Abstract
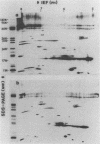
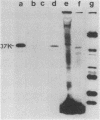
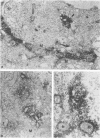
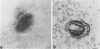
A 37,000-dalton polypeptide (p37K) present on purified extracellular vaccinia virus but absent from intracellular virus particles of classical morphology (G. Hiller et al., J. Virol. 39:903-913, 1981; L. G. Payne, J. Virol. 27:28-37, 1978) was further characterized. The polypeptide was only expressed in infected cells after onset of viral DNA replication. Phase partition experiments showed that it is relatively hydrophobic. Although p37K apparently is not a glycoprotein, in vivo radioisotope labeling detected tightly associated palmitic acid. Antibodies to p37K were used to monitor its distribution within infected cells at the light and electron microscopic levels. After synthesis p37K first accumulated in the Golgi region due to a tight membrane association. During progressing infection p37K-carrying membranes were used to form double-walled envelopes around brick-shaped vaccinia particles. Within these specialized vesicles vaccinia particles were moved through the cytoplasm toward the cell's surface, presumably along cellular routes for certain secretory products. Finally, single enveloped viruses were released into the extracellular space by an exocytotic process.
Full text
PDF



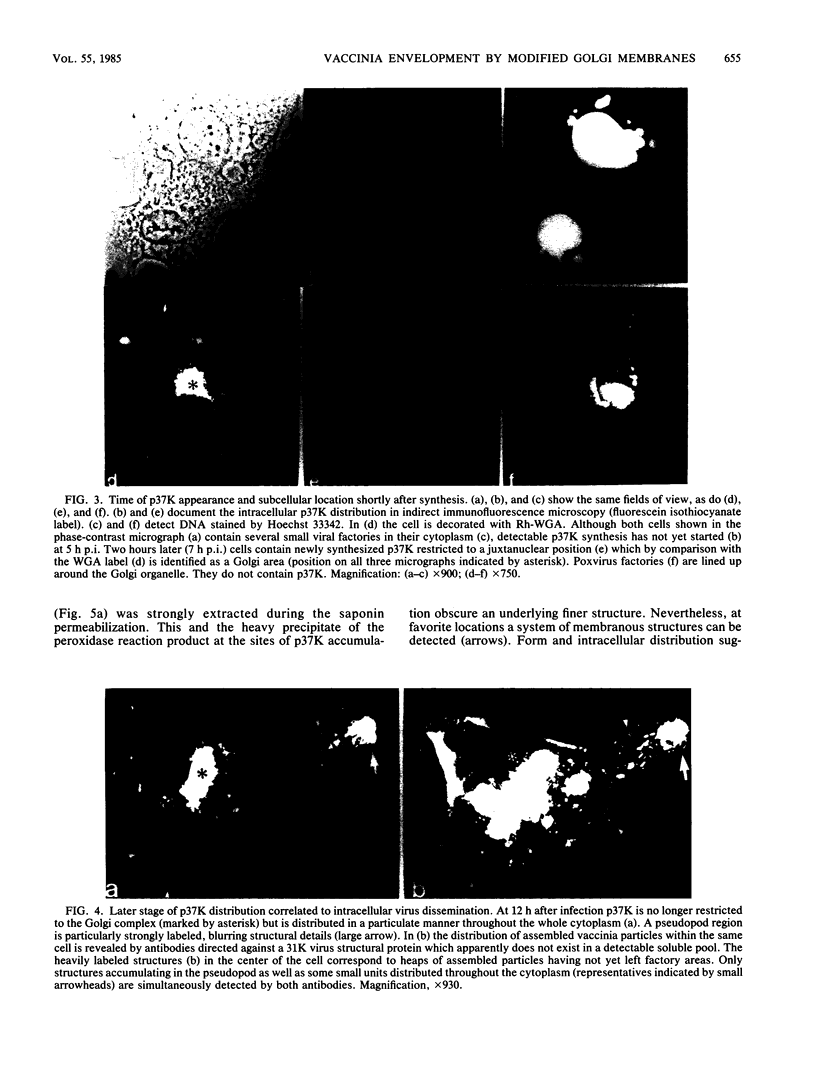




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- GAYLORD W. H., Jr, MELNICK J. L. Intracellular forms of pox viruses as shown by the electron microscope (Vaccinia, Ectromelia, Molluscum Contagiosum). J Exp Med. 1953 Aug;98(2):157–172. doi: 10.1084/jem.98.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Moss B. Glycoprotein synthesis in cells infected with vaccinia virus. II. A glycoprotein component of the virion. Virology. 1971 Nov;46(2):233–246. doi: 10.1016/0042-6822(71)90026-2. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Brands R., Burke B., Louvard D., Warren G. Viral membrane proteins acquire galactose in trans Golgi cisternae during intracellular transport. J Cell Biol. 1982 Dec;95(3):781–792. doi: 10.1083/jcb.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford C. G., Hamlin A., Rieders E. Electron microscopic autoradiography of DNA synthesis in cells infected with vaccinia virus. Exp Cell Res. 1966 Apr;42(1):50–57. doi: 10.1016/0014-4827(66)90318-1. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Hiller G., Eibl H., Weber K. Characterization of intracellular and extracellular vaccinia virus variants: N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine interferes with cytoplasmic virus dissemination and release. J Virol. 1981 Sep;39(3):903–913. doi: 10.1128/jvi.39.3.903-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller G., Weber K. A phosphorylated basic vaccinia virion polypeptide of molecular weight 11,000 is exposed on the surface of mature particles and interacts with actin-containing cytoskeletal elements. J Virol. 1982 Nov;44(2):647–657. doi: 10.1128/jvi.44.2.647-657.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller G., Weber K. Golgi detection in mitotic and interphase cells by antibodies to secreted galactosyltransferase. Exp Cell Res. 1982 Nov;142(1):85–94. doi: 10.1016/0014-4827(82)90412-8. [DOI] [PubMed] [Google Scholar]
- Hiller G., Weber K., Schneider L., Parajsz C., Jungwirth C. Interaction of assembled progeny pox viruses with the cellular cytoskeleton. Virology. 1979 Oct 15;98(1):142–153. doi: 10.1016/0042-6822(79)90533-6. [DOI] [PubMed] [Google Scholar]
- Holowczak J. A. Glycopeptides of vaccinia virus. I. Preliminary characterization and hexosamine content. Virology. 1970 Sep;42(1):87–99. doi: 10.1016/0042-6822(70)90241-2. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- Kato N., Eggers H. J., Rolly H. Inhibition of release of vaccinia virus by N1-isonicotinoly-N2-3-methyl-4-chlorobenzoylhydrazine. J Exp Med. 1969 Apr 1;129(4):795–808. doi: 10.1084/jem.129.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses observed in the electron microscope. II. Vaccinia and fowl pox viruses. J Exp Med. 1954 Sep 1;100(3):301–310. doi: 10.1084/jem.100.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976 Aug;73(1):43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Payne L. G. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J Virol. 1979 Jul;31(1):147–155. doi: 10.1128/jvi.31.1.147-155.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Kristenson K. Mechanism of vaccinia virus release and its specific inhibition by N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine. J Virol. 1979 Nov;32(2):614–622. doi: 10.1128/jvi.32.2.614-622.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Kristensson K. Effect of glycosylation inhibitors on the release of enveloped vaccinia virus. J Virol. 1982 Feb;41(2):367–375. doi: 10.1128/jvi.41.2.367-375.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Presence of haemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976 Jul;32(1):63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- Payne L. G. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980 Sep;50(1):89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- Payne L. Polypeptide composition of extracellular enveloped vaccinia virus. J Virol. 1978 Jul;27(1):28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov I., Joklik W. K. Studies on the nature and location of the capsid polypeptides of vaccinia virions. Virology. 1972 Nov;50(2):579–592. doi: 10.1016/0042-6822(72)90409-6. [DOI] [PubMed] [Google Scholar]
- Shida H., Dales S. Biogenesis of vaccinia: carbohydrate of the hemagglutinin molecules. Virology. 1981 May;111(1):56–72. doi: 10.1016/0042-6822(81)90653-x. [DOI] [PubMed] [Google Scholar]
- Simons K., Garoff H. The budding mechanisms of enveloped animal viruses. J Gen Virol. 1980 Sep;50(1):1–21. doi: 10.1099/0022-1317-50-1-1. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Ekblom P., Laurila P. Subcellular compartmentalization of saccharide moieties in cultured normal and malignant cells. J Cell Biol. 1980 May;85(2):429–434. doi: 10.1083/jcb.85.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Bibring T., Osborn M. Specific visualization of tubulin-containing structures in tissue culture cells by immunofluorescence. Cytoplasmic microtubules, vinblastine-induced paracrystals, and mitotic figures. Exp Cell Res. 1975 Oct 1;95(1):111–120. doi: 10.1016/0014-4827(75)90615-1. [DOI] [PubMed] [Google Scholar]
- Weintraub S., Stern W., Dales S. Biogenesis of vaccinia. Effects of inhibitors of glycosylation on virus-mediated activities. Virology. 1977 May 1;78(1):315–322. doi: 10.1016/0042-6822(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]