Abstract
In the assembly of bacteriophage P22, precursor particles containing two major proteins, the gene 5 coat protein and the gene 8 scaffolding protein, package the DNA molecule. During the encapsidation reaction all of the scaffolding protein molecules are released intact and subsequently participate in further rounds of DNA encapsidation. We have previously shown that even though it lies in the center of the late region of the genetic map, the scaffolding protein gene is not always expressed coordinately with the remainder of the late proteins and that some feature of the phage assembly process affects its expression. We present here in vivo experiments which show that there is an inverse correlation between the amount of unassembled scaffolding protein and the rate of scaffolding protein synthesis and that long amber fragments of the scaffolding protein can turn down the synthesis of intact scaffolding protein in trans. These results support a model for scaffolding protein regulation in which the feature of the assembly process which modulates the rate of scaffolding protein synthesis is the amount of unassembled scaffolding protein itself.
Full text
PDF
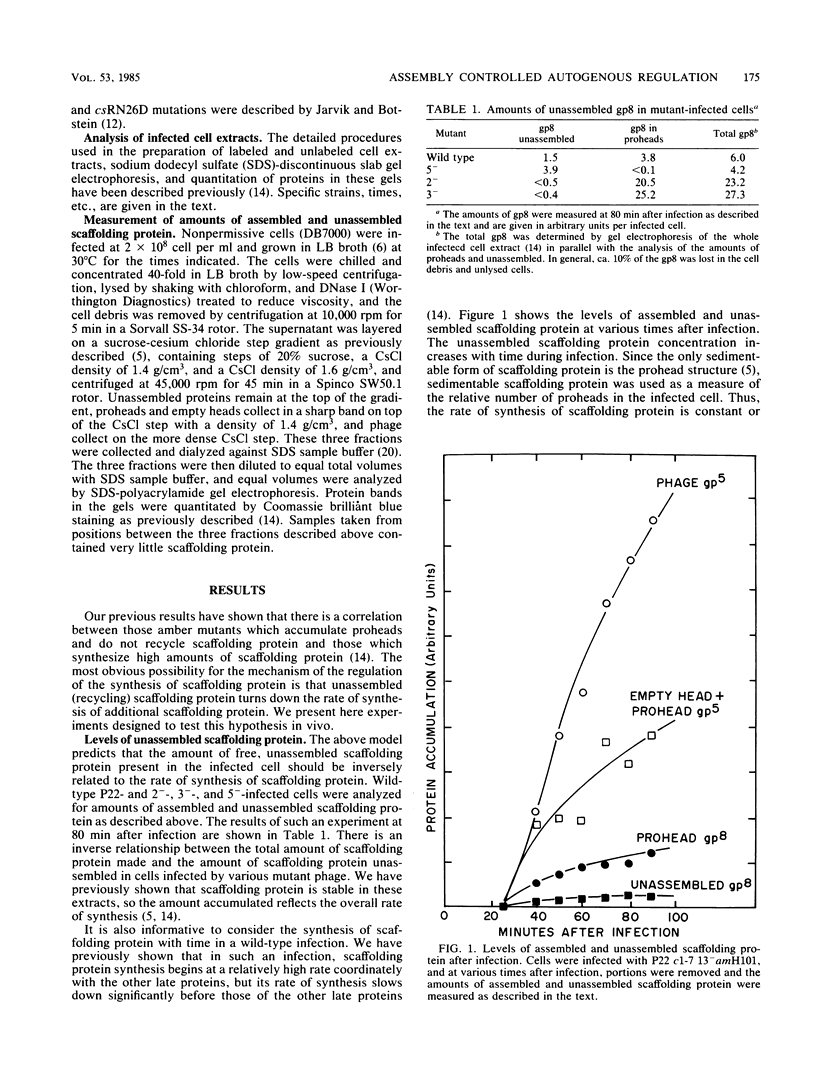
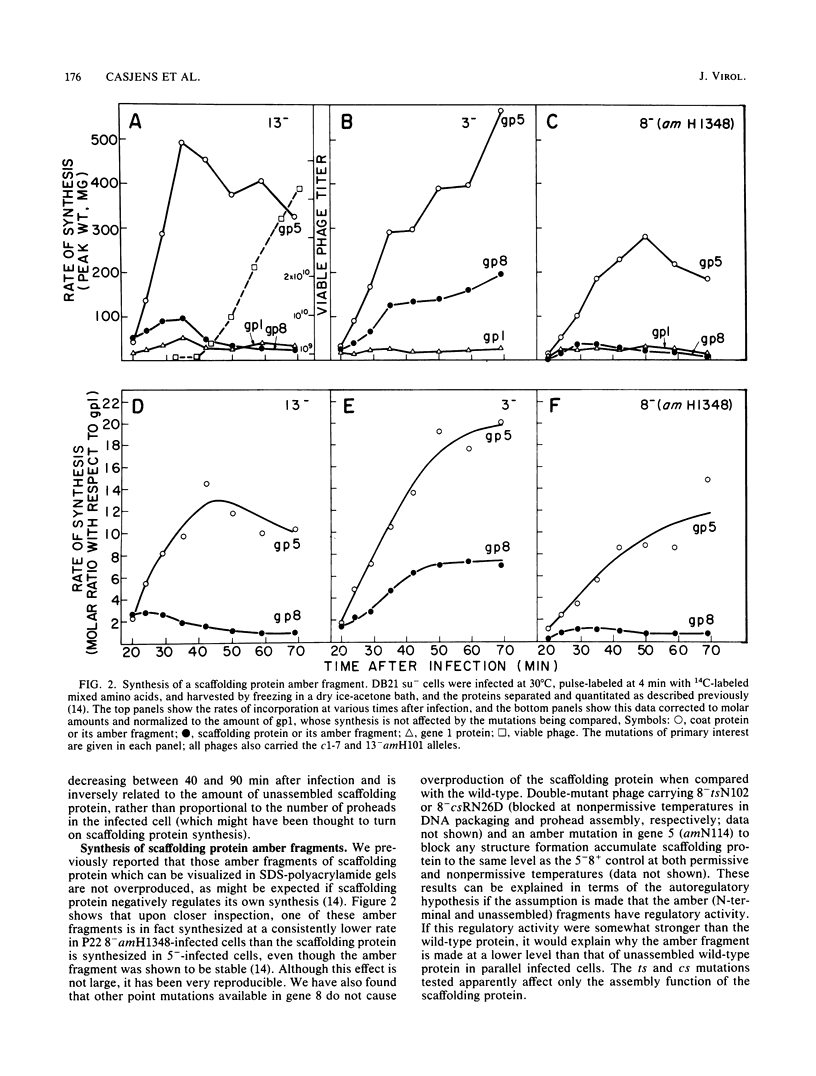
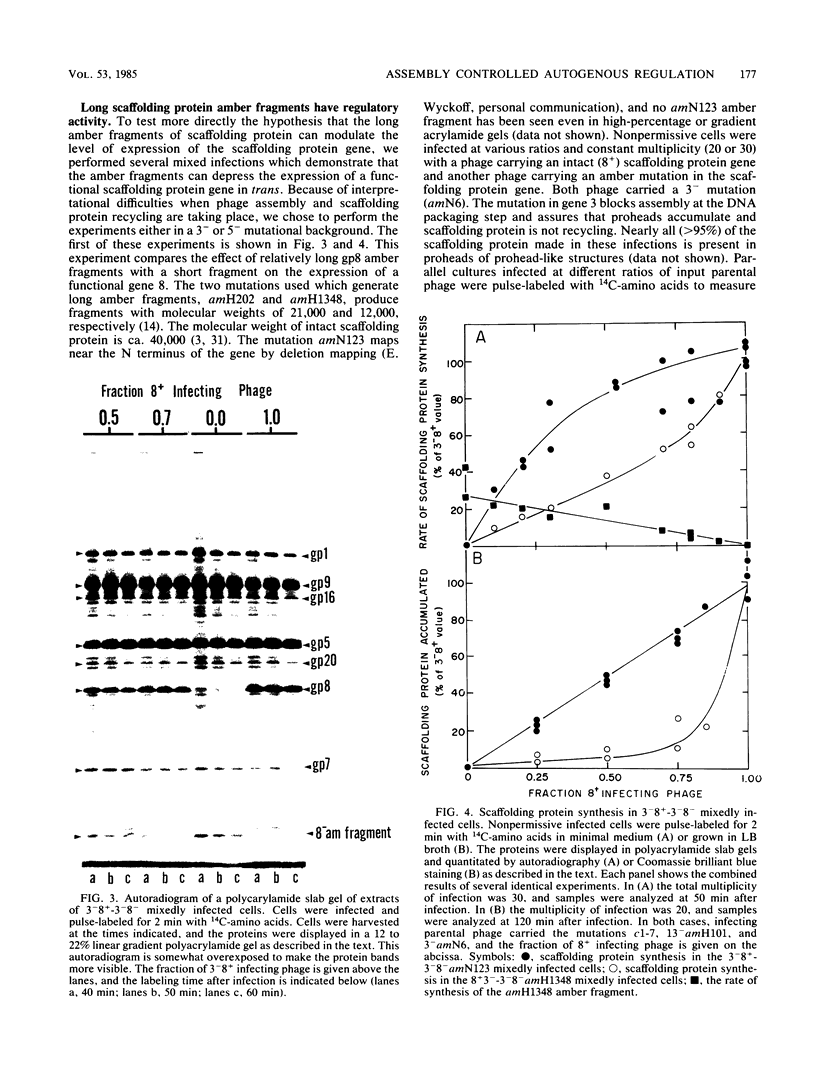

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Botstein D., Herskowitz I. Properties of hybrids between Salmonella phage P22 and coliphage lambda. Nature. 1974 Oct 18;251(5476):584–589. doi: 10.1038/251584a0. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Casjens S., Adams M. B. Posttranscriptional modulation of bacteriophage P22 scaffolding protein gene expression. J Virol. 1985 Jan;53(1):185–191. doi: 10.1128/jvi.53.1.185-191.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. P22 morphogenesis. I: Catalytic scaffolding protein in capsid assembly. J Supramol Struct. 1974;2(2-4):202–224. doi: 10.1002/jss.400020215. [DOI] [PubMed] [Google Scholar]
- Chan R. K., Botstein D. Genetics of bacteriophage P22. I. Isolation of prophage deletions which affect immunity to superinfection. Virology. 1972 Jul;49(1):257–267. doi: 10.1016/s0042-6822(72)80027-8. [DOI] [PubMed] [Google Scholar]
- Earnshaw W., Casjens S., Harrison S. C. Assembly of the head of bacteriophage P22: x-ray diffraction from heads, proheads and related structures. J Mol Biol. 1976 Jun 25;104(2):387–410. doi: 10.1016/0022-2836(76)90278-3. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Taketo M., Ishihama A. Autogenous regulation of RNA polymerase beta subunit synthesis in vitro. J Biol Chem. 1978 Jul 10;253(13):4501–4504. [PubMed] [Google Scholar]
- Glass R. E. Identification of an amber fragment of the beta subunit of Escherichia coli RNA polymerase: a yardstick for measuring controls on RNA polymerase subunit synthesis. Mol Gen Genet. 1977 Feb 28;151(1):83–88. doi: 10.1007/BF00446916. [DOI] [PubMed] [Google Scholar]
- Jarvik J., Botstein D. Conditional-lethal mutations that suppress genetic defects in morphogenesis by altering structural proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2738–2742. doi: 10.1073/pnas.72.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Casjens S. Catalytic head assembling protein in virus morphogenesis. Nature. 1974 Sep 13;251(5471):112–119. doi: 10.1038/251112a0. [DOI] [PubMed] [Google Scholar]
- King J., Hall C., Casjens S. Control of the synthesis of phage P22 scaffolding protein is coupled to capsid assembly. Cell. 1978 Oct;15(2):551–560. doi: 10.1016/0092-8674(78)90023-5. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- Komeda Y., Iino T. Regulation of expression of the flagellin gene (hag) in Escherichia coli K-12: analysis of hag-lac gene fusions. J Bacteriol. 1979 Sep;139(3):721–729. doi: 10.1128/jb.139.3.721-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M., Nathans D. Translation of the genome of a ribonucleic acid bacteriophage. Bacteriol Rev. 1972 Mar;36(1):109–134. doi: 10.1128/br.36.1.109-134.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew K., Casjens S. Identification of early proteins coded by bacteriophage P22. Virology. 1975 Dec;68(2):525–533. doi: 10.1016/0042-6822(75)90292-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Poteete A. R., Botstein D. Purification and properties of proteins essential to DNA encapsulation by phage P22. Virology. 1979 Jun;95(2):565–573. doi: 10.1016/0042-6822(79)90509-9. [DOI] [PubMed] [Google Scholar]
- Poteete A. R., Jarvik V., Botstein D. Encapsulation of phage P22 DNA in vitro. Virology. 1979 Jun;95(2):550–564. doi: 10.1016/0042-6822(79)90508-7. [DOI] [PubMed] [Google Scholar]
- Poteete A. R., King J. Functions of two new genes in Salmonella phage P22 assembly. Virology. 1977 Feb;76(2):725–739. doi: 10.1016/0042-6822(77)90254-9. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Transcription termination and late control in phage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3300–3304. doi: 10.1073/pnas.72.9.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D. Molecular genetics of bacteriophage P22. Microbiol Rev. 1978 Jun;42(2):385–413. doi: 10.1128/mr.42.2.385-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., Riggs P. D., Botstein D. Genetics of bacteriophage P22. III. The late operon. Virology. 1980 Oct 15;106(1):82–91. doi: 10.1016/0042-6822(80)90223-8. [DOI] [PubMed] [Google Scholar]
- Winston F., Botstein D., Miller J. H. Characterization of amber and ochre suppressors in Salmonella typhimurium. J Bacteriol. 1979 Jan;137(1):433–439. doi: 10.1128/jb.137.1.433-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff E., Casjens S. Autoregulation of the bacteriophage P22 scaffolding protein gene. J Virol. 1985 Jan;53(1):192–197. doi: 10.1128/jvi.53.1.192-197.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youderian P., Susskind M. M. Identification of the products of bacteriophage P22 genes, including a new late gene. Virology. 1980 Nov;107(1):258–269. doi: 10.1016/0042-6822(80)90291-3. [DOI] [PubMed] [Google Scholar]