic ([Cl-]o = 94 mM) to isotonic ([Cl-]o = 144 mM) solutions. This was followed by a gradual decrease in both inward and outward current magnitudes (data not shown). However, recovery was thwarted with longer applications of hypotonic challenge.
Fig. 2.
Hypotonic challenge activates whole-cell chloride currents in astrocytes. A: Representative whole-cell recording of voltage steps from -80 mV to +80 mV for 200 ms from a 0-mV holding potential (inset) in ChCl bath and pipette solutions. B: Voltage steps after 2 min in hypotonic ChCl bath solution shows increased current magnitudes. C: Subtraction of A—B demonstrates hypotonically activated currents. D: Mean I-V plots of currents activated by hypotonic challenge at peak (filled squares) and ss (open squares). E: Time course of activation of currents induced by hypotonic challenge. Values were obtained from voltage ramps every 10 s and normalized to the mean absolute amplitudes before hypotonic challenge.
It should also be noted that in some cells, weakly outwardly rectifying currents activated spontaneously without application of hypotonic challenge. The spontaneous-activating currents appeared to have the same pharmacological properties as hypotonically activated currents in our cells. Additionally, although the occurrence of spontaneous currents was unpredictable, the activation invariably associated with what appeared to be spontaneous swelling or morphological change in the cells when viewed under the microscope. Also, as we never have seen activation of these currents in normal KCl pipette solution (Table 1), we suspected that the spontaneous changes might occur more frequently in the face of complete sodium (Na+) and potassium (K+) replacement with a larger cation.
Anion Substitution Reveals the Relative Permeability Sequence of the Hypotonically Activated Chloride Channel in Astrocytes
To determine the anion selectivity of the hypotonically activated Cl- channel, we performed anion substitution experiments. For these experiments, we activated Cl- currents by a 2-min hypotonic challenge while recording in NaCl bath and ChCl pipette solutions (Table 1) and then applied a superfusate of hypotonic NaCl bath containing 140 mM of the Na-salt of the test anion in place of 140 mM NaCl (Table 1). Currents were recorded every 10 s in response to voltage ramps ranging from -120 mV to +100 mV from a holding potential of 0 mV. We evaluated changes in the reversal potential as well as changes in outward current amplitude of the hypotonically activated currents in the presence of each test anion. Substitution of either I- or nitrate (NO3-) resulted in a negative shift, whereas substitution of either gluconate (Gluc-), methanesulfonate (MeS-), or isethionate (Ise-) resulted in a positive shift of the reversal potential of the hypotonically activated currents. A negative shift suggests the test anion is more permeable than Cl- and a positive shift suggests the test anion is less permeable than Cl- through the hypotonically activated channel. From the changes in reversal potentials, we calculated permeability ratios using the Goldman-Hodgkin-Katz (GHK) equation (see Data Analysis for Electrophysiology in Materials and Methods), and the results are shown in Table 2. On the basis of these data, the overall permeability sequence for the hypotonically-activated Cl- channel was: I-> NO3-> Cl-> Gluc ≥ MeS-> Ise-. The reversal potential measured for each test anion was significantly different (P < 0.05) from the reversal potential in Cl-.
TABLE 2.
Anion Selectivity of Hypotonically Activated Chloride Channels
| Anion | Measured ΔErev(mV) |
Jnx Potmeas (mV) |
actual ΔErev (mV) |
Panion/PCl- | N |
|---|---|---|---|---|---|
| Cl- | — | — | — | — | — |
| Gluc- | 11.25 | 2.4 | 8.85 ± 1.52 | 0.74 | 4 |
| I- | -6.74 | 0.5 | -7.24 ± 0.52 | 1.39 | 4 |
| Ise- | 17.04 | 1.5 | 15.54 ± 3.08 | 0.57 | 4 |
| MeS- | 10.52 | 1.3 | 9.22 ± 1.34 | 0.73 | 4 |
| NO3- | -3.75 | 0.4 | -4.15 ± 0.27 | 1.23 | 4 |
Consistent with Gluc-, MeS-, and Ise- being less permeable and NO3- being more permeable than Cl- through the hypotonically activated Cl- channel, we also observed a decrease in outward current amplitude, corresponding to a decrease in relative conductance, upon substitution of Gluc-, MeS-, or Ise- for Cl-. We noted an increase in outward current amplitude, corresponding to an increase in relative conductance, upon substitution of NO3- for Cl- (data not shown). However, there was a discrepancy between the relative permeability of I- to Cl- determined by shift in reversal potential and by change in current amplitude, and this is further examined below. Such a discrepancy was previously described for a Cl- current in guinea pig cardiac myocytes (Vandenberg et al., 1994).
Extracellular Iodide Substitution Inhibits the Hypotonically Activated Chloride Channel in Astrocytes
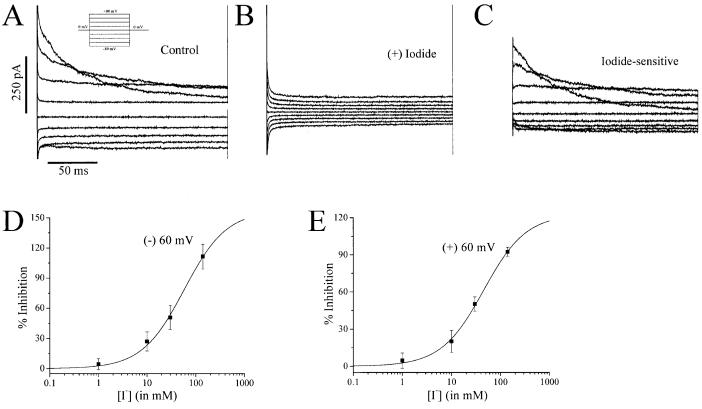
Interestingly, although I- substitution resulted in a leftward shift in the reversal potential of the hypotonically activated currents, substitution of I- for Cl- in the bathing solution also resulted in almost complete abolishment of the hypotonically activated Cl- currents. Therefore, it appeared that I- was also exerting a blocking effect on the hypotonically activated currents. Figure 3A shows a representative example of currents recorded in response to series of voltage steps following a 2-min hypotonic challenge. Figure 3B shows the currents recorded in the same cell after I- substitution but still in the presence of hypotonic challenge. Figure 3C shows a subtraction of the currents before and after I- substitution in order to isolate the I- -sensitive currents. We also determined the dose-response relationships for I- inhibition of the hypotonically activated currents by analyzing the current amplitudes in response to voltage ramps before and after application of four different I- concentrations, namely 1, 10, 30, and 140 mM. Plotting the percentage inhibition at -60 mV and +60 mV as a function of I- concentration and then fitting the data with binding isotherms (see Data Analysis for Electrophysiology in Materials and Methods) suggested apparent IC50 values of 58 mM and 45 mM at -60 mV and +60 mV, respectively (Fig 3D,E).
Fig. 3.
Extracellular iodide substitution inhibits the hypotonically activated chloride channel in astrocytes. A-C: Representative whole-cell recording of voltage steps from -80 mV to +80 mV for 200 ms from a 0-mV holding potential (inset) in ChCl bath and pipette solutions following 2-min hypotonic challenge. B: Voltage steps after substitution of 140 mM NaI for 140 mM NaCl in the bath solution. C: Subtraction of A—B demonstrates iodide-sensitive currents. D,E: Dose-response curves of current inhibition by iodide at +60 mV (D) and -60 mV (E). Binding isotherms suggest apparent IC50 values of 45 mM and 58 mM at +60 mV and -60 mV, respectively.
Chloride Channel Blockers Inhibit the Hypotonically Activated Chloride Channel in Astrocytes
To determine the pharmacology of the hypotonically activated chloride channel, we investigated the effects of several chloride channel blockers on current amplitudes. For these experiments, we activated Cl- currents by 2-min hypotonic challenge while recording in ChCl bath and pipette solutions (Table 1) and then applied a superfusate of hypotonic ChCl bath containing either 123 μM NPPB, 200 μM niflumic acid, 200 μM DIDS, 500 μM Cd2+, or 200 μM DIDS in combination with 500 μM Cd2+. Currents were recorded either every 10 s in response to voltage ramps ranging from -120 mV to +100 mV from a holding potential of 0 mV or before and after drug application in response to voltage steps ranging from -80 to +80 in +20 mV increments from a holding potential of 0 mV. Figure 4A–C shows subtraction of the currents before and after exposure to NPPB, niflumic acid, or DIDS in order to isolate the NPPB, niflumic acid or DIDS-sensitive currents, respectively. Of note is that NPPB and niflumic acid appeared to produce a voltage-independent block of both outward and inward currents, whereas DIDS appeared to produce predominantly a voltage-dependent block of outward currents only. Such a voltage-dependent block by DIDS has been demonstrated previously for swelling-activated chloride currents in other cell types (Braun and Schulman, 1996; Ehring et al., 1994; Winpenny et al., 1996; Mitchell et al., 1997). We compared the percentage inhibition by each test drug in Figure 4D,E. A negative current block is shown for control cells because the currents continued to increase in hypotonic solution between two minutes in hypotonicity and during the time period of drug application. A negative current block is also shown for cells in the presence of Cd2+ only, and Cd2+ did not significantly block the hypotonically activated Cl- channel. In contrast, NPPB, niflumic acid, DIDS, or DIDS in combination with Cd2+ produced a significant inhibition of currents at both +60 and -60 mV. However, as discussed above, the current block of DIDS at -60 mV was not nearly as complete as that of NPPB or niflumic acid. Also, DIDS in combination with Cd2+ did not produce a block that was significantly different from that of DIDS alone at either +60 mV or -60 mV.
Fig. 4.
Chloride channel blockers inhibit the hypotonically-activated chloride channel in astrocytes. A–C: Representative whole-cell recordings of NPPB-sensitive (A), NA-sensitive (B), and DIDS-sensitive (C) currents obtained from subtraction of current traces in response to voltage steps from -80 mV to +80 mV from a holding potential of 0 mV (inset) in ChCl hypotonic bath and ChCl pipette solutions before and after NPPB, NA or DIDS application, respectively. D,E: Percentage current block at +60 mV (D) and -60 mV (E) for different chloride channel blockers of interest. Values were obtained by comparison of current amplitudes in response to voltage steps following 2-min hypotonic challenge only and in the presence of the blocker of interest. Note that negative current block was obtained for control cells, as the current amplitudes continued to increase with time in hypotonic challenge. Asterisks indicate a statistically significant (*P < 0.05, **P < 0.001) change from control amplitudes.
Chloride Ion Efflux Through Hypotonically Activated Chloride Channels Accounts for Solute Efflux During Volume Regulation of Astrocytes
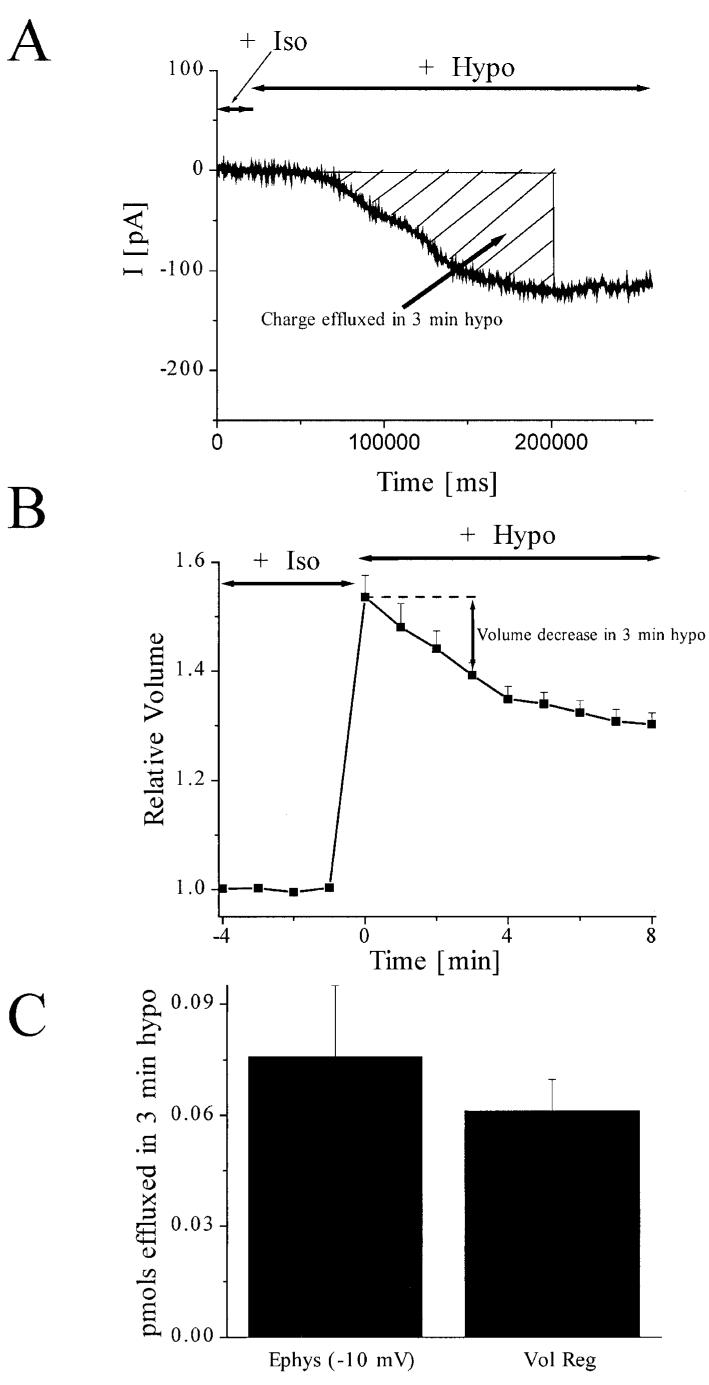
In addition to the examination of the biophysical and pharmacological properties of the hypotonically activated Cl- channel, we also wanted to consider how Cl- ion efflux through hypotonically activated Cl- channels compared to that of solute efflux required during volume regulation. To do so, we first decided to perform gap-free electrophysiological recordings and to calculate the total number of mols effluxed through hypotonically activated Cl- channels during a given period of time. Figure 5A shows a representative example of hypotonically activated Cl- currents recorded in the gap-free mode at a holding potential of -10 mV. We chose to hold the cells at -10 mV in order to slightly hyperpolarize the cells from ECl- (set to -1.3 mV in our cells) and to mimic the situation of astrocytes depolarized towards EClminus when exposed to hypotonic solution. Cells were recorded in ChCl bath and pipette solutions (Table 1), and hypotonic challenge was applied where indicated. As expected, hyperpolarization from ECl- resulted in inward Cl- currents that corresponded to an efflux of Cl-. We calculated the charge effluxed in 3 min of hypotonic challenge by integrating under the curve during this time period. This value, determined in Clampfit (Axon Instruments), was 7.3 × 103 pA • s(±1.8 × 103, n = 3). Using Faraday’s constant, we converted the charge effluxed into mols, which resulted in an average of 0.076 pmol (±0.019, n = 3) of Cl- ions effluxed during a 3-min hypotonic challenge.
Fig. 5.
Magnitude of chloride effluxed through hypotonically activated chloride channels accounts for mols of solute effluxed during volume regulation of astrocytes. A: Representative example of gap-free currents obtained at a -10 mV holding potential during 3-min hypotonic challenge. Note that the -10 mV holding potential corresponds to an 8.7-mV hyperpolarization from the calculated ECl. The total charge effluxed during this time was calculated by integrating the area under the curve (hatched). B: Normal volume regulation plotted for 10 min following exposure to hypotonic challenge. The volume decrease within the 3 minutes of interest is shown with an arrow. C: Comparison of pmols effluxed during 3-min hypotonic challenge in gap-free electrophysiological recordings at -10 mV holding potential and during volume regulation. An explanation of the calculations is given in the Results section.
We next calculated the mols effluxed during volume regulation in response to a three minute 50% hypotonic challenge. Figure 5B shows the mean normalized cell volume of astrocytes in response to hypotonic challenge. Each point represents the mean cell volume of 15,000 cells monitored every minute by electronic sizing using a Coulter counter (see Materials and Methods) for an average of nine experiments. It should be noted that the hypotonic challenge was slightly stronger in these volume regulation experiments than in the electrophysiological experiments. In the volume regulation experiments, the cells contained a finite intra-cellular volume to equilibrate with the hypotonic bath. The cells exhibited an instantaneous peak volume followed by a volume decrease. In contrast, in the electro-physiology experiments, the cells were exposed to an infinite pipette volume, and the cells appeared to continue swelling throughout the experiment. Therefore, we chose a slightly less dilute hypotonic challenge for the electrophysiology experiments in order to best compare the solute efflux between the two distinct experimental setups. Further, the cells in the electrophysiology experiments were much more stable at 35% hypotonic challenge than at 50% hypotonic challenge, whereas a 50% hypotonic challenge produced the most consistent and stable volume regulation curves.
An arrow depicts the volume decrease during 3 min in hypotonicity (Fig. 5B). The average volume decrease during this time period was 320.2 fL (±52.4, n = 9). In order to convert volume decrease to number of mols effluxed, we made the following assumptions and calculations. We assumed that the initial starting osmolarity within the cells was 300 mOsm. From this, we calculated the average total mols of solute within the cells in isotonic solution. Then, at t = 0 (immediately following hypotonic challenge), we assumed that the same number of solute mols should be diluted into a larger volume due to an influx of water and cell swelling. This gave us an average osmolarity of solutes within the cells at t = 0 of 196.2 mOsm (± 5.1, n = 9). During the following 3 min, we assumed that the cells would maintain this osmolarity while losing solutes and water to volume regulate. Therefore, the number of mols of solute lost during a three minute hypotonic challenge should be equal to the volume decrease during this time period multiplied by the average osmolarity within the cells at t = 0. This resulted in an average of 0.061 pmol (±0.009, n = 9) of solute effluxed during a 3-min hypotonic challenge. This number is remarkably similar to our calculation of the number of mols of Cl- effluxed through hypotonically activated Cl- channels during the same time period, and these values are compared in Figure 5C.
Astrocytes Express Previously Cloned Chloride Channels
In an initial attempt to identify potential molecular candidates for the hypotonically activated Cl- channel in cultured cortical astrocytes, we performed a combination of RT-PCR and immunocytochemistry. To date, a comprehensive list of Cl- channel expression in rat cortical astrocytes has not been published, although there have been reports of ClC-2 and ClC-3 (Sik et al., 2000; Stegen et al., 2000), members of the ClC family of voltage-dependent Cl- channels, of CFTR (Ballerini et al., 2002), the sole member of the ATP-binding cassette channels and of BR1-VDAC (Dermietzel et al., 1994), a member of the voltage-dependent anion channels in these cells. We decided to focus on the expression of these previously identified channels in our cells, as well as to examine the expression of other Cl- channels that have been sequenced from rat, at both the RNA and protein levels. At the protein level, we were specifically interested in those channels that were present on the membrane surface, as only these should be able to contribute to whole-cell hypotonically activated Cl- currents.
Figure 6 shows the results of RT-PCR performed with sequence specific primers for ClC-1 through ClC-7, ClC-K1, ClC-K2, CFTR, and VDAC (Table 3). All the primers used were previously shown to recognize bands at the correct number of base pairs in rat tissues and to correspond to the channel of interest upon sequencing. Of the channels tested, we showed the presence of ClC-2, 3, 4, 5, and 7 (Fig. 6A), CFTR (Fig. 6B) and VDAC (Fig. 6C) in our cells. As controls, we also showed the presence or absence of β-actin product with and without addition of reverse transcriptase, respectively (Fig. 6D).
Fig. 6.
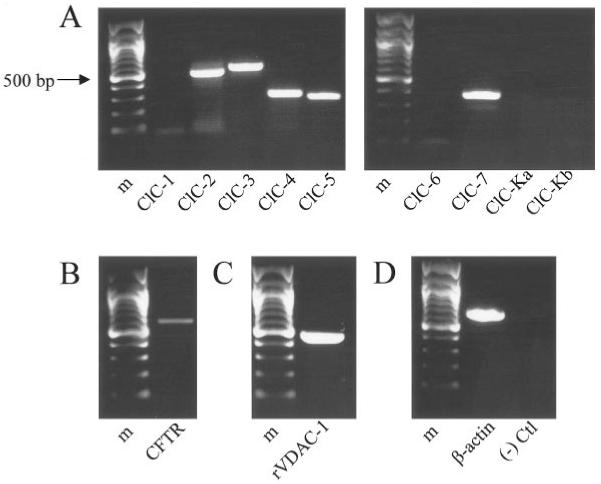
Astrocytes express RNA encoding previously cloned chloride channels. All panels show ethidium bromide-stained products in a 1% agarose gel following RT-PCR amplification of rat cortical astrocyte RNA. Sequence specific primers for the gene of interest were used for each reaction. A 100-bp ladder is included in each panel for reference; Bands are visible at the expected number of base pairs for ClC-2, 3, 4, 5, and 7 (A), CFTR (B), VDAC (C), and β-actin (D). As control, product is not observed in the absence of reverse transcriptase (D).
TABLE 3.
Oligonucleotide Primers for RT-PCR
| Gene | Sense primer (5′ to 3′) | Antisense primer (5′ to 3′) | Expected length |
Reference |
|---|---|---|---|---|
| ClC-1 | TGTGGAACGCTCAGAACTGCAGTC | TCTAGTGCCAAGACACCTCTGAGC | 656 | Kulka et al. (2002) |
| ClC-2 | CAAGTTCCTCTCCCTCTTTG | GAACTGTCCAAAGCCAGGG | 499 | Enz et al. (1999) |
| ClC-3 | CCTCTTTCCAAAGTATAGCAC | TTACTGGCATTCATGTCATTTC | 552 | Enz et al. (1999) |
| ClC-4 | GGTACATGGCTGAACTCTTC | GAGTCATGTTGGGGTCATTG | 297 | Enz et al. (1999) |
| ClC-5 | TGCTGACTGTCCTTACTCAG | CAGGATGTTCCGAAGCTTCA | 269 | Kulka et al. (2002) |
| ClC-6 | TCTGTGCTGCTGCTGCAGGTGGTG | TGGCTGCACTCCTCCACCGATGTC | 345 | Kulka et al. (2002) |
| ClC-7 | ATGAGCACGCCTGTGACCTGCCTG | CGAGGAAGAGATGCCTCCTGTGGC | 377 | Kulka et al. (2002) |
| ClC-K1 | TTCAGCATCGAGGTCATGTC | GCCTCTCCCAAGAGGCG | 665 | Enz et al. (1999) |
| ClC-K2 | TTCAGCATCGAGGTCATGTC | CCCCAAAGAGGCGCCCG | 661 | Enz et al. (1999) |
| CFTR | CGCAGGTTCTCAGTGGACGATGCC | CCTCAACCAGAAAAACCAGCACGCA | 607 | Huber et al. (1998) |
| VDAC-1 | GGACCGAGTATGGGCTGACG | GCTGCTATTCCAAAGCGAGTGTTAC | 478 | Bres et al. (2000) |
| β-Actin | CCATGTACGTAGCCATCCA | GATAGAGCCACCAATCCAC | 625 | Kulka et al. (2002) |
Figure 7 shows the results of immunostaining with antibodies against each of the channels found at the RNA level in our cells. We performed the immunostaining without a permeabilization step in order to limit reactivity to the cell surface. The strongest surface expression was observed for ClC-2 (Fig. 7A) and ClC-3 (Fig. 7B). Strong immunostaining was also observed for ClC-5 (Fig. 7D) and VDAC (Fig. 7F). Weak immunostaining was observed for ClC-7 (Fig. 7E) and very weak immunostaining was observed for ClC-4 (Fig. 7C) and CFTR (Fig. 7G). Staining was negative in the absence of primary antibody (Fig. 7H).
Fig. 7.
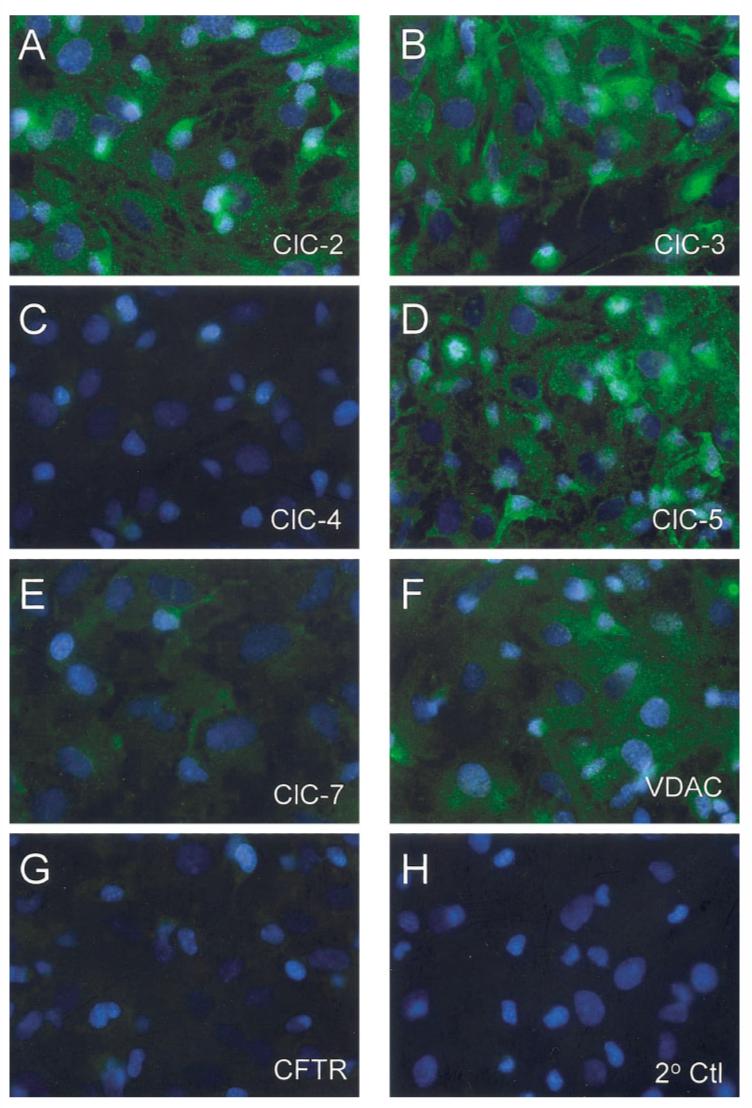
Immunocytochemical detection of previously cloned chloride channels in astrocytes. Cultured rat cortical astrocytes were evaluated for the presence of chloride channels proteins that correlated with those detected at the RNA level in Fig. 6. These included ClC channels (A-E), VDAC (F), and CFTR (G). The strongest staining was visible for ClC-2 (A) and ClC-3 (B). Strong staining was also visible for ClC-5 (C) and VDAC (F). Weak staining was visible for ClC-7 (E) and very weak staining was visible for ClC-4 (C) and CFTR (G). Note that cells were not permeabilized and that staining represented protein localized to the cell membrane. Control stainings were performed by exclusion of primary antibody (H).
DISCUSSION
This study examined the biophysical and pharmacological properties of the hypotonically activated Cl- currents in rat cortical astrocytes. One of the main goals of the study was to provide a basis on which to compare and contrast the properties of the hypotonically activated Cl- channels with the properties of Cl- channels involved in volume regulation. Therefore, we tested many of the same anion substitutions and chloride channel blockers as in a previous study where we characterized the Cl- channels involved in volume regulation (Parkerson and Sontheimer, 2003). As described below, we found that the hypotonically activated Cl- channel shared several key properties with the Cl- channels involved in volume regulation of cortical astrocytes and led us to propose the hypotonically activated Cl- channel in cortical astrocytes as a major contributor to volume regulation in these cells.
Comparison of the Hypotonically Activated Chloride Channel With Chloride Channels Involved in Volume Regulation of Cortical Astrocytes
In a recent study, we characterized the pharmacological properties and ionic dependence of volume regulation in the same astrocyte preparation (Parkerson and Sontheimer, 2003). Comparing those results to this electrophysiological examination suggests that the here described Cl- channel is largely responsible for volume regulation. First, we performed ion substitution experiments with several different anions. The effects of Ise- and Gluc- were notably similar for both the hypotonically activated Cl- channel and volume regulation. Ise- was about one-half and Gluc- was about three-quarters as permeable as Cl- through the hypotonically activated Cl- channel. Both Ise- and Gluc- partially inhibited volume regulation, with Ise- showing somewhat greater inhibition.
The effect of I- on both the hypotonically-activated Cl- channel and volume regulation was also similar. Despite a leftward shift in the reversal potential and a calculated permeability of >1 upon substitution of I- for Cl- in our electrophysiological recordings, extracellular substitution of I- for Cl- almost completely blocked both the hypotonically activated Cl- currents and volume regulation.
Substitution of NO3 - produced slightly, different results between the electrophysiological and volume regulation studies. NO3- was slightly more permeable than Cl- through the hypotonically activated Cl- channels. However, the presence of NO3- did not enhance volume regulation. Rather, volume regulation was supported similarly to Cl-. Interestingly, none of the anion substitutions resulted in a significant increase in volume regulation when compared to Cl-. This may suggest an intrinsic limit in the magnitude of volume regulation under the experimental conditions. For example, volume regulation might be limited by accompanying cation or water flux. In this case, even if the test anion were more permeable than Cl- through the anion channels involved in volume regulation, the magnitude of volume regulation would not increase. We attempted to test a limitation of cation flux by inclusion of a cation ionophore, gramicidin, in the bath solution. However, addition of gramicidin resulted repeatedly in a significant precipitate in the beaker, and we judged the experiments to be unreliable.
Substitution of MeS- also produced slightly different results between the electrophysiological and volume regulation studies. MeS- was about three-quarters as permeable as Cl- through the hypotonically activated Cl- channel. However, MeS- also supported volume regulation similarly to Cl-. The latter finding surprised us when we initially performed the volume regulation experiments, as we expected MeS- to behave similarly to other large anions such as Gluc- or Ise-. We previously attributed the unexpected behavior to a potential enhanced permeability of the channels to taurine and its analogues, including MeS-, as cortical astrocytes have been shown to release a significant percentage of their intracellular osmolyte pool during volume regulation (Pasantes-Morales et al., 1993). However, our present results warrant further examination of such enhanced permeability. It should be noted, though, that our present results still suggest a significant permeability of the hypotonically activated Cl- channel to MeS- and therefore do not contradict either a loss of intracellular osmolytes through these channels or a role for these channels in volume regulation.
Next, we tested the effects of several chloride channel blockers on the hypotonically activated Cl- channel. In our previous paper (Parkerson and Sontheimer, 2003), we showed that several typical Cl- channel blockers inhibited volume regulation. Specifically, NPPB was the most effective inhibitor of volume regulation. Niflumic acid also significantly inhibited, DIDS slightly but not significantly inhibited, and Cd2+ did not inhibit volume regulation. These Cl- channel blockers exhibited a similar order of effects on the inward currents (corresponding to the efflux of Cl- ions) through the hypotonically activated Cl- channel. Indeed, currents at -60 mV were almost completely inhibited by NPPB, significantly inhibited by niflumic acid, slightly inhibited by DIDS and not inhibited by Cd2+.
A discrepancy between the pharmacology of the volume regulation and electrophysiological studies pertained to the effect of Cd2+ in combination with NPPB, niflumic acid, or DIDS. Although Cd2+ had no effect on either volume regulation or the hypotonically activated Cl- channel when applied alone, it did synergistically inhibit volume regulation when applied in combination with NPPB, niflumic acid, or DIDS. We previously postulated that this might suggest a minor role for a second type of Cl- channel in volume regulation (Parkerson and Sontheimer, 2003), as Cd2+ typically blocks channels distinct from those inhibited by NPPB, niflumic acid or DIDS. Therefore, we tested the effects of DIDS in combination with Cd2+ on our hypotonically activated currents. As our currents appeared to be composed of one channel type only, we obtained inhibition that was similar to that of DIDS alone. One plausible explanation could be the invasiveness of the whole-cell patch clamp technique as compared with our volume regulation studies. The Cd2+ -sensitive Cl- channels may depend on second messenger cascades that are disrupted in the patch-clamp recordings. In agreement with this explanation, inwardly rectifying Cd2+ -sensitive currents have been reported in cortical astrocytes, and their presence depended on long-term preincubation with dBcAMP (Ferroni et al, 1997). Further experiments will be required in order to determine whether these channels could represent a second Cl- channel involved in volume regulation.
Since we were ultimately interested in identifying the channels that mediate volume regulation, we sought more evidence that the hypotonically activated channel is involved in this process. Therefore, we evaluated whether mols of Cl- effluxed through the hypotonically activated Cl- channel correlated with mols of solute efflux required for volume regulation of cortical astrocytes. As described under Results, it was not possible to produce completely identical conditions between the volume regulation and patch clamp studies. However, our experimental setups did allow for a reasonable first approximation. Overall, we found that during a three minute hypotonic challenge, the two values were remarkably close to each other.
Therefore, the ion substitution and Cl- channel blocker experiments combined with the comparison of Cl- and solute efflux provided us with significant evidence that the hypotonically activated Cl- channel likely represented the major contributor to volume regulation. Ultimately, we would like to confirm this further by successful inhibition of both volume regulation and hypotonically activated currents upon knockdown of the hypotonically activated channel. However, this will require molecular identification of the channel.
Chloride Channel Gene Candidates Expressed in Cortical Astrocytes
As a first step toward examining the diversity of Cl- channels found in astrocytes, we used a combination of RT-PCR and immunocytochemical studies. Our results indicated the presence of at least seven different Cl- channel transcripts within our cells and some protein product of each of these localized to the cell surface. This suggested that the hypotonically activated Cl- channel could be the product of one or more gene candidates. Additionally, based on RT-PCR and immunocytochemistry alone, we could not eliminate the possibility that the hypotonically activated Cl- channel could also be the product of a gene from a novel Cl- channel family not cloned to date.
We decided to compare the physiological properties of the hypotonically activated Cl- channel with those of each candidate channel found on the cell surface. The properties for each channel as described previously in heterologous expression studies, overexpression studies, or antisense knockdown studies are outlined in Table 4. With the exception of I- sensitivity which has been demonstrated for ClC-1 (Fahlke et al., 1997), but not ClC-3, ClC-3 appears to be one viable candidate. However, conclusive identification of the molecular identity of the hypotonically activated Cl- channel in cortical astrocytes will have to be determined using more definitive techniques such as antisense knockdown or RNA interference.
TABLE 4.
Properties of Chloride Channels Expressed on the Cell Surface of Cortical Astrocytes
| Channel | Rectification | Activation | Ion selectivity | Inhibitors | Modifiers | References |
|---|---|---|---|---|---|---|
| ClC-2 | Inward | Activated by strong hyperpolarization |
Cl≥ Br> I- | NPPB,Cd2+, Zn2+, 9-AC, DPC, not DIDS |
Increased current amplitudes by hypotonicity and EC acidification |
Thiemann et al., 1992; Grunder et al., 1992; Jordt and Jentsch, 1997; Furukawa et al., 1998; Schwiebert et al., 1998 |
| ClC-3 | Weakly outward |
Activated with hyper/ depolarizing steps; time-dep inactivation >+80 mV |
I>Cl≥Br> Ace- >Gluc- or I > Cl- > Asp |
DIDS, NPPB, tamoxifen, ATP, PKC activation, not 9-AC or DPC |
Increased current amplitudes by hypotonicity |
Jordt and Jentsch, 1997; Duan et al., 1997; Olsen et al., 2003 |
| ClC-4 | Strongly outward |
Activated by depolarization > 20 mV |
SCN->>NO3- >Cl- >Br->I->>Asp- or I->Cl->F- |
DIDS | Decreased current amplitudes by EC acidification |
Friedrich et al., 1999; Kawasaki et al., 1999; Vanoye and George 2002 |
| ClC-5 | Strongly outward |
Activated by depolarization > +20 mV |
NO3->Cl-> Br->I- >Glut- or I- > Cl- > F- |
±DPC, ±DIDS | Decreased current amplitudes by EC acidification |
Steinmeyer et al., 1995; Sakamoto et al., 1996; Friedrich et al., 1999 |
| ClC-7 | Strongly outward |
Activated by depolarization and acidic pH (≈4.0–6.0) |
DIDS, SITS | Diewald et al., 2002 | ||
| CFTR | Linear | Regulated by hydrolyzable nucleoside triphosphates and cAMP-dependent phosphorylation |
Br-≥Cl->I->F- | Glibenclamide, tolbutamide, I-, DPC, flufenamic acid, intracellular DIDS, or DNDS |
Anderson et al., 1991; Berger et al., 1991; Sheppard and Welsh, 1992; Tabcharani et al., 1992; McCarty et al., 1993; Linsdell and Hanrahan, 1996 |
|
| VDAC | Linear | Activated near 0 mV; closure > ±25 mV |
Perm to adenine nucleotides |
Dextran sulfate | Increased range of activation by Al3+ |
Guibert et al., 1998 |
The most intriguing observation from these molecular studies was the finding that astrocytes express at least seven different Cl- channels from three gene families. Yet, surprisingly, we did not observe any Cl- currents unless our cells were challenged by hypotonic swelling. This suggests to us that astrocytic Cl- channels must be highly regulated in their activity, and may function only under certain physiological or patho-physiological conditions. Indeed, Cl- channels can be regulated by a number of intracellular and extracellular signals, including protein kinase A (PKA) (Berger et al., 1991; Tewari et al., 2000), PKC (Berger et al., 1993; Kawasaki et al., 1994; Uchida et al., 1994), pH (Kajita and Brown, 1997; Sauve et al., 2000; Tewari et al., 2000; Diewald et al., 2002), and Ca2+ (Sauve et al., 2000; Stewart et al., 2001). Moreover, several studies have demonstrated that the differentiation and/or proliferation stage of a cell can alter the expression and/or the activity of Cl- channels (Christensen and Strange, 2001; Rutledge et al., 2001; Furukawa et al., 2002; Zheng et al., 2002; Makara et al., 2003). Therefore, it appears likely that different Cl- channels will function under different biological conditions. Clearly, much further work is needed to gain a better understanding of the involvement of Cl- channels in the various facets of astrocyte biology.
ACKNOWLEDGMENTS
This work was supported by NIH grant RO1-NS36692 (to H.S.). K.A. Parkerson was supported by the NIH Medical Scientist Training Program.
Grant sponsor: National Institutes of Health. Grant number: R01-NS36692.
REFERENCES
- Ackerman MJ, Krapivinsky GB, Gordon E, Krapivinsky L, Clapham DC. Characterization of a native swelling-induced chloride current, ICl, swell, and its regulatory protein, pICln, in Xenopus oocytes. Jpn J Physiol. 1994a;44:S17–S24. [PubMed] [Google Scholar]
- Ackerman MJ, Wickman KD, Clapham DE. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994b;103:153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Ballerini P, Di Iorio P, Ciccarelli R, Nargi E, D’Alimonte I, Traversa U, Rathbone MP, Caciagli F. Glial cells express multiple ATP binding cassette proteins which are involved in ATP release. NeuroReport. 2002;13:1789–1792. doi: 10.1097/00001756-200210070-00019. [DOI] [PubMed] [Google Scholar]
- Berger HA, Anderson MP, Gregory RJ, Thompson S, Howard PW, Maurer RA, Mulligan R, Smith AE, Welsh MJ. Identification and regulation of the cystic fibrosis transmembrane conductance regulator-generated chloride channel. J Clin Invest. 1991;88:1422–1431. doi: 10.1172/JCI115450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger HA, Travis SM, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by specific protein kinases and protein phosphatases. J Biol Chem. 1993;268:2037–2047. [PubMed] [Google Scholar]
- Bevan S, Chiu SY, Gray PTA, Ritchie JM. The presence of voltage-gated sodium, potassium and chloride channels in rat cultured astrocytes. Proc R Soc Lond. 1985;B225:299–313. doi: 10.1098/rspb.1985.0063. [DOI] [PubMed] [Google Scholar]
- Bond TD, Ambikapathy S, Mohammad S, Valverde MA. Osmo-sensitive Cl- currents and their relevance to regulatory volume decrease in human intestinal t84 cells: outwardly vs. inwardly rectifying currents. J Physiol (Lond) 1998;511:45–54. doi: 10.1111/j.1469-7793.1998.045bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AP, Schulman H. Distinct voltage-dependent gating behaviours of a swelling-activated chloride current in human epithelial cells. J Physiol. 1996;495:743–753. doi: 10.1113/jphysiol.1996.sp021630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bres V, Hurbin A, Duvoid A, Orcel H, Moos FC, Rabie A, Hussy N. Pharmacological characterization of volume-sensitive, taurine permeable anion channels in rat supraoptic glial cells. Br J Pharmacol. 2000;130:1976–1982. doi: 10.1038/sj.bjp.0703492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M, Strange K. Developmental regulation of a novel outwardly rectifying mechanosensitive anion channel in Caenorhabditis elegans. J Biol Chem. 2001;276:45024–45030. doi: 10.1074/jbc.M107652200. [DOI] [PubMed] [Google Scholar]
- Crepel V, Panenka W, Kelly ME, MacVicar BA. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. J Neurosci. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Hwang T-K, Buettner R, Hofer A, Dotzler E, Kremer M, Deutzmann R, Thinnes FP, Fishman GI, Spray DC, Siemen D. Cloning and in situ localization of a brain-derived porin that constitutes a large-conductance anion channel in astrocytic plasma membranes. Proc Natl Acad Sci USA. 1994;91:499–503. doi: 10.1073/pnas.91.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diewald L, Rupp J, Dreger M, Hucho F, Gillen C, Nawrath H. Activation by acidic pH of CLC-7 expressed in oocytes from Xenopus laevis. Biochem Biophys Res Commun. 2002;291:421–424. doi: 10.1006/bbrc.2002.6462. [DOI] [PubMed] [Google Scholar]
- Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- Ehring GR, Osipchuk YV, Cahalan MD. Swelling-activated chloride channels in multidrug-sensitive and -resistant cells. J Gen Physiol. 1994;104:1129–1161. doi: 10.1085/jgp.104.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R, Ross BJ, Cutting GR. Expression of the voltage-gated chloride channel clc-2 in rod bipolar cells of the rat retina. J Neurosci. 1999;19:9841–9847. doi: 10.1523/JNEUROSCI.19-22-09841.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Yu HT, Beck CL, Rhodes TH, AL George., Jr. Pore forming segments in voltage-gated chloride channels. Nature. 1997;390:529–532. doi: 10.1038/37391. [DOI] [PubMed] [Google Scholar]
- Ferroni S, Marchini C, Nobile M, Rapisarda C. Characterization of an inwardly rectifying chloride conductance expressed by cultured rat cortical astrocytes. Glia. 1997;21:217–227. doi: 10.1002/(sici)1098-1136(199710)21:2<217::aid-glia5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Friedrich T, Breiderhoff T, Jentsch TJ. Mutational analysis demonstrates that ClC-4 and ClC-5 directly mediate plasma membrane currents. J Biol Chem. 1999;274:896–902. doi: 10.1074/jbc.274.2.896. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Ogura T, Katayama Y, Hiraoka M. Characteristics of rabbit ClC-2 current expressed in Xenopus oocytes and its contribution to volume regulation. Am J Physiol. 1998;274:C500–C512. doi: 10.1152/ajpcell.1998.274.2.C500. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Ogura T, Zheng YJ, Tsuchiya H, Nakaya H, Katayama Y, Inagaki N. Phosphorylation and functional regulation of ClC-2 chloride channels expressed in Xenopus oocytes by M cyclin-dependent protein kinase. J Physiol. 2002;540(Pt 3):883–893. doi: 10.1113/jphysiol.2001.016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PT, Ritchie JM. A voltage-gated chloride conductance in rat cultured astrocytes. Proc R Soc Lond B. 1986;228:267–288. doi: 10.1098/rspb.1986.0055. [DOI] [PubMed] [Google Scholar]
- Grunder S, Thiemann A, Pusch M, Jentsch TJ. Regions involved in the opening of ClC-2 chloride channel by voltage and cell volume. Nature. 1992;360:759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- Guibert B, Dermietzel R, Siemen D. Large conductance channel in plasma membranes of astrocytic cells is functionally related to mitochondrial VDAC-channels. Int J Biochem Cell Biol. 1998;30:379–391. doi: 10.1016/s1357-2725(97)00137-4. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hermoso M, Satterwhite CM, Andrade YN, Hidalgo J, Wilson SM, Horowitz B, Hume JR. ClC-3 is a fundamental molecular component of volume-sensitive outwardly rectifying Cl- channels and volume regulation in HeLa cells and Xenopus laevis oocytes. J Biol Chem. 2002;277:40066–40074. doi: 10.1074/jbc.M205132200. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- Hoffmann EK, Dunham PB. Membrane mechanisms and intra-cellular signalling in cell volume regulation. Int Rev Cytol. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- Huber S, Braun G, Burger-Kentischer A, Reinhart B, Luckow B, Horster M. CFTR mRNA and its truncated splice variant (TRN-CFTR) are differentially expressed during collecting duct ontogeny. FEBS Lett. 1998;423:362–366. doi: 10.1016/s0014-5793(98)00112-4. [DOI] [PubMed] [Google Scholar]
- Hubert MD, Levitan I, Hoffman MM, Zraggen M, Hofreiter ME, Garber SS. Modulation of volume regulated anion current by I(Cln) Biochim Biophys Acta. 2000;1466:105–114. doi: 10.1016/s0005-2736(00)00177-2. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Jentsch TJ. Molecular dissection of gating in the ClC-2 chloride channel. EMBO J. 1997;16:1582–1592. doi: 10.1093/emboj/16.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita H, Brown PD. Inhibition of the inward-rectifying Cl- channel in rat choroid plexus by a decrease in extracellular pH. J Physiol. 1997;498(Pt 3):703–707. doi: 10.1113/jphysiol.1997.sp021894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M, Uchida S, Monkawa T, Miyawaki A, Mikoshiba K, Marumo F, Sasaki S. Cloning and expression of a protein kinase C-regulated chloride channel abundantly expressed in rat brain neuronal cells. Neuron. 1994;12:597–604. doi: 10.1016/0896-6273(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Fukuma T, Yamauch K, Sakamoto H, Marumo F, Sasaki S. Identification of an acid activated (Cl-) channel from human skeletal muscles. Am J Physiol. 1999;277:C948–C954. doi: 10.1152/ajpcell.1999.277.5.C948. [DOI] [PubMed] [Google Scholar]
- Krapivinsky GB, Ackerman MJ, Gordon EA, Krapivinsky LD, Clapham DE. Molecular characterization of a swelling-induced chloride conductance regulatory protein, picln. Cell. 1994;76:439–448. doi: 10.1016/0092-8674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Kulka M, Schwingshackl A, Befus AD. Mast cells express chloride channels of the ClC family. Inflamm Res. 2002;51:451–456. doi: 10.1007/pl00012411. [DOI] [PubMed] [Google Scholar]
- Lascola CD, Kraig RP. Whole-cell chloride currents in rat astrocytes accompany changes in cell morphology. J Neurosci. 1996;16:2532–2545. doi: 10.1523/JNEUROSCI.16-08-02532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascola CD, Nelson DJ, Kraig RP. Cytoskeletal actin gates a Cl- channel in neocortical astrocytes. J Neurosci. 1998;18:1679–1692. doi: 10.1523/JNEUROSCI.18-05-01679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I, Christian AE, Tulenko TN, Rothblat GH. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J Gen Physiol. 2000;115:405–416. doi: 10.1085/jgp.115.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsdell P, Hanrahan JW. Disulphonic stilbene block of cystic fibrosis transmembrane conductance regulator Cl- channels expressed in a mammalian cell line and its regulation by a critical pore residue. J Physiol. 1996;496:687–693. doi: 10.1113/jphysiol.1996.sp021719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makara JK, Rappert A, Matthias K, Steinhauser C, Spat A, Ketten-mann H. Astrocytes from mouse brain slices express ClC-2-mediated Cl- currents regulated during development and after injury. Mol Cell Neurosci. 2003;23:521–530. doi: 10.1016/s1044-7431(03)00080-0. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, deVellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Physiol (Lond) 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty NA, McDonough S, Cohen BN, Riordan JR, Davidson N, Lester HA. Voltage-dependent block of the cystic fibrosis transmembrane conductance regulator Cl- channel by two closely related arylaminobenzoates. J Gen Physiol. 1993;102:1–23. doi: 10.1085/jgp.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Zhang JJ, Wang L, Jacob TJ. Volume-sensitive chloride current in pigmented ciliary epithelial cells: role of phospholipases. Am J Physiol. 1997;272:C212–C222. doi: 10.1152/ajpcell.1997.272.1.C212. [DOI] [PubMed] [Google Scholar]
- Nilius B, Oike M, Zahradnik I, Droogmans G. Activation of a Cl- current by hypotonic volume increase in human endothelial cells. J Gen Physiol. 1994;103:787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Sehrer J, De Smet P, Van Driessche W, Droogmans G. Volume regulation in a toad epithelial cell line: role of coactivation of K+ and Cl- channels. J Physiol. 1995;487:367–378. doi: 10.1113/jphysiol.1995.sp020886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Droogmans G. Volume-activated Cl- channels. Gen Pharm. 1996;27:1131–1140. doi: 10.1016/s0306-3623(96)00061-4. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Olsen M, Schade S, Lyons SA, Amaral MD, Sontheimer H. Expression of voltage-gated chloride channels in human glioma cells. J Neurosci. 2003;23:5572–5582. doi: 10.1523/JNEUROSCI.23-13-05572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkerson KA, Sontheimer H. Contribution of chloride channels to volume regulation of cortical astrocytes. Am J Physiol Cell Physiol. 2003;284:C1460–C1467. doi: 10.1152/ajpcell.00603.2002. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Alavez S, Sanchez OR, Moran J. Contribution of organic and inorganic osmolytes to volume regulation in rat brain cells in culture. Neurochem Res. 1993;18:445–452. doi: 10.1007/BF00967248. [DOI] [PubMed] [Google Scholar]
- Rutledge E, Bianchi L, Christensen M, Boehmer C, Morrison R, Broslat A, Beld AM, George AL, Greenstein D, Strange K. CLH-3, a ClC-2 anion channel ortholog activated during meiotic maturation in C. elegans oocytes. Curr Biol. 2001;11:161–70. doi: 10.1016/s0960-9822(01)00051-3. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Kawasaki M, Uchida S, Sasaki S, Marumo F. Identification of a new outwardly rectifying Cl- channel that belongs to a subfamily of the ClC Cl- channels. J Biol Chem. 1996;271:10210–10216. doi: 10.1074/jbc.271.17.10210. [DOI] [PubMed] [Google Scholar]
- Sarkadi B, Parker JC. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991;1071:407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Sauve R, Cai S, Garneau L, Klein H, Parent L. pH and external Ca2+ regulation of a small conductance Cl- channel in kidney distal tubule. Biochim Biophys Acta. 2000;1509:73–85. doi: 10.1016/s0005-2736(00)00287-x. [DOI] [PubMed] [Google Scholar]
- Schlichter LC, Sakellaropoulos G, Ballyk B, Pennefather PS, Phipps DJ. Properties of K+ and Cl- channels and their involvement in proliferation of rat microglial cells. Glia. 1996;17:225–236. doi: 10.1002/(SICI)1098-1136(199607)17:3<225::AID-GLIA5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Cid-Soto LP, Stafford D, Carter M, Blaisdell CJ, Zeitlin PL, Guggino WB, Cutting GR. Analysis of ClC-2 channels as an alternative pathway for chloride conduction in cystic fibrosis airway cells. Proc Natl Acad Sci USA. 1998;95:3879–3884. doi: 10.1073/pnas.95.7.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MR, Droogmans G, Eggermont J, Voets T, Ellory JC, Nilius B. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J Physiol. 2000;529:385–394. doi: 10.1111/j.1469-7793.2000.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Smith RL, Freund TF. Distribution of chloride channel-2-immunoreactive neuronal and astrocytic processes in the hippocampus. Neuroscience. 2000;101:51–65. doi: 10.1016/s0306-4522(00)00360-2. [DOI] [PubMed] [Google Scholar]
- Smitherman KA, Sontheimer H. Inhibition of glial Na+ and K+ currents by tamoxifen. J Membr Biol. 2001;181:125–135. doi: 10.1007/s00232-001-0016-2. [DOI] [PubMed] [Google Scholar]
- Stegen C, Matskevich I, Wagner CA, Paulmichl M, Lang F, Broer S. Swelling-induced taurine release without chloride channel activity in Xenopus laevis oocytes expressing anion channels and transporters. Biochim Biophys Acta. 2000;1467:91–100. doi: 10.1016/s0005-2736(00)00209-1. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K, Schwappach B, Bens M, Vandewalle A, Jentsch TJ. Cloning and functional expression of rat clc-5, a chloride channel related to kidney disease. J Biol Chem. 1995;270:31172–31177. doi: 10.1074/jbc.270.52.31172. [DOI] [PubMed] [Google Scholar]
- Stewart GS, Glanville M, Aziz O, Simmons NL, Gray MA. Regulation of an outwardly rectifying chloride conductance in renal epithelial cells by external and internal calcium. J Membr Biol. 2001;180:49–64. doi: 10.1007/s002320010058. [DOI] [PubMed] [Google Scholar]
- Tabcharani JA, Chang XB, Riordan JR, Hanrahan JW. The cystic fibrosis transmembrane conductance regulator chloride channel: iodide block and permeation. Biophys J. 1992;62:1–4. doi: 10.1016/S0006-3495(92)81759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari KP, Malinowska DH, Sherry AM, Cuppoletti J. PKA and arachidonic acid activation of human recombinant ClC-2 chloride channels. Am J Physiol Cell Physiol. 2000;279:C40–50. doi: 10.1152/ajpcell.2000.279.1.C40. [DOI] [PubMed] [Google Scholar]
- Thiemann A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Uchida S, Kawasaki M, Sasaki S, Marumo F. Cloning and expression of a PKC-regulated chloride channel. Jpn J Physiol. 1994;44:S55–62. [PubMed] [Google Scholar]
- Vandenberg JI, Yoshida A, Kirk K, Powell T. Swelling-activated and isoprenaline-activated chloride currents in guinea pig cardiac myocytes have distinct electrophysiology and pharmacology. J Gen Physiol. 1994;104:997–1017. doi: 10.1085/jgp.104.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoye CG, George AL., Jr. Functional characterization of recombinant human ClC-4 channels in cultured mammalian cells. J Physiol. 2002;539(Pt 2):373–383. doi: 10.1113/jphysiol.2001.013115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Szucs G, Droogmans G, Nilius B. Blockers of volume-activated Cl- currents inhibit endothelial cell proliferation. Pflugers Arch. 1995;431:132–134. doi: 10.1007/BF00374387. [DOI] [PubMed] [Google Scholar]
- Voets T, Wei L, De Smet P, Van Driessche W, Eggermont J, Droogmans G, Nilius B. Downregulation of volume-activated Cl- currents during muscle differentiation. Am J Physiol. 1997;272:C667–674. doi: 10.1152/ajpcell.1997.272.2.C667. [DOI] [PubMed] [Google Scholar]
- Winpenny JP, Mathews CJ, Verdon B, Wardle CJ, Chambers JA, Harris A, Argent BE, Gray MA. Volume-sensitive chloride currents in primary cultures of human fetal vas deferens epithelial cells. Pflugers Arch. 1996;432:644–654. doi: 10.1007/s004240050181. [DOI] [PubMed] [Google Scholar]
- Wondergem R, Gong W, Monen SH, Dooley SN, Gonce JL, Conner TD, Houser M, Ecay TW, Ferslew KE. Blocking swelling-activated chloride current inhibits mouse liver cell proliferation. J Physiol. 2001;532:661–672. doi: 10.1111/j.1469-7793.2001.0661e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YJ, Furukawa T, Ogura T, Tajimi K, Inagaki N. M phase-specific expression and phosphorylation-dependent ubiquitination of the ClC-2 channel. J Biol Chem. 2002;277:32268–32273. doi: 10.1074/jbc.M202105200. [DOI] [PubMed] [Google Scholar]