Abstract
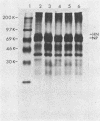
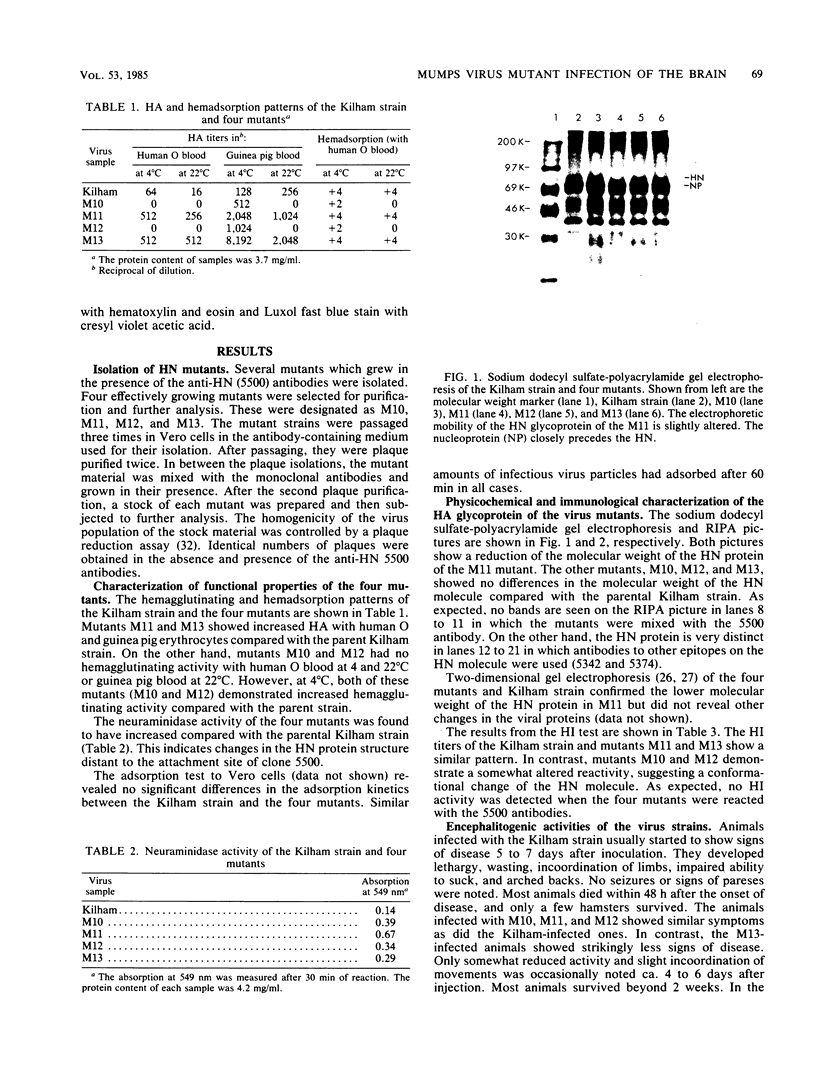
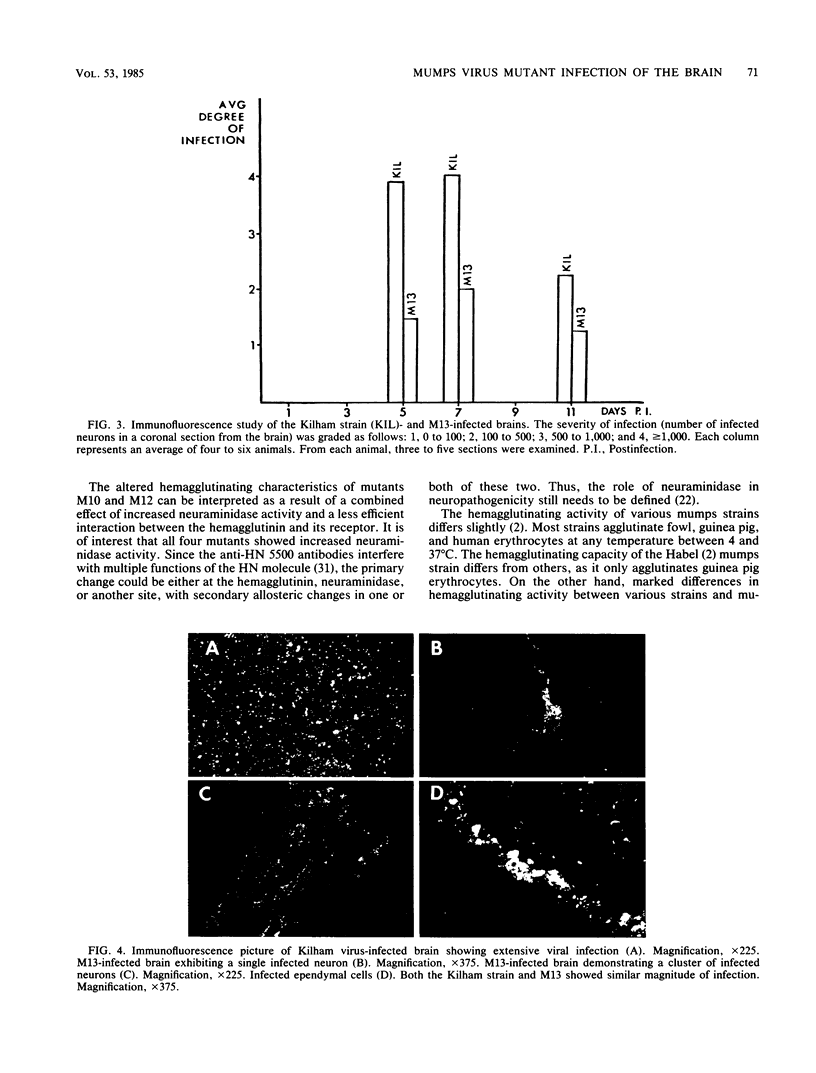
With the aid of monoclonal antibodies directed against a specific site on the hemagglutinin-neuraminidase surface glycoprotein, four mutants of the Kilham neurotropic strain of mumps virus were isolated. All four mutants had increased neuraminidase activity. Two mutants (M10 and M12) lost their hemagglutination capacity with human O erythrocytes but retained their ability to agglutinate guinea pig erythrocytes at 4 degrees C. A third mutant (M11) showed a change in the molecular weight of the hemagglutinin-neuraminidase glycoprotein. These three mutants (M10, M11, and M12) showed unaltered capacity to infect tissue cultures and to cause encephalitis in newborn hamsters. A fourth mutant (M13) retained its hemagglutination activity and capacity to infect Vero cell cultures but showed significantly lower neurovirulence in the suckling hamster brain than did the parental Kilham strain and the other three mutants. Both the number of infected neurons and the amount of infectious virus in the brain was reduced. On the other hand, there were no apparent differences in the occurrence of viral antigen in ependymal cells, indicating a selective change in affinity for neurons in the brain. These results suggest that certain changes in the hemagglutinin-neuraminidase glycoprotein may lead to an alteration of the neuropathogenicity of the Kilham strain of mumps virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breschkin A. M., Walmer B., Rapp F. Hemagglutination variant of measles virus. Virology. 1977 Jul 15;80(2):441–444. doi: 10.1016/s0042-6822(77)80021-4. [DOI] [PubMed] [Google Scholar]
- CANTELL K. Mumps virus. Adv Virus Res. 1961;8:123–164. doi: 10.1016/s0065-3527(08)60684-3. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Coulon P., Rollin P., Aubert M., Flamand A. Molecular basis of rabies virus virulence. I. Selection of avirulent mutants of the CVS strain with anti-G monoclonal antibodies. J Gen Virol. 1982 Jul;61(Pt 50):97–100. doi: 10.1099/0022-1317-61-1-97. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Jameson B. A., Lewis A. J., Larsen G. R., Wimmer E. Poliovirus neutralization epitopes: analysis and localization with neutralizing monoclonal antibodies. J Virol. 1982 Sep;43(3):997–1005. doi: 10.1128/jvi.43.3.997-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N., Greene M. I. Genetic and molecular mechanisms of viral pathogenesis: implications for prevention and treatment. Nature. 1982 Nov 4;300(5887):19–23. doi: 10.1038/300019a0. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P. Synthesis and maturation of glycoproteins of enveloped animal viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):26–39. doi: 10.1093/clinids/2.1.26. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensik S. C., Silver S. Polypeptides of mumps virus. J Virol. 1976 Feb;17(2):363–373. doi: 10.1128/jvi.17.2.363-373.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus following viral infection: the pathology of aqueductal stenosis developing after experimental mumps virus infection. J Neuropathol Exp Neurol. 1968 Oct;27(4):591–606. [PubMed] [Google Scholar]
- KILHAM L., OVERMAN J. R. Natural pathogenicity of mumps virus for suckling hamsters on intracerebral inoculation. J Immunol. 1953 Feb;70(2):147–151. [PubMed] [Google Scholar]
- Klenk H. D., Garten W., Bosch F. X., Rott R. Viral glycoproteins as determinants of pathogenicity. Med Microbiol Immunol. 1982;170(3):145–153. doi: 10.1007/BF02298195. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Orvell C., Leestma J., Norrby E. Sendai virus infection in the brains of mice: distribution of viral antigens studied with monoclonal antibodies. J Infect Dis. 1983 Feb;147(2):297–301. doi: 10.1093/infdis/147.2.297. [DOI] [PubMed] [Google Scholar]
- Lafon M., Wiktor T. J., Macfarlan R. I. Antigenic sites on the CVS rabies virus glycoprotein: analysis with monoclonal antibodies. J Gen Virol. 1983 Apr;64(Pt 4):843–851. doi: 10.1099/0022-1317-64-4-843. [DOI] [PubMed] [Google Scholar]
- Margolis G., Kilham L., Baringer J. R. A new look at mumps encephalitis: inclusion bodies and cytopathic effects. J Neuropathol Exp Neurol. 1974 Jan;33(1):13–28. doi: 10.1097/00005072-197401000-00002. [DOI] [PubMed] [Google Scholar]
- McCarthy M., Johnson R. T. A comparison of the structural polypeptides of five strains of mumps virus. J Gen Virol. 1980 Jan;46(1):15–27. doi: 10.1099/0022-1317-46-1-15. [DOI] [PubMed] [Google Scholar]
- McCarthy M., Jubelt B., Fay D. B., Johnson R. T. Comparative studies of five strains of mumps virus in vitro and in neonatal hamsters: evaluation of growth, cytopathogenicity, and neurovirulence. J Med Virol. 1980;5(1):1–15. doi: 10.1002/jmv.1890050102. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Server A. C., Waxham M. N., Wolinsky J. S. Biosynthesis of mumps virus F glycoprotein: non-fusing strains efficiently cleave the F glycoprotein precursor. J Gen Virol. 1983 Jul;64(Pt 7):1457–1467. doi: 10.1099/0022-1317-64-7-1457. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Wolinsky J. S. Biochemical features of mumps virus neuraminidases and their relationship with pathogenicity. Virology. 1981 Oct 15;114(1):218–227. doi: 10.1016/0042-6822(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Minor P. D., Schild G. C., Bootman J., Evans D. M., Ferguson M., Reeve P., Spitz M., Stanway G., Cann A. J., Hauptmann R. Location and primary structure of a major antigenic site for poliovirus neutralization. Nature. 1983 Feb 24;301(5902):674–679. doi: 10.1038/301674a0. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. 4. A simple procedure for production of high potency antigen for hemagglutination-inhibition (HI) tests. Proc Soc Exp Biol Med. 1962 Dec;111:814–818. doi: 10.3181/00379727-111-27930. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- OVERMAN J. R., PEERS J. H., KILHAM L. Pathology of mumps virus meningoencephalitis in mice and hamsters. AMA Arch Pathol. 1953 Jun;55(6):457–465. [PubMed] [Google Scholar]
- Orvell C., Grandien M. The effects of monoclonal antibodies on biologic activities of structural proteins of Sendai virus. J Immunol. 1982 Dec;129(6):2779–2787. [PubMed] [Google Scholar]
- Orvell C. Identification of paramyxovirus-specific haemolysis-inhibiting antibodies separate from haemagglutinating-inhibiting and neuraminidase-inhibiting antibodies. 1. Sendai virus haemolysis-inhibiting antibodies. Acta Pathol Microbiol Scand B. 1976 Dec;84B(6):441–450. [PubMed] [Google Scholar]
- Orvell C. Immunological properties of purified mumps virus glycoproteins. J Gen Virol. 1978 Dec;41(3):517–526. doi: 10.1099/0022-1317-41-3-517. [DOI] [PubMed] [Google Scholar]
- Orvell C., Norrby E. Immunological relationships between homologous structural polypeptides of measles and canine distemper virus. J Gen Virol. 1980 Oct;50(2):231–245. doi: 10.1099/0022-1317-50-2-231. [DOI] [PubMed] [Google Scholar]
- Orvell C. Structural polypeptides of mumps virus. J Gen Virol. 1978 Dec;41(3):527–539. doi: 10.1099/0022-1317-41-3-527. [DOI] [PubMed] [Google Scholar]
- Orvell C. The reactions of monoclonal antibodies with structural proteins of mumps virus. J Immunol. 1984 May;132(5):2622–2629. [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Adsorption and penetration of enveloped and naked vaccinia virus particles. J Virol. 1978 Jul;27(1):19–27. doi: 10.1128/jvi.27.1.19-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Marx P. S., Kingsbury D. W. A temperature-sensitive mutant of Sendai virus with an altered hemagglutinin-neuraminidase polypeptide: consequences for virus assembly and cytopathology. Virology. 1975 Sep;67(1):179–187. doi: 10.1016/0042-6822(75)90415-8. [DOI] [PubMed] [Google Scholar]
- Portner A., Webster R. G., Bean W. J. Similar frequencies of antigenic variants in Sendai, vesicular stomatitis, and influenza A viruses. Virology. 1980 Jul 15;104(1):235–238. doi: 10.1016/0042-6822(80)90382-7. [DOI] [PubMed] [Google Scholar]
- Rammohan K. W., McFarland H. F., Bellini W. J., Gheuens J., McFarlin D. E. Antibody-mediated modification of encephalitis induced by hamster neurotropic measles virus. J Infect Dis. 1983 Mar;147(3):546–550. doi: 10.1093/infdis/147.3.546. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Sheshberadaran H., Chen S. N., Norrby E. Monoclonal antibodies against five structural components of measles virus. I. Characterization of antigenic determinants on nine strains of measles virus. Virology. 1983 Jul 30;128(2):341–353. doi: 10.1016/0042-6822(83)90261-1. [DOI] [PubMed] [Google Scholar]
- Shida H., Dales S. Biogenesis of vaccinia: molecular basis for the hemagglutination-negative phenotype of the IHD-W strain. Virology. 1982 Feb;117(1):219–237. doi: 10.1016/0042-6822(82)90521-9. [DOI] [PubMed] [Google Scholar]
- Shida H., Matsumoto S. Analysis of the hemagglutinin glycoprotein from mutants of vaccinia virus that accumulates on the nuclear envelope. Cell. 1983 Jun;33(2):423–434. doi: 10.1016/0092-8674(83)90424-5. [DOI] [PubMed] [Google Scholar]
- Shirodaria P. V., Dermott E., Gould E. A. Some characteristics of salt-dependent haemagglutinating measles viruses. J Gen Virol. 1976 Oct;33(1):107–115. doi: 10.1099/0022-1317-33-1-107. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Bronson R. T., Fields B. N. Hemagglutinin variants of reovirus type 3 have altered central nervous system tropism. Science. 1983 Apr 29;220(4596):505–507. doi: 10.1126/science.6301010. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Laver W. G. Selection and analysis of antigenic variants of the neuraminidase of N2 influenza viruses with monoclonal antibodies. Virology. 1982 Feb;117(1):93–104. doi: 10.1016/0042-6822(82)90510-4. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wolinsky J. S., Baringer J. R., Margolis G., Kilham L. Ultrastructure of mumps virus replication in newborn hamster central nervous system. Lab Invest. 1974 Oct;31(4):403–412. [PubMed] [Google Scholar]
- Wolinsky J. S., Klassen T., Baringer J. R. Persistence of neuroadapted mumps virus in brains of newborn hamsters after intraperitoneal inoculation. J Infect Dis. 1976 Mar;133(3):260–267. doi: 10.1093/infdis/133.3.260. [DOI] [PubMed] [Google Scholar]
- Wolinsky J. S., Stroop W. G. Virulence and persistence of three prototype strains of mumps virus in newborn hamsters. Arch Virol. 1978;57(4):355–359. doi: 10.1007/BF01320075. [DOI] [PubMed] [Google Scholar]