Abstract
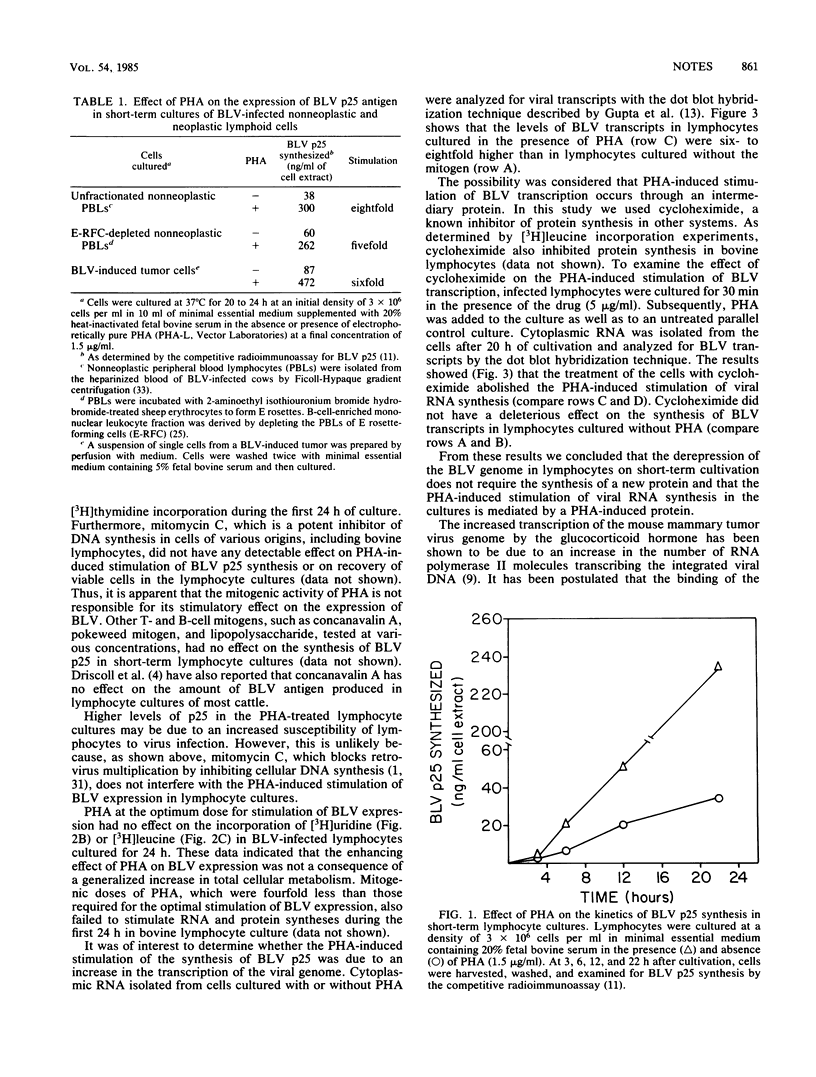
Addition of supramitogenic doses of phytohemagglutinin (PHA) to short-term cultures of neoplastic or nonneoplastic lymphocytes infected with bovine leukemia virus increased the synthesis of the major core virion antigen (p25) by 5- to 10-fold. Such stimulation was not due to the mitogenic effect of PHA or to a generalized increase in cellular RNA or protein synthesis but rather to enhanced transcription of the viral genome by a PHA-induced protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADER J. P. THE REQUIREMENT FOR DNA SYNTHESIS IN THE GROWTH OF ROUS SARCOMA AND ROUS-ASSOCIATED VIRUSES. Virology. 1965 Jun;26:253–261. doi: 10.1016/0042-6822(65)90272-2. [DOI] [PubMed] [Google Scholar]
- Baliga V., Ferrer J. F. Expression of the bovine leukemia virus and its internal antigen in blood lymphocytes. Proc Soc Exp Biol Med. 1977 Nov;156(2):388–391. doi: 10.3181/00379727-156-39942. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Driscoll D. M., Baumgartener L. E., Olson C. Concanavalin A and the production of bovine leukemia virus antigen in short-term lymphocyte cultures. J Natl Cancer Inst. 1977 May;58(5):1513–1514. doi: 10.1093/jnci/58.5.1513. [DOI] [PubMed] [Google Scholar]
- Duc-Nguyen H., Henle W. Replication of mumps virus in human leukocyte cultures. J Bacteriol. 1966 Jul;92(1):258–265. doi: 10.1128/jb.92.1.258-265.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman R., Wheelock E. F. Specific role of each human leukocyte type in viral infections. II. Phytohemagglutinin-treated lymphocytes as host cells for vesicular stomatitis virus replication in vitro. J Virol. 1968 May;2(5):440–448. doi: 10.1128/jvi.2.5.440-448.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J. F. Bovine lymphosarcoma. Adv Vet Sci Comp Med. 1980;24:1–68. [PubMed] [Google Scholar]
- Firzlaff J. M., Diggelmann H. Dexamethasone increases the number of RNA polymerase II molecules transcribing integrated mouse mammary tumor virus DNA and flanking mouse sequences. Mol Cell Biol. 1984 Jun;4(6):1057–1062. doi: 10.1128/mcb.4.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y. I., Sugamura K., Hinuma Y. Healthy carriers of a human retrovirus, adult T-cell leukemia virus (ATLV): demonstration by clonal culture of ATLV-carrying T cells from peripheral blood. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4780–4782. doi: 10.1073/pnas.79.15.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Ferrer J. F. Comparison of various serological and direct methods for the diagnosis of BLV infection in cattle. Int J Cancer. 1981 Aug 15;28(2):179–184. doi: 10.1002/ijc.2910280211. [DOI] [PubMed] [Google Scholar]
- Gupta P., Ferrer J. F. Expression of bovine leukemia virus genome is blocked by a nonimmunoglobulin protein in plasma from infected cattle. Science. 1982 Jan 22;215(4531):405–407. doi: 10.1126/science.6276975. [DOI] [PubMed] [Google Scholar]
- Gupta P., Kashmiri S. V., Ferrer J. F. Transcriptional control of the bovine leukemia virus genome: role and characterization of a non-immunoglobulin plasma protein from bovine leukemia virus-infected cattle. J Virol. 1984 Apr;50(1):267–270. doi: 10.1128/jvi.50.1.267-270.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Sodroski J., Patarca R., Briggs D., Perkins D., Wong-Staal F. Structure of 3' terminal region of type II human T lymphotropic virus: evidence for new coding region. Science. 1984 Jul 27;225(4660):419–421. doi: 10.1126/science.6330894. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Miller S. E., Palker T. J., Moore J. O., Dunn P. H., Bolognesi D. P., Metzgar R. S. Identification of human T cell leukemia virus in a Japanese patient with adult T cell leukemia and cutaneous lymphomatous vasculitis. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2054–2058. doi: 10.1073/pnas.80.7.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashmiri S. V., Mehdi R., Ferrer J. F. Molecular cloning of covalently closed circular DNA of bovine leukemia virus. J Virol. 1984 Feb;49(2):583–587. doi: 10.1128/jvi.49.2.583-587.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon S. J., Piper C. E. Properties of density gradient-fractionated peripheral blood leukocytes from cattle infected with bovine leukemia virus. Infect Immun. 1977 Jun;16(3):898–903. doi: 10.1128/iai.16.3.898-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Cleuter Y., Couez D., Burny A., Marbaix G. Leukemogenesis by bovine leukemia virus: proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3' proximate cellular sequences. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2465–2469. doi: 10.1073/pnas.79.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Marbaix G., Cleuter Y., Portetelle D., Mammerickx M., Burny A. Genomic integration of bovine leukemia provirus and lack of viral RNA expression in the target cells of cattle with different responses to BLV infection. Leuk Res. 1980;4(6):509–519. doi: 10.1016/0145-2126(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Kleinman L. F., Kibrick S., Ennis F., Polgar P. Herpes simplex virus replication in human lymphocyte cultures stimulated with phytomitogens and anti-lymphocyte globulin. Proc Soc Exp Biol Med. 1972 Dec;141(3):1095–1099. doi: 10.3181/00379727-141-36941. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P. S., Pomeroy K. A., Castro A. E., Johnson D. W., Muscoplat C. C., Sorensen D. K. Detection of bovine leukemia virus in B-lymphocytes by the syncytia induction assay. J Natl Cancer Inst. 1977 Oct;59(4):1269–1272. doi: 10.1093/jnci/59.4.1269. [DOI] [PubMed] [Google Scholar]
- Paul P. S., Senogles D. R., Muscoplat C. C., Johnson D. W. Enumeration of T cells, B cells and monocytes in the peripheral blood of normal and lymphocytotic cattle. Clin Exp Immunol. 1979 Feb;35(2):306–316. [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Stock N. D., Ferrer J. F. Replicating C-type virus in phytohemagglutinin-treated buffy-coat cultures of bovine origin. J Natl Cancer Inst. 1972 Apr;48(4):985–996. [PubMed] [Google Scholar]
- Takashima I., Olson C., Driscoll D. M., Baumgartener L. E. B-lymphocytes and T-lymphocytes in three types of bovine lymphosarcoma. J Natl Cancer Inst. 1977 Oct;59(4):1205–1209. doi: 10.1093/jnci/59.4.1205. [DOI] [PubMed] [Google Scholar]
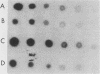
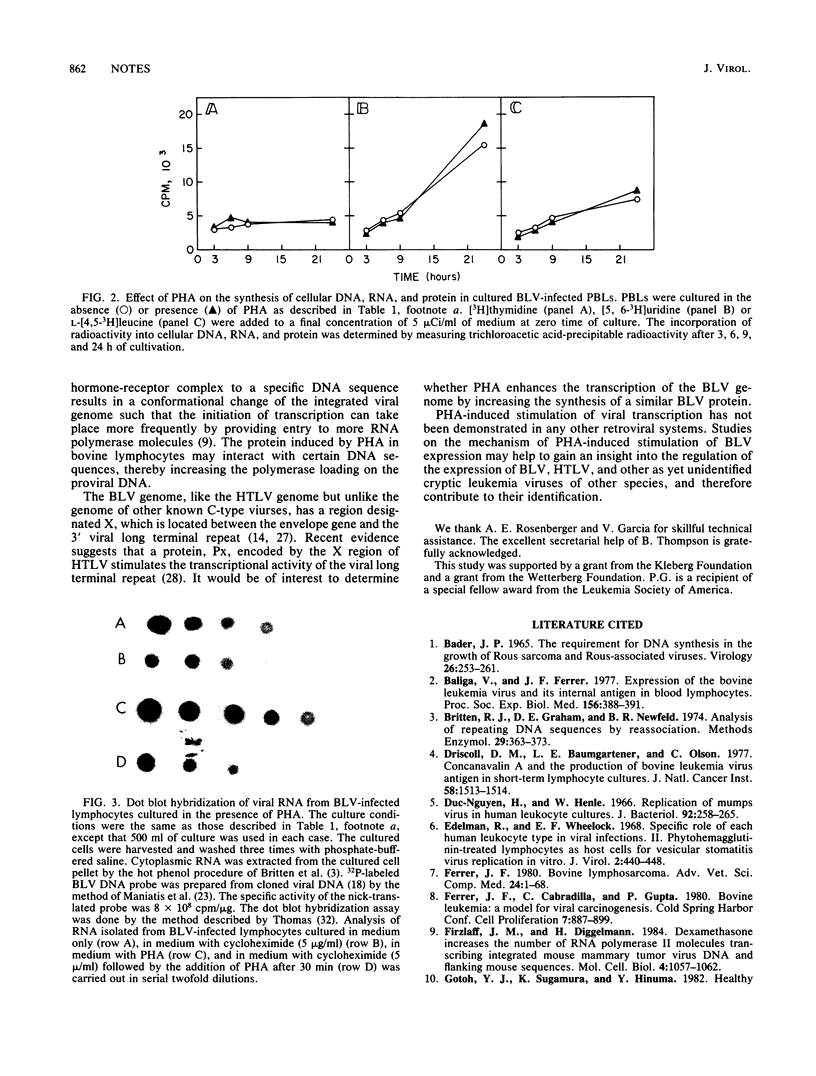
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn R. M., Gupta P., Kenyon S. J., Ferrer J. F. Evidence that the spontaneous blastogenesis of lymphocytes from bovine leukemia virus-infected cattle is viral antigen specific. Infect Immun. 1981 Oct;34(1):84–89. doi: 10.1128/iai.34.1.84-89.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Replication of poliovirus in phytohemagglutinin-stimulated human lymphocytes. J Virol. 1969 May;3(5):451–457. doi: 10.1128/jvi.3.5.451-457.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]