Abstract
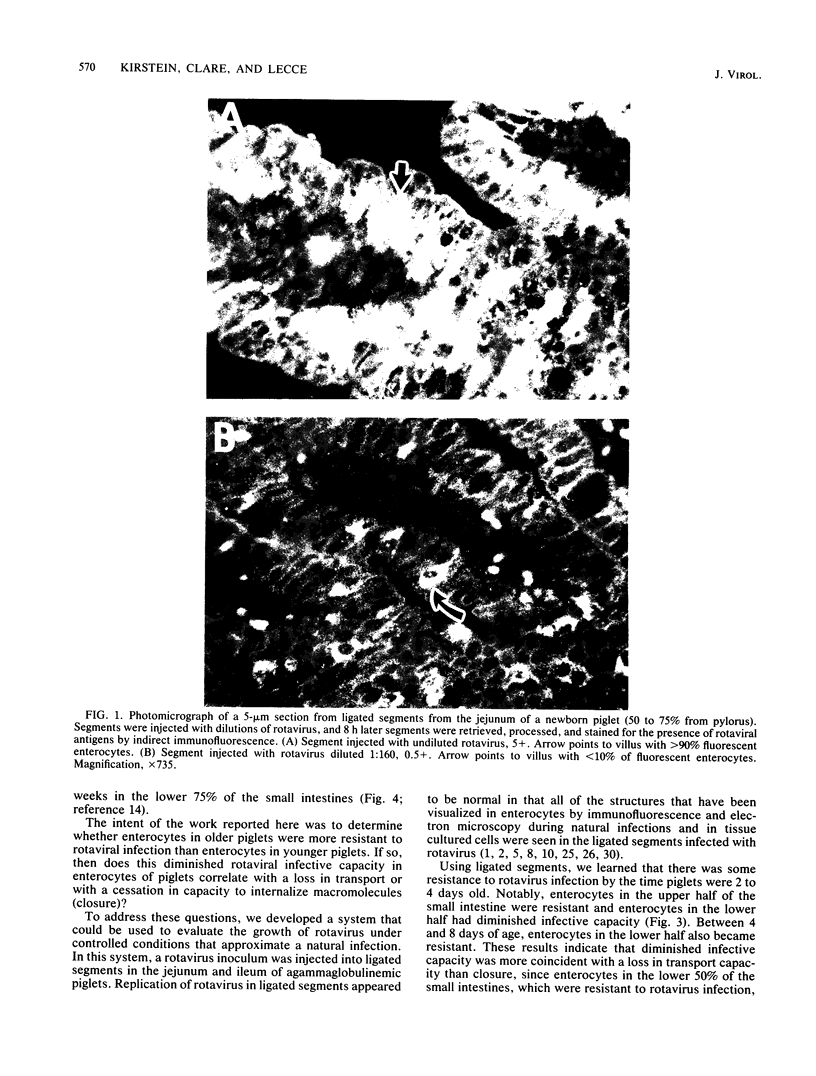
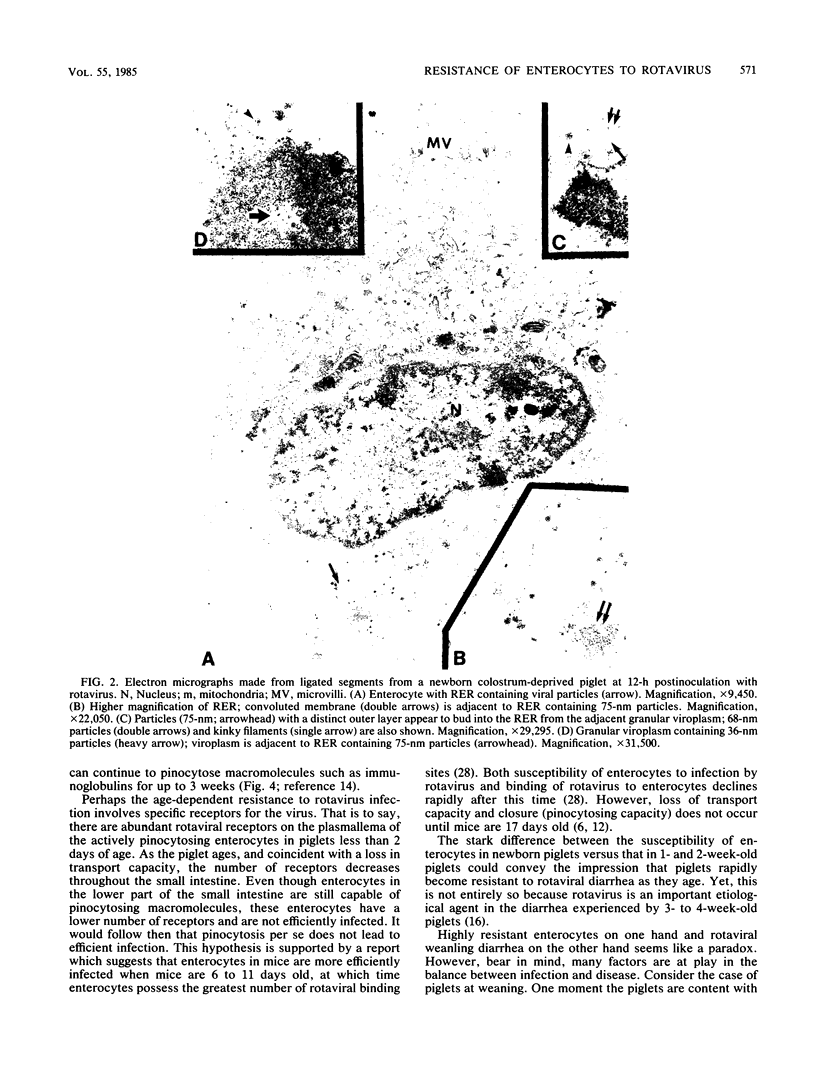
The first part of this report describes the development of a technique for evaluating the growth of rotavirus under controlled conditions that approximate a natural infection. A standard dose of rotavirus (approximately 10(9) viral particles) was injected into ligated segments in the small intestine of newborn, agammaglobulinemic, colostrum-deprived piglets. After various periods postinoculation, the segments were retrieved and the enterocytes were evaluated for the presence of rotaviral antigens by immunofluorescence and rotaviral particles by transmission electron microscopy. Peak immunofluorescence in enterocytes was detected at 8 h postinoculation in the upper and middle jejunum and ileum. Transmission electron microscopy at this time revealed fully formed virions which were not seen in sections examined before this 8-h period. The second part of our study describes the use of ligated segments in determining the susceptibility to rotavirus of enterocytes in piglets ranging in age from newborn to 2 weeks. By the time piglets were 2 days old, enterocytes in the upper half of the small intestines appeared to be resistant to rotavirus, whereas those in the lower half seemed partially resistant. Between 4 and 8 days of age, enterocytes in the lower half also became resistant. Resistance paralleled the loss in capacity of piglets to transport macromolecules through enterocytes and was not correlated with the loss in capacity to internalize macromolecules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. R., Kraft L. M. Electron-Microscopic Study of the Intestinal Epithelium of Mice Infected with the Agent of Epizootic Diarrhea of Infant Mice (EDIM Virus). Am J Pathol. 1967 Jul;51(1):39–60. [PMC free article] [PubMed] [Google Scholar]
- Altenburg B. C., Graham D. Y., Estes M. K. Ultrastructural study of rotavirus replication in cultured cells. J Gen Virol. 1980 Jan;46(1):75–85. doi: 10.1099/0022-1317-46-1-75. [DOI] [PubMed] [Google Scholar]
- Barnett B. B., Spendlove R. S., Clark M. L. Effect of enzymes on rotavirus infectivity. J Clin Microbiol. 1979 Jul;10(1):111–113. doi: 10.1128/jcm.10.1.111-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R. F., Davidson G. P., Holmes I. H., Ruck B. J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973 Dec 8;2(7841):1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Broughton C. W., Lecce J. G. Electron-microscopic studies of the jejunal epithelium from neonatal pigs fed different diets. J Nutr. 1970 Apr;100(4):445–449. doi: 10.1093/jn/100.4.445. [DOI] [PubMed] [Google Scholar]
- Bégin M. E. Enhanced production of infectious rotavirus in BSC-1 cell cultures by various factors in the presence of absence of trypsin. J Gen Virol. 1980 Dec;51(Pt 2):263–270. doi: 10.1099/0022-1317-51-2-263. [DOI] [PubMed] [Google Scholar]
- Coalson J. A., Lecce J. G. Herd differences in the expression of fatal diarrhea in artificially reared piglets weaned after 12 hours vs. 36 hours of nursing. J Anim Sci. 1973 Jun;36(6):1114–1121. doi: 10.2527/jas1973.3661114x. [DOI] [PubMed] [Google Scholar]
- Esparza J., Gorziglia M., Gil F., Römer H. Multiplication of human rotavirus in cultured cells: an electron microscopic study. J Gen Virol. 1980 Apr;47(2):461–472. doi: 10.1099/0022-1317-47-2-461. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Estes M. K. Proteolytic enhancement of rotavirus infectivity: biology mechanism. Virology. 1980 Mar;101(2):432–439. doi: 10.1016/0042-6822(80)90456-0. [DOI] [PubMed] [Google Scholar]
- LECCE J. G., MATRONE G. Porcine neonatal nutrition: the effect of diet on blood serum proteins and performance of the baby pig. J Nutr. 1960 Jan;70:13–20. doi: 10.1093/jn/70.1.13. [DOI] [PubMed] [Google Scholar]
- LECCE J. G., MORGAN D. O. Effect of dietary regimen on cessation of intestinal absorption of large molecules (closure) in the neonatal pig and lamb. J Nutr. 1962 Nov;78:263–268. doi: 10.1093/jn/78.3.263. [DOI] [PubMed] [Google Scholar]
- Lecce J. G. Absorption of macromolecules by neonatal intestine. Biol Neonat. 1965;9(1):50–61. [PubMed] [Google Scholar]
- Lecce J. G., Balsbaugh R. K., Clare D. A., King M. W. Rotavirus and hemolytic enteropathogenic Escherichia coli in weanling diarrhea of pigs. J Clin Microbiol. 1982 Oct;16(4):715–723. doi: 10.1128/jcm.16.4.715-723.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecce J. G., Coalson J. A. Diets for rearing colostrum-free piglets with an automatic feeding device. J Anim Sci. 1976 Mar;42(3):622–629. doi: 10.2527/jas1976.423622x. [DOI] [PubMed] [Google Scholar]
- Lecce J. G., King M. W., Mock R. Reovirus-like agent associated with fatal diarrhea in neonatal pigs. Infect Immun. 1976 Sep;14(3):816–825. doi: 10.1128/iai.14.3.816-825.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecce J. G. Rearing piglets artificially in a farm environment: a promise unfulfilled. J Anim Sci. 1975 Aug;41(2):659–666. doi: 10.2527/jas1975.412659x. [DOI] [PubMed] [Google Scholar]
- Leece J. G. Effect of dietary regimen on cessation of uptake of macromolecules by piglet intestinal epithelium (closure) and transport to the blood. J Nutr. 1973 May;103(5):751–756. doi: 10.1093/jn/103.5.751. [DOI] [PubMed] [Google Scholar]
- Loria R. M., Kibrick S., Broitman S. A. Peroral infection with group B coxsackievirus in the adult mouse: protective functions of the gut. J Infect Dis. 1974 Nov;130(5):539–543. doi: 10.1093/infdis/130.5.539. [DOI] [PubMed] [Google Scholar]
- McLean B. S., Holmes I. H. Effects of antibodies, trypsin, and trypsin inhibitors on susceptibility of neonates to rotavirus infection. J Clin Microbiol. 1981 Jan;13(1):22–29. doi: 10.1128/jcm.13.1.22-29.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty M. S., Curran W. L., McFerran J. B. The morphogenesis of a cytopathic bovine rotavirus in Madin-Darby bovine kidney cells. J Gen Virol. 1976 Dec;33(3):503–508. doi: 10.1099/0022-1317-33-3-503. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., McNulty M. S. Ultrastructural changes in small intestinal epithelium of neonatal pigs infected with pig rotavirus. Arch Virol. 1979;59(1-2):127–136. doi: 10.1007/BF01317902. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Lee P. C., Carmody P. J., Barrett H. J., Ogra P. L. Age-dependent rotavirus-enterocyte interactions. Proc Soc Exp Biol Med. 1982 Jun;170(2):146–154. doi: 10.3181/00379727-170-41410. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Ferguson A., Allan F., Angus K. W., Mitchell B. Small intestinal morphology and epithelial cell kinetics in lamb rotavirus infections. Gastroenterology. 1979 Mar;76(3):477–481. [PubMed] [Google Scholar]
- Wolf J. L., Cukor G., Blacklow N. R., Dambrauskas R., Trier J. S. Susceptibility of mice to rotavirus infection: effects of age and administration of corticosteroids. Infect Immun. 1981 Aug;33(2):565–574. doi: 10.1128/iai.33.2.565-574.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Hall G., Dennis M. J. The isolation of a reovirus-like agent associated with diarrhoea in colostrum-deprived calves in Great Britain. Res Vet Sci. 1974 Jan;16(1):102–105. [PubMed] [Google Scholar]