Abstract
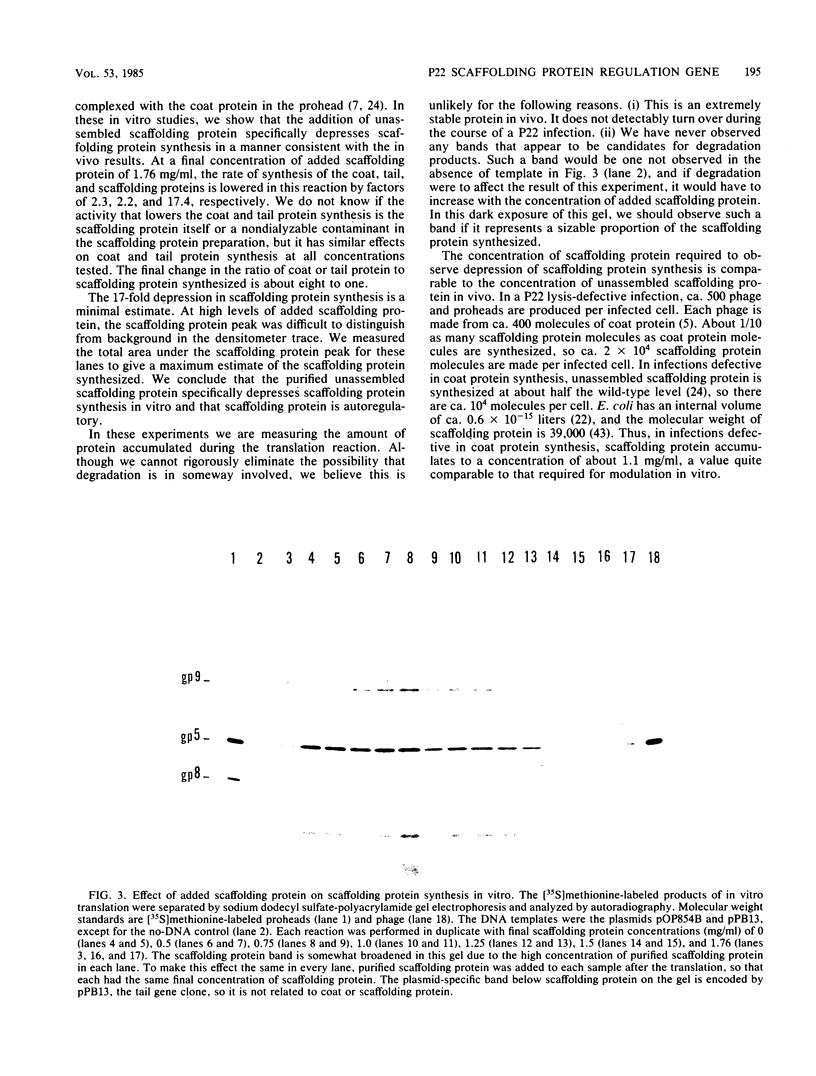
During the formation of each bacteriophage P22 head, about 250 molecules of the product of gene 8, scaffolding protein, coassemble with and dictate correct assembly of the coat protein into a proper shell structure. At approximately the time that DNA is inserted inside the coat protein shell, all of the scaffolding protein molecules leave the structure. They remain active and participate in several subsequent rounds of shell assembly. Previous work has shown that scaffolding protein gene expression is affected by the head assembly process and has generated the hypothesis that unassembled scaffolding protein negatively modulates the expression of its own gene but that it lacks this activity when complexed with coat protein in proheads. To test this model, a P22 restriction fragment containing the scaffolding and coat protein genes was cloned under control of the lac promoter. These cloned genes were then expressed in an in vitro DNA-dependent transcription-translation reaction. The addition of purified scaffolding protein to this reaction resulted in reduced scaffolding protein synthesis relative to coat and tail protein synthesis to an extent and at a protein concentration that was consistent with the observed reduction in vivo. We conclude that scaffolding protein synthesis is autoregulated and that scaffolding protein is the only phage-coded protein required for this process. In addition, these experiments provide additional evidence that this autoregulation is posttranscriptional.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Berget P. B., Poteete A. R., Sauer R. T. Control of phage P22 tail protein expression by transcription termination. J Mol Biol. 1983 Mar 15;164(4):561–572. doi: 10.1016/0022-2836(83)90050-5. [DOI] [PubMed] [Google Scholar]
- Botstein D., Herskowitz I. Properties of hybrids between Salmonella phage P22 and coliphage lambda. Nature. 1974 Oct 18;251(5476):584–589. doi: 10.1038/251584a0. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Casjens S., Adams M. B., Hall C., King J. Assembly-controlled autogenous modulation of bacteriophage P22 scaffolding protein gene expression. J Virol. 1985 Jan;53(1):174–179. doi: 10.1128/jvi.53.1.174-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., Adams M. B. Posttranscriptional modulation of bacteriophage P22 scaffolding protein gene expression. J Virol. 1985 Jan;53(1):185–191. doi: 10.1128/jvi.53.1.185-191.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. P22 morphogenesis. I: Catalytic scaffolding protein in capsid assembly. J Supramol Struct. 1974;2(2-4):202–224. doi: 10.1002/jss.400020215. [DOI] [PubMed] [Google Scholar]
- Casjens S. Molecular organization of the bacteriophage P22 coat protein shell. J Mol Biol. 1979 Jun 15;131(1):1–14. doi: 10.1016/0022-2836(79)90298-5. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans R. J., Jackson E. N. Restriction endonuclease Hin dIII cleavage site map of bacteriophage P22. Virology. 1979 Jun;95(2):359–372. doi: 10.1016/0042-6822(79)90491-4. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Earnshaw W., Casjens S., Harrison S. C. Assembly of the head of bacteriophage P22: x-ray diffraction from heads, proheads and related structures. J Mol Biol. 1976 Jun 25;104(2):387–410. doi: 10.1016/0022-2836(76)90278-3. [DOI] [PubMed] [Google Scholar]
- Forbes D., Herskowitz I. Polarity suppression by the Q gene product of bacteriophage lambda. J Mol Biol. 1982 Oct 5;160(4):549–569. doi: 10.1016/0022-2836(82)90314-x. [DOI] [PubMed] [Google Scholar]
- Fuller F. A family of cloning vectors containing the lacUV5 promoter. Gene. 1982 Jul-Aug;19(1):43–54. doi: 10.1016/0378-1119(82)90187-1. [DOI] [PubMed] [Google Scholar]
- Fuller M. T., King J. Regulation of coat protein polymerization by the scaffolding protein of bacteriophage P22. Biophys J. 1980 Oct;32(1):381–401. doi: 10.1016/S0006-3495(80)84963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Goldberger R. F. Autogenous regulation of gene expression. Science. 1974 Mar 1;183(4127):810–816. doi: 10.1126/science.183.4127.810. [DOI] [PubMed] [Google Scholar]
- Grayhack E. J., Roberts J. W. The phage lambda Q gene product: activity of a transcription antiterminator in vitro. Cell. 1982 Sep;30(2):637–648. doi: 10.1016/0092-8674(82)90260-4. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Jackson D. A., Deans R. J. EcoRI analysis of bacteriophage P22 DNA packaging. J Mol Biol. 1978 Jan 25;118(3):365–388. doi: 10.1016/0022-2836(78)90234-6. [DOI] [PubMed] [Google Scholar]
- Johnson A. D., Poteete A. R., Lauer G., Sauer R. T., Ackers G. K., Ptashne M. lambda Repressor and cro--components of an efficient molecular switch. Nature. 1981 Nov 19;294(5838):217–223. doi: 10.1038/294217a0. [DOI] [PubMed] [Google Scholar]
- Kennell D., Riezman H. Transcription and translation initiation frequencies of the Escherichia coli lac operon. J Mol Biol. 1977 Jul;114(1):1–21. doi: 10.1016/0022-2836(77)90279-0. [DOI] [PubMed] [Google Scholar]
- King J., Casjens S. Catalytic head assembling protein in virus morphogenesis. Nature. 1974 Sep 13;251(5471):112–119. doi: 10.1038/251112a0. [DOI] [PubMed] [Google Scholar]
- King J., Hall C., Casjens S. Control of the synthesis of phage P22 scaffolding protein is coupled to capsid assembly. Cell. 1978 Oct;15(2):551–560. doi: 10.1016/0092-8674(78)90023-5. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- Klein R. D., Selsing E., Wells R. D. A rapid microscale technique for isolation of recombinant plasmid DNA suitable for restriction enzyme analysis. Plasmid. 1980 Jan;3(1):88–91. doi: 10.1016/s0147-619x(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Allet B. Nucleotide sequences involved in bacteriophage T4 gene 32 translational self-regulation. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4937–4941. doi: 10.1073/pnas.79.16.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Expression of ribosomal genes in bacteria. Adv Genet. 1982;21:53–121. doi: 10.1016/s0065-2660(08)60297-7. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W., Yanisch-Perron C. R. Purified lexA protein is a repressor of the recA and lexA genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4199–4203. doi: 10.1073/pnas.78.7.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteete A. R., Botstein D. Purification and properties of proteins essential to DNA encapsulation by phage P22. Virology. 1979 Jun;95(2):565–573. doi: 10.1016/0042-6822(79)90509-9. [DOI] [PubMed] [Google Scholar]
- Poteete A. R., King J. Functions of two new genes in Salmonella phage P22 assembly. Virology. 1977 Feb;76(2):725–739. doi: 10.1016/0042-6822(77)90254-9. [DOI] [PubMed] [Google Scholar]
- Rutila J. E., Jackson E. N. Physical map of the bacteriophage P22 genome. Virology. 1981 Sep;113(2):769–775. doi: 10.1016/0042-6822(81)90206-3. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D. Molecular genetics of bacteriophage P22. Microbiol Rev. 1978 Jun;42(2):385–413. doi: 10.1128/mr.42.2.385-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Huberman J. A., Botstein D. Non-random circular permutation of phage P22 DNA. J Mol Biol. 1974 Jan 5;85(4):501–528. doi: 10.1016/0022-2836(74)90312-x. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., Riggs P. D., Botstein D. Genetics of bacteriophage P22. III. The late operon. Virology. 1980 Oct 15;106(1):82–91. doi: 10.1016/0042-6822(80)90223-8. [DOI] [PubMed] [Google Scholar]
- Winston F., Botstein D., Miller J. H. Characterization of amber and ochre suppressors in Salmonella typhimurium. J Bacteriol. 1979 Jan;137(1):433–439. doi: 10.1128/jb.137.1.433-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youderian P., Susskind M. M. Identification of the products of bacteriophage P22 genes, including a new late gene. Virology. 1980 Nov;107(1):258–269. doi: 10.1016/0042-6822(80)90291-3. [DOI] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Kowalczykowski S. C., Lonberg N., Newport J. W., Paul L. S., Stormo G. D., Gold L. Autoregulation of gene expression. Quantitative evaluation of the expression and function of the bacteriophage T4 gene 32 (single-stranded DNA binding) protein system. J Mol Biol. 1982 Dec 25;162(4):795–818. doi: 10.1016/0022-2836(82)90548-4. [DOI] [PubMed] [Google Scholar]