Abstract
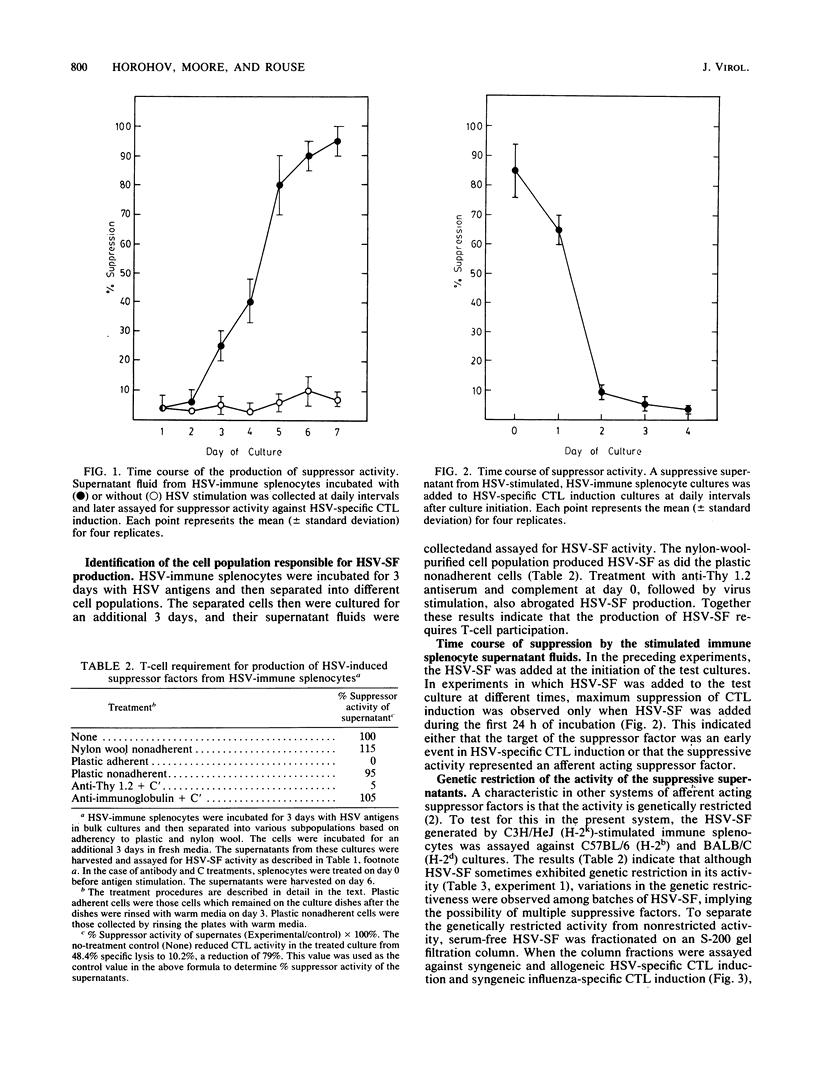
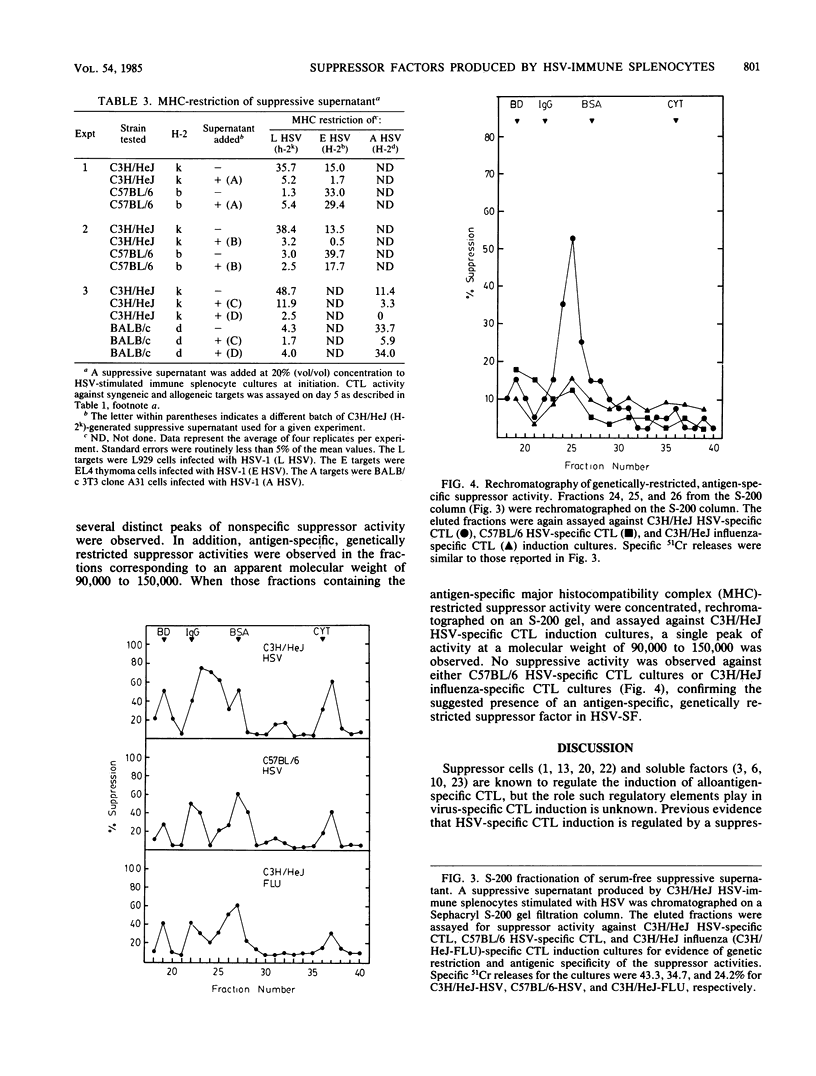
Indirect evidence indicates that herpes simplex virus (HSV)-specific cytotoxic-T-lymphocyte induction is regulated by suppressor cells. To search for such suppressor effects, supernatant fluids from splenocyte cultures from normal and HSV-immune mice cultured either with or without viral stimulation were tested for their ability to inhibit HSV-specific cytotoxic-T-lymphocyte induction. Only the supernatant fluid from the HSV-stimulated, HSV-immune cultures contained a suppressor activity (HSV-SF). HSV-SF was produced by nylon-wool-purified Thy 1+ cells. HSV-SF was detectable after 3 days of culture and would only suppress cytotoxic-T-lymphocyte induction if HSV-SF was added within 24 h of initiation of the test cultures. HSV-SF was neither dialyzable nor heat stable. Molecular sieve chromatography of HSV-SF yielded multiple peaks of suppressor activity. Although most of these peaks exhibited nonspecific suppressor activity, the suppression mediated by the 90,000 to 150,000-molecular-weight fractions was antigen specific and genetically restricted. These results provide direct evidence for the regulation of HSV-cytotoxic-T-lymphocyte induction by a novel suppressor factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Adra A. R., Pilarski L. M. Antigen-specific suppression of cytotoxic T cell responses in mice. I. Suppressor T cells are not cytotoxic cells. Eur J Immunol. 1978 Jul;8(7):504–511. doi: 10.1002/eji.1830080710. [DOI] [PubMed] [Google Scholar]
- Aoki I., Usui M., Minami M., Dorf M. E. A genetically restricted suppressor factor that requires interaction with two distinct targets. J Immunol. 1984 Apr;132(4):1735–1740. [PubMed] [Google Scholar]
- Aune T. M., Pierce C. W. Preparation of soluble immune response suppressor and macrophage-derived suppressor factor. J Immunol Methods. 1982 Aug 27;53(1):1–14. doi: 10.1016/0022-1759(82)90235-6. [DOI] [PubMed] [Google Scholar]
- Benacerraf B., Germain R. N. A single major pathway of T-lymphocyte interactions in antigen-specific immune suppression. Scand J Immunol. 1981;13(1):1–10. doi: 10.1111/j.1365-3083.1981.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Bone D. R., Courtney R. J. A temperature-sensitive mutant of herpes simplex virus type 1 defective in the synthesis of the major capsid polypeptide. J Gen Virol. 1974 Jul;24(1):17–27. doi: 10.1099/0022-1317-24-1-17. [DOI] [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. II. The effect on the in vitro generation of cytolytic T cells. J Immunol. 1979 Oct;123(4):1632–1638. [PubMed] [Google Scholar]
- Green D. R., Flood P. M., Gershon R. K. Immunoregulatory T-cell pathways. Annu Rev Immunol. 1983;1:439–463. doi: 10.1146/annurev.iy.01.040183.002255. [DOI] [PubMed] [Google Scholar]
- Iwasaka T., Sheridan J. F., Aurelian L. Immunity to herpes simplex virus type 2: recurrent lesions are associated with the induction of suppressor cells and soluble suppressor factors. Infect Immun. 1983 Dec;42(3):955–964. doi: 10.1128/iai.42.3.955-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Henkel T. J., Braciale V., Braciale T. J. Mycoplasma infection of cell cultures: thymidine incorporation of culture supernatants as a screening test. J Immunol. 1984 Jan;132(1):9–11. [PubMed] [Google Scholar]
- Kramer M., Koszinowski U. T cell-specific suppressor factor(s) with regulatory influence on interleukin 2 production and function. J Immunol. 1982 Feb;128(2):784–790. [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C., Kirkpatrick D., Fitzgerald P. A., Ching C. Y., Pahwa R. N., Good R. A., Smithwick E. M. Studies of the cell lineage of the effector cells that spontaneously lyse HSV-1 infected fibroblasts (NK(HSV-1)). J Immunol. 1982 Aug;129(2):824–828. [PubMed] [Google Scholar]
- Miller R. G. An immunological suppressor cell inactivating cytotoxic T-lymphocyte precursor cells recognizing it. Nature. 1980 Oct 9;287(5782):544–546. doi: 10.1038/287544a0. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Field H. J., Quartey-Papafio R. Cell-mediated immunity in herpes simplex virus-infected mice: induction, characterization and antiviral effects of delayed type hypersensitivity. J Gen Virol. 1980 Jun;48(Pt 2):351–357. doi: 10.1099/0022-1317-48-2-351. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Gell P. G. Membrane phenotype of murine effector and suppressor T cells involved in delayed hypersensitivity and protective immunity to herpes simplex virus. Cell Immunol. 1983 Feb 1;75(2):348–355. doi: 10.1016/0008-8749(83)90332-5. [DOI] [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Rouse B. T., Wagner H. Frequency of herpes simplex virus-specific cytotoxic T lymphocyte precursors in lymph node cells of infected mice. Immunology. 1984 Jan;51(1):57–64. [PMC free article] [PubMed] [Google Scholar]
- Schmid D. S., Rouse B. T. Cellular interactions in the cytotoxic T lymphocyte response to herpes simplex virus antigens: differential antigen activation requirements for the helper T lymphocyte and cytotoxic T lymphocyte precursors. J Immunol. 1983 Jul;131(1):479–484. [PubMed] [Google Scholar]
- Schwartz A., Sutton S. L., Gershon R. K. Regulation of in vitro cytotoxic T lymphocyte generation. II. Demonstration of noncytotoxic alloantigen-specific suppressor T lymphocytes. Eur J Immunol. 1982 May;12(5):380–386. doi: 10.1002/eji.1830120505. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susskind B. M., Merluzzi V. J., Faanes R. B., Palladino M. A., Choi Y. S. Regulatory mechanisms in cytotoxic T lymphocyte development. I. A suppressor T cell subset that regulates the proliferative stage of CTL development. J Immunol. 1983 Feb;130(2):527–532. [PubMed] [Google Scholar]
- Truitt G. A., Rich R. R., Rich S. S. Suppression of cytotoxic lymphocyte responses in vitro by soluble products of alloantigen-activated spleen cells. J Immunol. 1978 Sep;121(3):1045–1051. [PubMed] [Google Scholar]
- Zembala M. A., Asherson G. L., James B. M., Stein V. E., Watkins M. C. Anti-haptene T suppressor factor acts through an I-J+, Ly1-2+, T acceptor cell that releases a nonspecific inhibitor of the transfer of contact sensitivity when exposed to antigen. J Immunol. 1982 Nov;129(5):1823–1829. [PubMed] [Google Scholar]


