Abstract
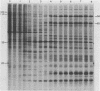
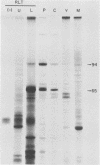
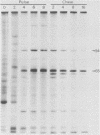
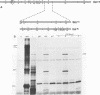
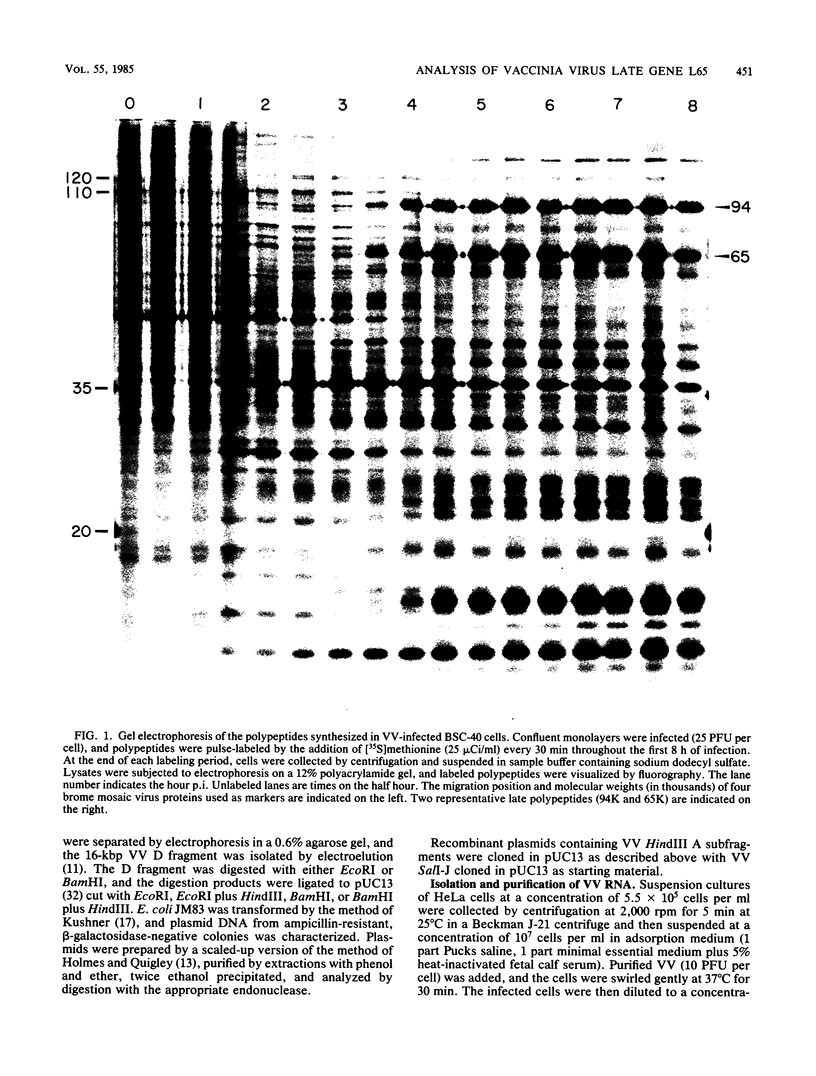
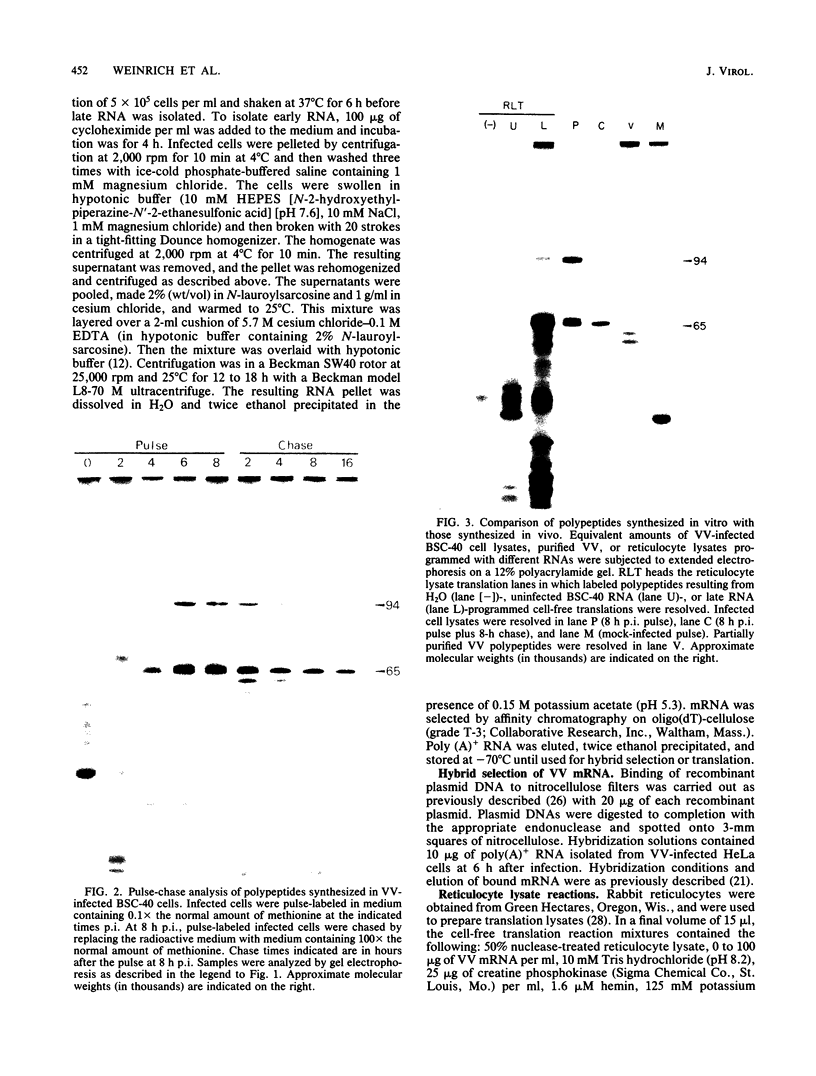
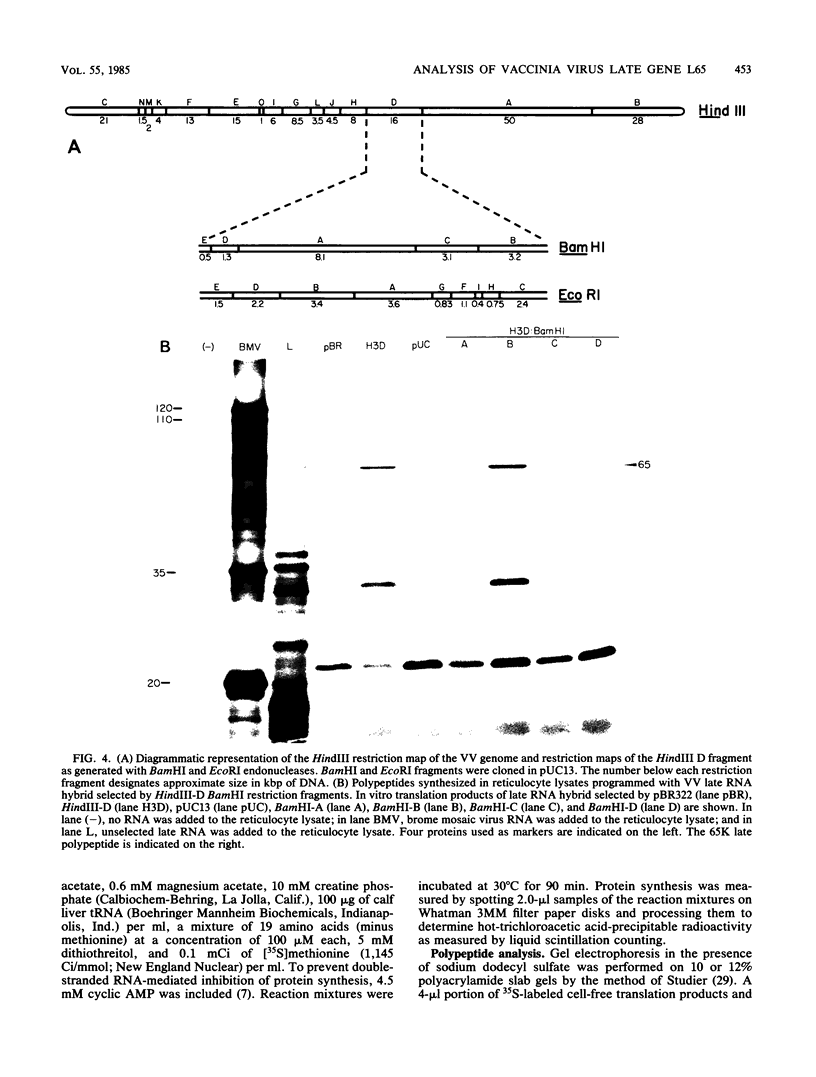
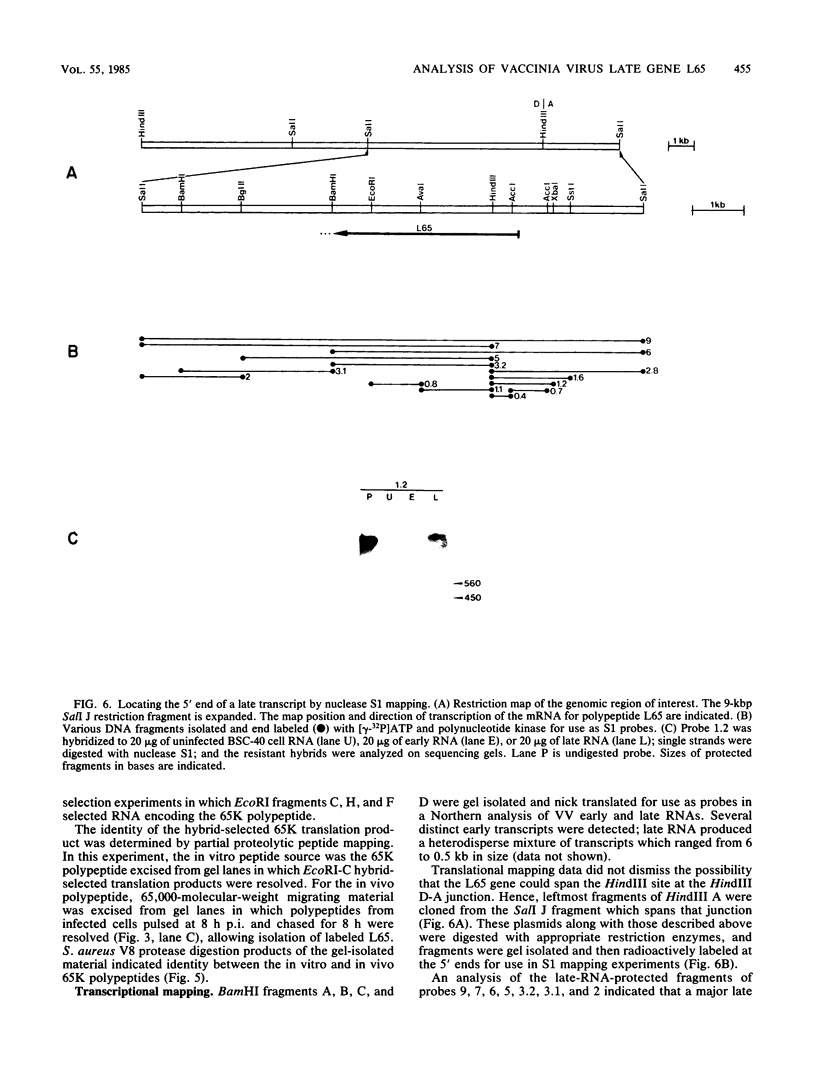
Among the products of vaccinia virus genes which are expressed late in infection is a major polypeptide (Mr, 65,000) designated L65. Pulse-chase analyses indicated that L65 is not subject to posttranslational cleavage as is the core polypeptide p4b which migrates to a similar position in sodium dodecyl sulfate-polyacrylamide gels. A polypeptide of 65,000 molecular weight produced in reticulocyte lysates programmed with viral mRNA isolated late in infection was identified as L65 by peptide mapping. L65 mRNA was purified by hybridization selection to restriction fragments of the viral genome and translated in vitro. This allowed the gene encoding L65 to be mapped to the rightmost 4.5 kilobase pairs of the HindIII D fragment. Transcriptional mapping of this region of the genome detected a late mRNA which was initiated at 450 base pairs to the right of the HindIII D-A junction, was transcribed in the leftward direction, and was terminated in the nondescript manner typical of vaccinia virus late mRNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone R. F., Moss B. Sequence complexity and relative abundance of vaccinia virus mRNA's synthesized in vivo and in vitro. J Virol. 1978 Jun;26(3):554–569. doi: 10.1128/jvi.26.3.554-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone R. F., Parr R. P., Moss B. Intermolecular duplexes formed from polyadenylylated vaccinia virus RNA. J Virol. 1979 Apr;30(1):365–374. doi: 10.1128/jvi.30.1.365-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipchase M., Schwendimann F., Wyler R. A map of the late proteins of vaccinia virus. Virology. 1980 Aug;105(1):261–264. doi: 10.1016/0042-6822(80)90176-2. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Colby C., Jurale C., Kates J. R. Mechanism of synthesis of vaccinia virus double-stranded ribonucleic acid in vivo and in vitro. J Virol. 1971 Jan;7(1):71–76. doi: 10.1128/jvi.7.1.71-76.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. In vitro translation of immediate early, early, and late classes of RNA from vaccinia virus-infected cells. Virology. 1979 Jul 30;96(2):368–380. doi: 10.1016/0042-6822(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. Translation of specific vaccinia virus RNAs purified as RNA-DNA hybrids on potassium iodide gradients. Nucleic Acids Res. 1979 Aug 10;6(11):3599–3612. doi: 10.1093/nar/6.11.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Wittek R., Moss B. Extension of the transcriptional and translational map of the left end of the vaccinia virus genome to 21 kilobase pairs. J Virol. 1981 Sep;39(3):733–745. doi: 10.1128/jvi.39.3.733-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Sedat J., Ziff E. Direct determination of DNA nucleotide sequences: structure of a fragment of bacteriophage phiX172 DNA. J Mol Biol. 1974 Aug 15;87(3):377–407. doi: 10.1016/0022-2836(74)90093-x. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A., Kates J. R. Vaccinia virus replication. I. Requirement for the host-cell nucleus. J Virol. 1979 Feb;29(2):705–715. doi: 10.1128/jvi.29.2.705-715.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Lynn D. L., Condit R. C., Kates J. R. Cellular differences in the molecular mechanisms of vaccinia virus host range restriction. J Gen Virol. 1980 Apr;47(2):485–488. doi: 10.1099/0022-1317-47-2-485. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Maki R. A., Miller D. B., Ball L. A. Fine structure analysis and nucleotide sequence of the vaccinia virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3411–3415. doi: 10.1073/pnas.80.11.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isle H. B., Venkatesan S., Moss B. Cell-free translation of early and late mRNAs selected by hybridization to cloned DNA fragments derived from the left 14 million to 72 million daltons of the vaccinia virus genome. Virology. 1981 Jul 15;112(1):306–317. doi: 10.1016/0042-6822(81)90636-x. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahr A., Roberts B. E. Arrangement of late RNAs transcribed from a 7.1-kilobase EcoRI vaccinia virus DNA fragment. J Virol. 1984 Feb;49(2):510–520. doi: 10.1128/jvi.49.2.510-520.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahr A., Roberts B. E. Organization of six early transcripts synthesized from a vaccinia virus EcoRI DNA fragment. J Virol. 1984 Feb;49(2):497–509. doi: 10.1128/jvi.49.2.497-509.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Letter: Protein cleavage and poxvirus morphogenesis: tryptic peptide analysis of core precursors accumulated by blocking assembly with rifampicin. J Mol Biol. 1973 Dec 5;81(2):267–269. doi: 10.1016/0022-2836(73)90195-2. [DOI] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Grady L. J. Transcriptional complexity of vaccinia virus in vivo and in vitro. J Virol. 1977 Sep;23(3):608–615. doi: 10.1128/jvi.23.3.608-615.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett C., Moss B. Selective transcription of vaccinia virus genes in template dependent soluble extracts of infected cells. Cell. 1983 Dec;35(2 Pt 1):441–448. doi: 10.1016/0092-8674(83)90177-0. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Baroudy B. M., Moss B. Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene. Cell. 1981 Sep;25(3):805–813. doi: 10.1016/0092-8674(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Complete nucleotide sequences of two adjacent early vaccinia virus genes located within the inverted terminal repetition. J Virol. 1982 Nov;44(2):637–646. doi: 10.1128/jvi.44.2.637-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Nucleotide sequence of the vaccinia virus thymidine kinase gene and the nature of spontaneous frameshift mutations. J Virol. 1983 May;46(2):530–537. doi: 10.1128/jvi.46.2.530-537.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Regulation of expression and nucleotide sequence of a late vaccinia virus gene. J Virol. 1984 Sep;51(3):662–669. doi: 10.1128/jvi.51.3.662-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Cooper J. A., Moss B. Transcriptional and translational mapping of a 6.6-kilobase-pair DNA fragment containing the junction of the terminal repetition and unique sequence at the left end of the vaccinia virus genome. J Virol. 1981 Sep;39(3):722–732. doi: 10.1128/jvi.39.3.722-732.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Hänggi M., Hiller G. Mapping of a gene coding for a major late structural polypeptide on the vaccinia virus genome. J Virol. 1984 Feb;49(2):371–378. doi: 10.1128/jvi.49.2.371-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R. Organization and expression of the poxvirus genome. Experientia. 1982 Mar 15;38(3):285–297. doi: 10.1007/BF01949349. [DOI] [PubMed] [Google Scholar]
- Wittek R., Richner B., Hiller G. Mapping of the genes coding for the two major vaccinia virus core polypeptides. Nucleic Acids Res. 1984 Jun 25;12(12):4835–4848. doi: 10.1093/nar/12.12.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]