Abstract
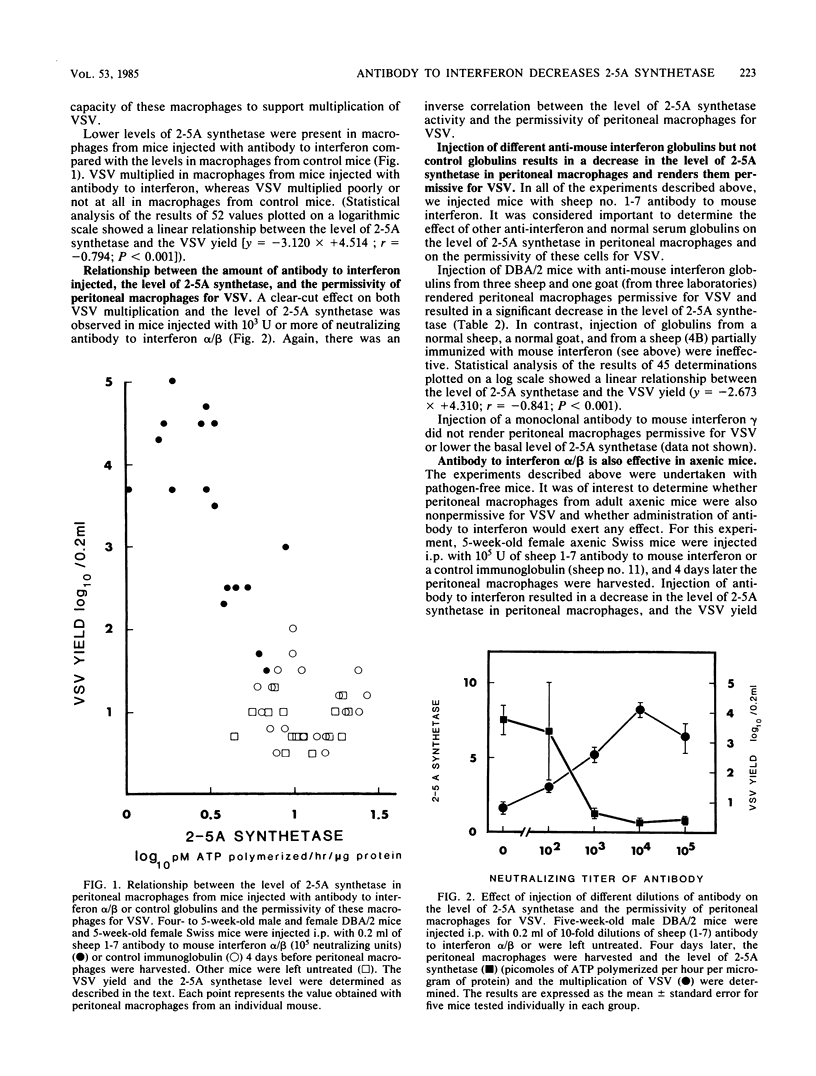
Injection of conventional or axenic weanling mice with potent sheep or goat antibody to mouse interferon alpha/beta resulted in a decrease in the basal level of 2-5A synthetase in resting peritoneal macrophages and rendered these cells permissive for vesicular stomatitis virus. There was a good inverse correlation between the level of 2-5A synthetase in peritoneal macrophages and the permissivity of these cells for vesicular stomatitis virus. The peritoneal macrophages of 1- and 2-week-old mice had low levels of 2-5A synthetase and were permissive for vesicular stomatitis virus, whereas at 3 weeks (and after) there was a marked increase in the level of 2-5A synthetase in peritoneal macrophages, and these cells were no longer permissive for vesicular stomatitis virus. We suggest that low levels of interferon alpha or beta or both are produced in normal mice, and that this interferon contributes to host defense by inducing and maintaining an antiviral state in some cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Nilsen T. W. Mechanisms of antiviral action of interferon. Interferon. 1983;5:23–42. [PubMed] [Google Scholar]
- Ball L. A., White C. N. Oligonucleotide inhibitor of protein synthesis made in extracts of interferon-treated chick embryo cells: comparison with the mouse low molecular weight inhibitor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1167–1171. doi: 10.1073/pnas.75.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardelli F., Vignaux F., Proietti E., Gresser I. Injection of mice with antibody to interferon renders peritoneal macrophages permissive for vesicular stomatitis virus and encephalomyocarditis virus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):602–606. doi: 10.1073/pnas.81.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffet-Janvresse C., Hovanessian A. G. Enzyme markers for the presence of circulating interferon: 2-5A synthetase in blood lymphocytes and protein kinase in platelet-rich plasma. Proc Soc Exp Biol Med. 1984 Feb;175(2):169–175. doi: 10.3181/00379727-175-41783. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J. Delayed hypersensitivity to Newcastle disease virus in high and low interferon-producing mice. J Immunol. 1983 May;130(5):2392–2396. [PubMed] [Google Scholar]
- Etienne-Smekens M., Vandenbussche P., Content J., Dumont J. E. (2'-5')Oligoadenylate in rat liver: modulation after partial hepatectomy. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4609–4613. doi: 10.1073/pnas.80.15.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Sen G. C., Dubois M. F., Ratner L., Slattery E., Lengyel P. Interferon action: two distinct pathways for inhibition of protein synthesis by double-stranded RNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5893–5897. doi: 10.1073/pnas.75.12.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bandu M. E., Maury C., Brouty-Boyé D. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. I. Rapid evolution of encephalomyocarditis virus infection. J Exp Med. 1976 Nov 2;144(5):1305–1315. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Brown R. E., Kerr I. M. Synthesis of low molecular weight inhibitor of protein synthesis with enzyme from interferon-treated cells. Nature. 1977 Aug 11;268(5620):537–540. doi: 10.1038/268537a0. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Hovanessian A. G. Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature. 1977 Aug 11;268(5620):540–542. doi: 10.1038/268540a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Hochman P. S., Haller O., Shearer G. M., Wigzell H., Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur J Immunol. 1977 Sep;7(9):655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- Kimchi A. Increased levels of interferon-induced (2'--5') oligo-isoadenylate synthetase in mature T-lymphocytes and in differentiated Friend-erythroleukemic cells. J Interferon Res. 1981;1(4):559–569. doi: 10.1089/jir.1981.1.559. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Anti-mitogenic function of interferon-induced (2'-5')oligo(adenylate) and growth-related variations in enzymes that synthesize and degrade this oligonucleotide. Eur J Biochem. 1981;114(1):5–10. doi: 10.1111/j.1432-1033.1981.tb06163.x. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Regulation of lymphocyte mitogenesis by (2'--5') oligo-isoadenylate. Nature. 1979 Dec 20;282(5741):849–851. doi: 10.1038/282849a0. [DOI] [PubMed] [Google Scholar]
- Krishnan I., Baglioni C. 2'5' oligo(A) polymerase activity in serum of mice infected with EMC virus or treated with interferon. Nature. 1980 Jun 12;285(5765):485–488. doi: 10.1038/285485a0. [DOI] [PubMed] [Google Scholar]
- Krust B., Rivière Y., Hovanessian A. G. p67K kinase in different tissues and plasma of control and interferon-treated mice. Virology. 1982 Jul 15;120(1):240–246. doi: 10.1016/0042-6822(82)90022-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meurs E., Hovanessian A. G., Montagnier L. Interferon-mediated antiviral state in human MRC5 cells in the absence of detectable levels of 2-5A synthetase and protein kinase. J Interferon Res. 1981 Feb;1(2):219–232. doi: 10.1089/jir.1981.1.219. [DOI] [PubMed] [Google Scholar]
- Mogensen K. E., Cantell K. Non-specific adsorption of interferon to immobilized serum immunoglobulin. J Gen Virol. 1979 Oct;45(1):171–175. doi: 10.1099/0022-1317-45-1-171. [DOI] [PubMed] [Google Scholar]
- Penn L. J., Williams B. R. Interferon-induced 2-5A synthetase activity in human peripheral blood mononuclear cells after immunization with influenza virus and rubella virus vaccines. J Virol. 1984 Mar;49(3):748–753. doi: 10.1128/jvi.49.3.748-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Rothko K., Klippel J. H., Friedman R. M., Johnston M. I. Interferon-induced 2'-5' adenylate synthetase in vivo and interferon production in vitro by lymphocytes from systemic lupus erythematosus patients with and without circulating interferon. J Exp Med. 1983 Jun 1;157(6):2140–2146. doi: 10.1084/jem.157.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Wiegand R. C., Farrell P. J., Sen G. C., Cabrer B., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978 Apr 14;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Saron M. F., Riviere Y., Hovanessian A. G., Guillon J. C. Chronic production of interferon in carrier mice congenitally infected with lymphocytic choriomeningitis virus. Virology. 1982 Feb;117(1):253–256. doi: 10.1016/0042-6822(82)90524-4. [DOI] [PubMed] [Google Scholar]
- Schattner A., Merlin G., Shapira A., Revel M., Wallach D. Comparison of (2'-5') oligo-adenylate synthetase and interferon blood-levels in mice early after viral infection. J Interferon Res. 1982;2(2):285–289. doi: 10.1089/jir.1982.2.285. [DOI] [PubMed] [Google Scholar]
- Schattner A., Wallach D., Merlin G., Hahn T., Levin S., Revel M. Assay of an interferon-induced enzyme in white blood cells as a diagnostic aid in viral diseases. Lancet. 1981 Sep 5;2(8245):497–500. doi: 10.1016/s0140-6736(81)90883-7. [DOI] [PubMed] [Google Scholar]
- Sen G. C. Mechanism of interferon action: progress toward its understanding. Prog Nucleic Acid Res Mol Biol. 1982;27:105–156. doi: 10.1016/s0079-6603(08)60599-1. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Sokawa Y. 2',5'-Oligoadenylate synthetase activity in lymphocytes from normal mouse. J Biol Chem. 1979 Dec 10;254(23):12034–12037. [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey M. G., Begon-Lours J., Gresser I. A method for the large scale production of potent interferon preparations. Proc Soc Exp Biol Med. 1974 Jul;146(3):809–815. doi: 10.3181/00379727-146-38196. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Dron M., Mogensen K. E., Lebleu B., Mechti N., Begonlours-Guymarho J. Isolation of Daudi cells with reduced sensitivity to interferon. II. On the mechanisms of resistance. J Gen Virol. 1983 Dec;64(Pt 12):2649–2653. doi: 10.1099/0022-1317-64-12-2649. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Kimchi A., Schmidt A., Revel M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4734–4738. doi: 10.1073/pnas.75.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]


