Abstract
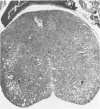
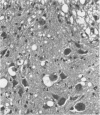
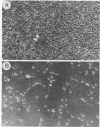
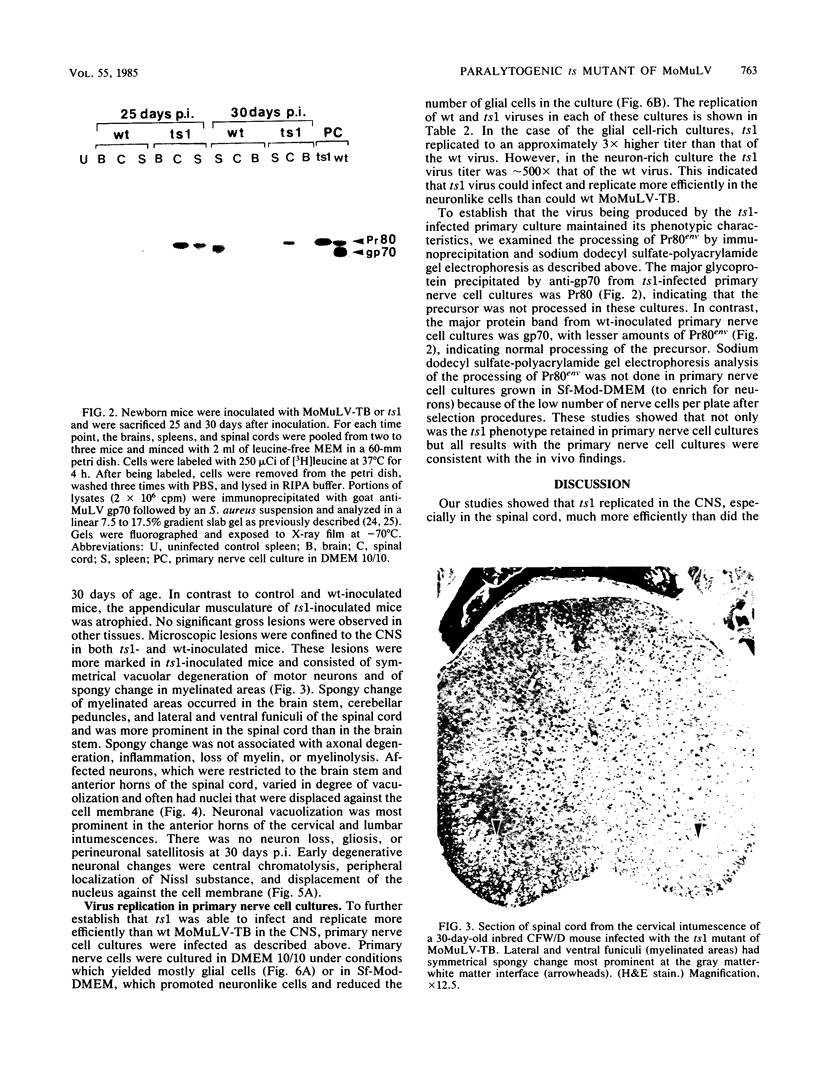
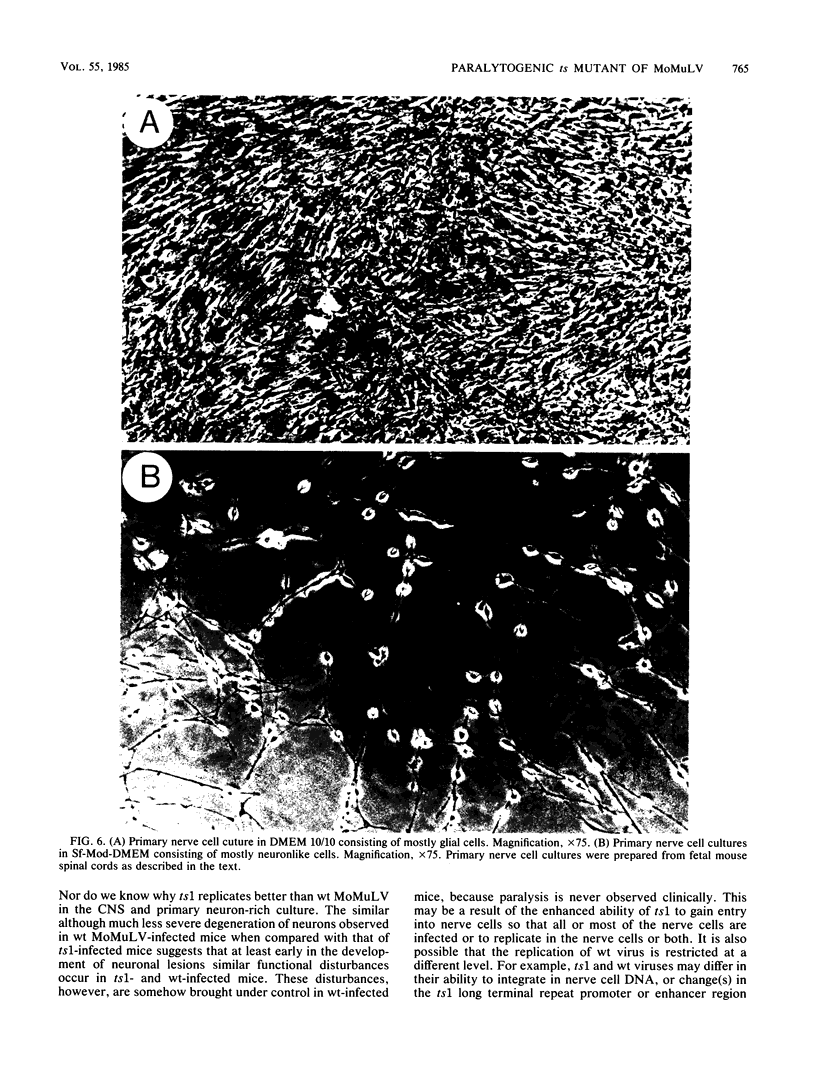
A temperature-sensitive mutant of Moloney murine leukemia virus TB (MoMuLV-TB), ts1, which is defective in intracellular processing of envelope precursor protein (Pr80env), also possesses the ability to induce hind-limb paralysis in infected mice. To investigate whether ts1 has acquired neurotropism and to determine to what extent it can replicate in the central nervous system, we compared viral titers in the spleen, plasma, spinal cord, and brain throughout the course of infection of mice infected with ts1 and parental wild-type (wt) MoMuLV-TB. In both the ts1- and wt-inoculated mice, the concentrations of infectious virus recovered from the plasma and spleen increased rapidly and reached a plateau by 10 days postinfection (p.i.). In contrast, virus concentrations in the spinal cord and brain of ts1-inoculated mice increased gradually and reached a titer comparable to that in the spleen and exceeding that in the plasma only at 25 to 30 days p.i. At this time, the virus titer was approximately 200X greater in ts1-infected spinal cord tissue and approximately 20X greater in ts1-infected brain tissue than in the same wt-infected tissues. Paralysis became evident at 25 to 30 days p.i. in ts1-inoculated mice, whereas the wt-inoculated mice were normal. In addition, a substantial amount of Pr80env was detected in the spinal cords of ts1-inoculated mice compared with that found in the spinal cords of wt-inoculated mice. The infectious virus isolated from ts1-infected nerve tissue was found to possess the characteristic phenotype of the ts1 virus. Microscopic lesions of ts1-inoculated mice at 30 days p.i. consisted of vacuolar degeneration of motor neurons and spongy change of white matter in the brain stem and spinal cord. Similar but less severe lesions were observed in wt-inoculated mice. With primary cultures of central nervous system tissue we showed that ts1 can infect and replicate in both neuron and glial cells. In contrast, although wt MoMuLV-TB replicated in glial cell-rich culture, viral replication was barely detectable in neuron-rich culture.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALL J. K., HUH T. Y., MCCARTER J. A. ON THE STATISTICAL DISTRIBUTION OF EPIDERMAL PAPILLOMATA IN MICE. Br J Cancer. 1964 Mar;18:120–123. doi: 10.1038/bjc.1964.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B. R., Swarz J. R., Johnson R. T. Spongiform polioencephalomyelopathy caused by a murine retrovirus. I. Pathogenesis of infection in newborn mice. Lab Invest. 1980 Nov;43(5):480–486. [PubMed] [Google Scholar]
- Gardner M. B. Retroviral spongiform polioencephalomyelopathy. Rev Infect Dis. 1985 Jan-Feb;7(1):99–110. doi: 10.1093/clinids/7.1.99. [DOI] [PubMed] [Google Scholar]
- Hooghe-Peters E., Dubois-Dalcq M., Schmechel D. Visna virus-induced fusion of nerve cells in vitro. Lab Invest. 1979 Sep;41(3):247–255. [PubMed] [Google Scholar]
- Kai K., Furuta T. Isolation of paralysis-inducing murine leukemia viruses from Friend virus passaged in rats. J Virol. 1984 Jun;50(3):970–973. doi: 10.1128/jvi.50.3.970-973.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. M., Barrett J. N. Serum factor supporting long-term survival of rat central neurons in culture. Science. 1983 Jun 24;220(4604):1394–1396. doi: 10.1126/science.6857258. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Gajdusek D. C., Gibbs C. J., Jr Subacute spongiform virus encephalopathies. Scrapie, Kuru and Creutzfeldt-Jakob disease: a review. Am J Pathol. 1972 Sep;68(3):626–652. [PMC free article] [PubMed] [Google Scholar]
- McCarter J. A., Ball J. K., Frei J. V. Lower limb paralysis induced in mice by a temperature-sensitive mutant of Moloney leukemia virus. J Natl Cancer Inst. 1977 Jul;59(1):179–183. doi: 10.1093/jnci/59.1.179. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Jensen F., Dixon F. J., Lampert P. W. Pathogenesis of the slow disease of the central nervous system associated with wild mouse virus. II. Role of virus and host gene products. Virology. 1980 Nov;107(1):180–193. doi: 10.1016/0042-6822(80)90283-4. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Jensen F., Elder J., Dixon F. J., Lampert P. W. Pathogenesis of the slow disease of the central nervous system associated with wild mouse virus. III. Role of input virus and MCF recombinants in disease. Virology. 1983 Jul 15;128(1):154–165. doi: 10.1016/0042-6822(83)90326-4. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Lampert P. W., Lee S., Dixon F. J. Pathogenesis of the slow disease of the central nervous system associated with WM 1504 E virus. I. Relationship of strain susceptibility and replication to disease. Am J Pathol. 1977 Jul;88(1):193–212. [PMC free article] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Skaper S. D., Adler R., Varon S. A procedure for purifying neuron-like cells in cultures from central nervous tissue with a defined medium. Dev Neurosci. 1979;2(5):233–237. doi: 10.1159/000112485. [DOI] [PubMed] [Google Scholar]
- Soong M. M., Yuen P. H., Wong P. K. Isolation and characterization of a Mo-MuSV-transformed TB cell line that produces noninfectious MuSV particles with uncleaved gag protein which is processed in the presence of Mo-MuLV. Virology. 1984 Jan 30;132(2):390–400. doi: 10.1016/0042-6822(84)90044-8. [DOI] [PubMed] [Google Scholar]
- Swarz J. R., Brooks B. R., Johnson R. T. Spongiform polioencephalomyelopathy caused by a murine retrovirus. II. Ultrastructural localization of virus replication and spongiform changes in the central nervous system. Neuropathol Appl Neurobiol. 1981 Sep-Oct;7(5):365–380. doi: 10.1111/j.1365-2990.1981.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Russ L. J., McCarter J. A. Rapid, selective procedure for isolation of spontaneous temperature-sensitive mutants of Moloney leukemia virus. Virology. 1973 Feb;51(2):424–431. doi: 10.1016/0042-6822(73)90441-8. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Soong M. M., MacLeod R., Gallick G. E., Yuen P. H. A group of temperature-sensitive mutants of Moloney leukemia virus which is defective in cleavage of env precursor polypeptide in infected cells also induces hind-limb paralysis in newborn CFW/D mice. Virology. 1983 Mar;125(2):513–518. doi: 10.1016/0042-6822(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Soong M. M., Yuen P. H. Replication of murine leukemia virus in heterologous cells: interaction between ecotropic and xenotropic viruses. Virology. 1981 Mar;109(2):366–378. doi: 10.1016/0042-6822(81)90507-9. [DOI] [PubMed] [Google Scholar]
- Yuen P. H., Malehorn D., Knupp C., Wong P. K. A 1.6-kilobase-pair fragment in the genome of the ts1 mutant of Moloney murine leukemia virus TB that is associated with temperature sensitivity, nonprocessing of Pr80env, and paralytogenesis. J Virol. 1985 May;54(2):364–373. doi: 10.1128/jvi.54.2.364-373.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen P. H., Malehorn D., Nau C., Soong M. M., Wong P. K. Molecular cloning of two paralytogenic, temperature-sensitive mutants, ts1 and ts7, and the parental wild-type Moloney murine leukemia virus. J Virol. 1985 Apr;54(1):178–185. doi: 10.1128/jvi.54.1.178-185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen P. H., Soong M. M., Kissil M. S., Wong P. K. Restriction of Moloney murine leukemia virus replication in Moloney murine sarcoma virus-infected cells. Virology. 1984 Jan 30;132(2):377–389. doi: 10.1016/0042-6822(84)90043-6. [DOI] [PubMed] [Google Scholar]