Abstract
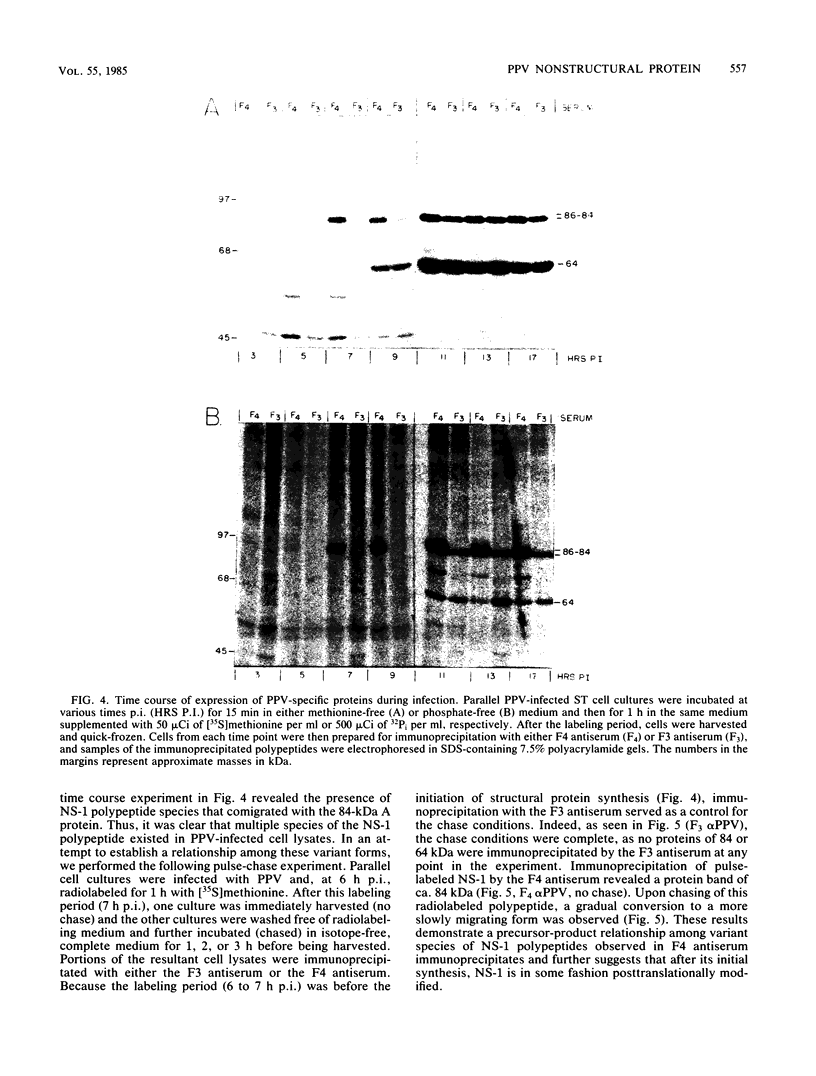
Sera from porcine parvovirus (PPV)-infected swine fetuses immunoprecipitated and 84- to 86-kilodalton polypeptide in addition to the A and B virion structural proteins. This polypeptide, designated NS-1, was present in PPV-infected cell lysates but not in purified virions. Partial proteolysis mapping revealed that NS-1 was not related to the A and B viral structural proteins. All three proteins in infected cells were phosphorylated at serine residues, and NS-1 also contained phosphothreonine. From pulse-labeling experiments with either 32Pi or [35S]methionine, NS-1 was found to first appear 5 to 7 h postinfection, whereas the viral structural polypeptides were first synthesized 9 to 11 h postinfection. Pulse-chase experiments revealed that NS-1 initially appeared as an 84-kilodalton protein and was subsequently structurally modified to forms of slower electrophoretic mobilities. The time of appearance of NS-1 after virus infection coincided with the initiation of viral DNA synthesis, suggesting that this polypeptide (and the modified forms thereof) may be involved in PPV replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Thomson M., Merchlinsky M., Ward D. C. The complete DNA sequence of minute virus of mice, an autonomous parvovirus. Nucleic Acids Res. 1983 Feb 25;11(4):999–1018. doi: 10.1093/nar/11.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Sturzenbecker L. J., Tattersall P. The autonomous parvovirus MVM encodes two nonstructural proteins in addition to its capsid polypeptides. Virology. 1983 Sep;129(2):333–343. doi: 10.1016/0042-6822(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Joo H. S., Donaldson-Wood C. R., Johnson R. H. A standardised haemagglutination inhibition test for porcine parvovirus antibody. Aust Vet J. 1976 Sep;52(9):422–424. doi: 10.1111/j.1751-0813.1976.tb09517.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederman M., Patton J. T., Stout E. R., Bates R. C. Virally coded noncapsid protein associated with bovine parvovirus infection. J Virol. 1984 Feb;49(2):315–318. doi: 10.1128/jvi.49.2.315-318.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga Y., Matsuno S. Structural and nonstructural proteins of a rabbit parvovirus. J Virol. 1983 Feb;45(2):627–633. doi: 10.1128/jvi.45.2.627-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengeling W. L. Prevalence of porcine parvovirus-induced reproductive failure: an abattoir study. J Am Vet Med Assoc. 1978 Jun 1;172(11):1291–1294. [PubMed] [Google Scholar]
- Mitra R., Wali T., Valdez V., Fabisch P., Salzman L. A. Transcription and translation in the autonomous parvovirus KRV. Virology. 1983 Mar;125(2):349–360. doi: 10.1016/0042-6822(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. Porcine parvovirus DNA: characterization of the genomic and replicative form DNA of two virus isolates. Virology. 1984 Sep;137(2):241–254. doi: 10.1016/0042-6822(84)90216-2. [DOI] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. Porcine parvovirus: virus purification and structural and antigenic properties of virion polypeptides. J Virol. 1983 Feb;45(2):842–854. doi: 10.1128/jvi.45.2.842-854.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso P. R. Identification of multiple forms of the noncapsid parvovirus protein NCVP1 in H-1 parvovirus-infected cells. J Virol. 1984 Oct;52(1):82–87. doi: 10.1128/jvi.52.1.82-87.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Dadachanji D., Astell C. R., Ward D. C. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 1983 Feb 25;11(4):1019–1038. doi: 10.1093/nar/11.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirtle E. C. Titration of two porcine respiratory viruses in mammalian cell cultures by direct fluorescent antibody staining. Am J Vet Res. 1974 Feb;35(2):249–250. [PubMed] [Google Scholar]
- Redman D. R., Bohl E. H., Ferguson L. C. Porcine parvovirus: natural and experimental infections of the porcine fetus and prevalence in mature swine. Infect Immun. 1974 Oct;10(4):718–723. doi: 10.1128/iai.10.4.718-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Complementation for replicative form DNA replication of a deletion mutant of H-1 by various parvoviruses. J Virol. 1982 Jun;42(3):1118–1122. doi: 10.1128/jvi.42.3.1118-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]