Abstract
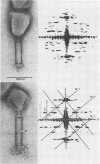
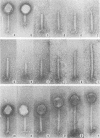
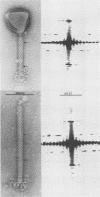
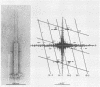
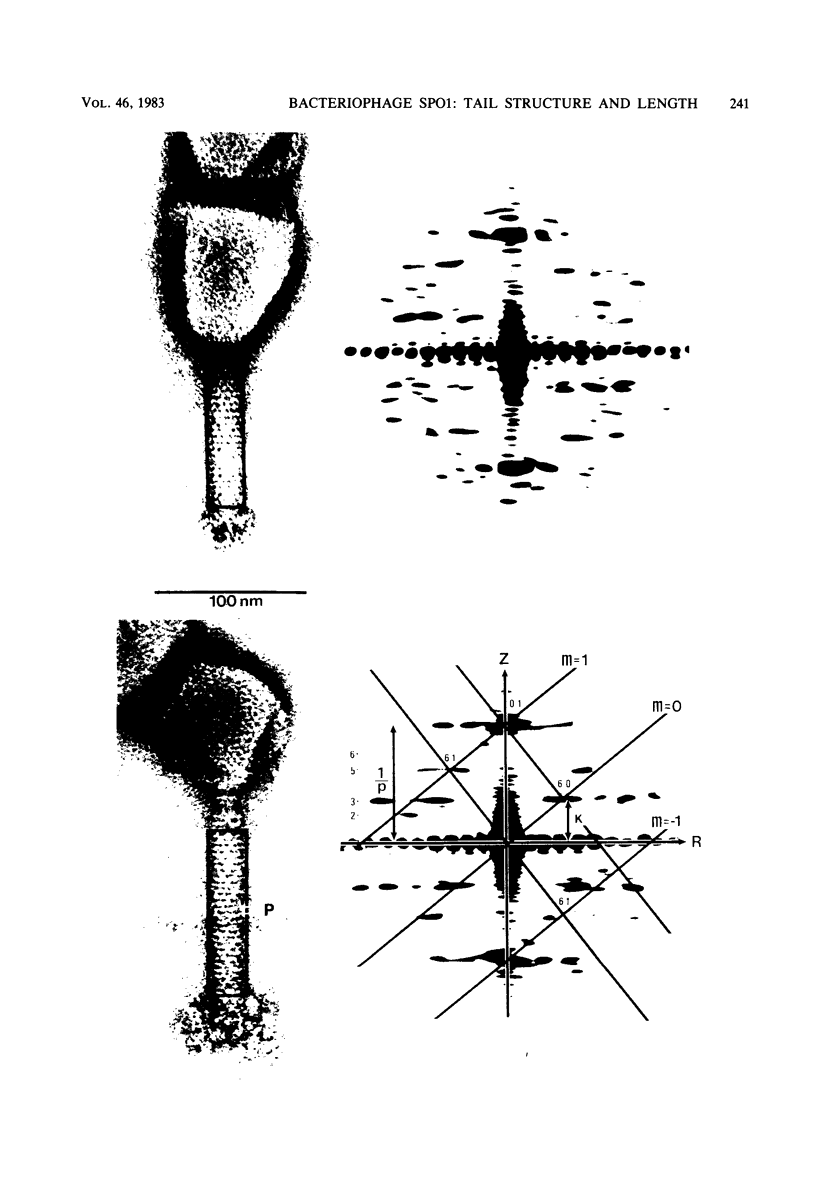
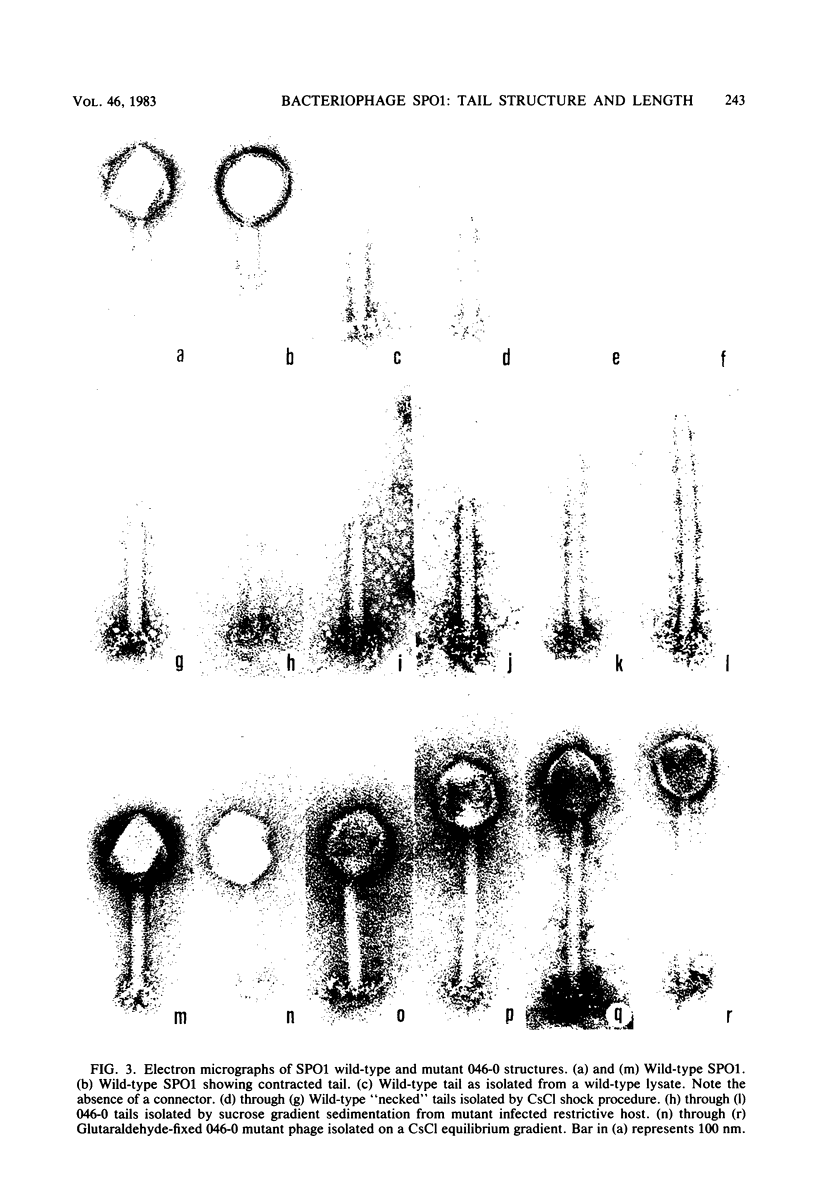
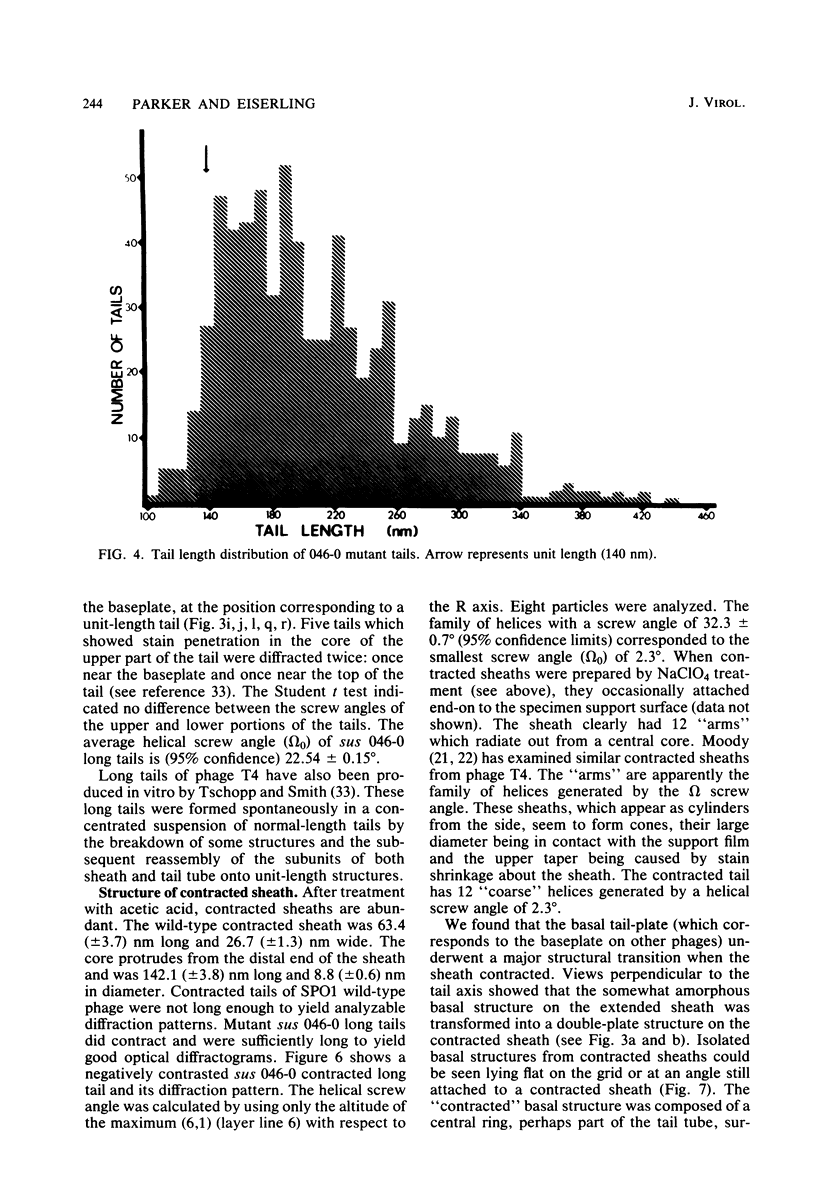
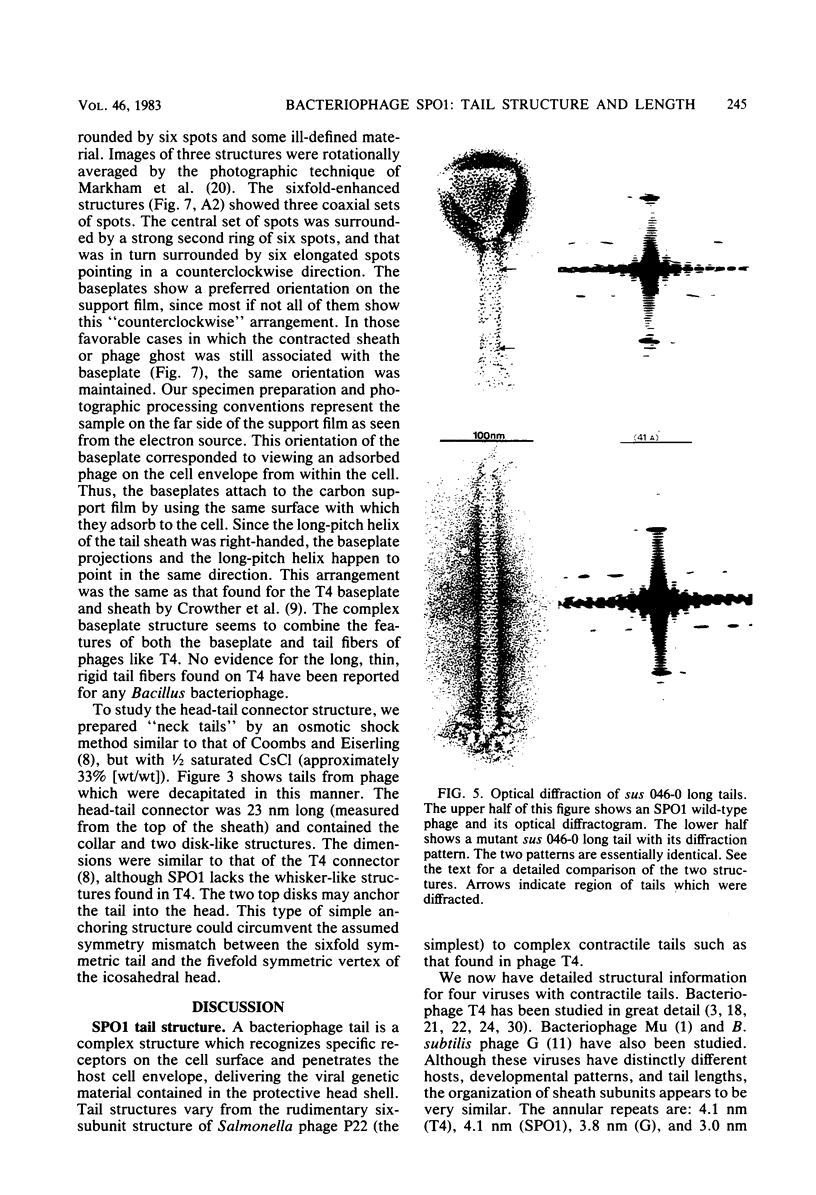
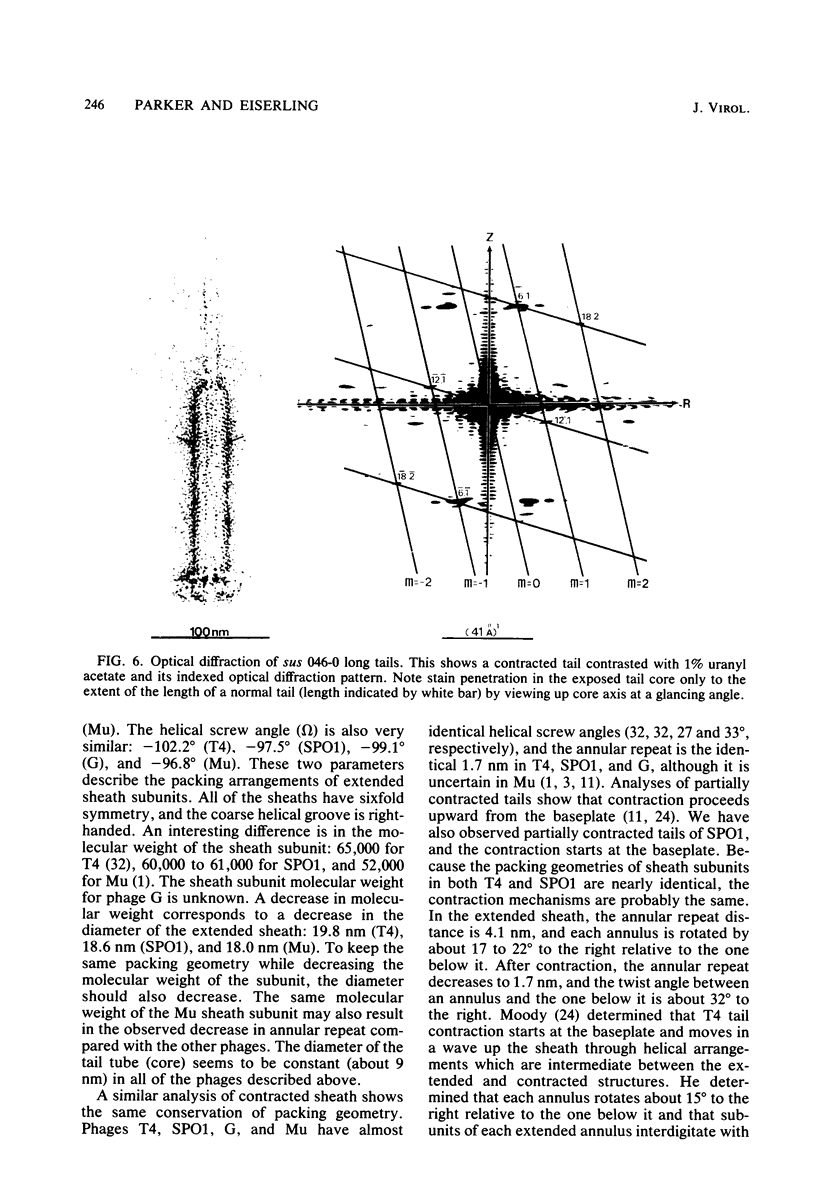
Bacteriophage SPO1, a structually complex phage with hydroxymethyl uracil replacing thymine, has been studied by structural and chemical methods with the aim of defining the virion organization. The contractile tail of SPO1 consists of a complex baseplate, a tail tube, and a 140-nm-long sheath composed of stacked disks (4.1 nm repeat), each containing six subunits of molecular weight 60,300. The subunits are arranged in six parallel helices, each with a helical screw angle (omega 0) of 22.5 degrees. The baseplate was shown to undergo a structural rearrangement during tail contraction into a hexameric pinwheel. A mutation in gene 8 which produced unattached heads and tails also produced tails of different lengths. The tail length distribution suggests that the smallest integral length increment is a single disk of subunits. The structural arrangement of subunits in long tails is identical to that of normal tails, and the tails can contract. Many of the long tails showed partial stain penetration within the tail tube to a point which coincides with the top of a unit-length tail. The implications of these findings with respect to tail length regulation are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admiraal G., Mellema J. E. The structure of the contractile sheath of bacteriophage Mu. J Ultrastruct Res. 1976 Jul;56(1):48–64. doi: 10.1016/s0022-5320(76)80140-2. [DOI] [PubMed] [Google Scholar]
- Amos L. A., Klug A. Three-dimensional image reconstructions of the contractile tail of T4 bacteriophage. J Mol Biol. 1975 Nov 25;99(1):51–64. doi: 10.1016/s0022-2836(75)80158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Bacteriophage T2 as seen with the freeze-etching technique. Virology. 1970 Mar;40(3):703–718. doi: 10.1016/0042-6822(70)90215-1. [DOI] [PubMed] [Google Scholar]
- Benz W. C., Goldberg E. B. Interactions between modified phage T4 particles and spheroplasts. Virology. 1973 May;53(1):225–235. doi: 10.1016/0042-6822(73)90481-9. [DOI] [PubMed] [Google Scholar]
- Bijlenga R. K., Aebi U., Kellenberger E. Properties and structure of a gene 24-controlled T4 giant phage. J Mol Biol. 1976 May 25;103(3):469–498. doi: 10.1016/0022-2836(76)90213-8. [DOI] [PubMed] [Google Scholar]
- Coombs D. H. Density gradient fractionation by piston displacement. Anal Biochem. 1975 Sep;68(1):95–101. doi: 10.1016/0003-2697(75)90683-1. [DOI] [PubMed] [Google Scholar]
- Coombs D. H., Eiserling F. A. Studies on the structure, protein composition and aseembly of the neck of bacteriophage T4. J Mol Biol. 1977 Nov 5;116(3):375–405. doi: 10.1016/0022-2836(77)90076-6. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Lenk E. V., Kikuchi Y., King J. Molecular reorganization in the hexagon to star transition of the baseplate of bacteriophage T4. J Mol Biol. 1977 Nov 5;116(3):489–523. doi: 10.1016/0022-2836(77)90081-x. [DOI] [PubMed] [Google Scholar]
- DAVISON P. F. THE STRUCTURE OF BACTERIOPHAGE SP8. Virology. 1963 Oct;21:146–151. doi: 10.1016/0042-6822(63)90250-2. [DOI] [PubMed] [Google Scholar]
- Donelli G., Guglielmi F., Paoletti L. Structure and physico-chemical properties of bacteriophage G. I. Arrangement of protein subunits and contraction process of tail sheath. J Mol Biol. 1972 Nov 14;71(2):113–125. doi: 10.1016/0022-2836(72)90341-5. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura I. Morphogenesis of bacteriophage lambda tail. Polymorphism in the assembly of the major tail protein. J Mol Biol. 1976 Nov 5;107(3):307–326. doi: 10.1016/s0022-2836(76)80007-1. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- Krimm S., Anderson T. F. Structure of normal and contracted tail sheaths of T4 bacteriophage. J Mol Biol. 1967 Jul 28;27(2):197–202. doi: 10.1016/0022-2836(67)90015-0. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Moody M. F., Makowski L. X-ray diffraction study of tail-tubes from bacteriophage T2L. J Mol Biol. 1981 Aug 5;150(2):217–244. doi: 10.1016/0022-2836(81)90450-2. [DOI] [PubMed] [Google Scholar]
- Moody M. F. Sheath of bacteriophage T4. 3. Contraction mechanism deduced from partially contracted sheaths. J Mol Biol. 1973 Nov 15;80(4):613–635. doi: 10.1016/0022-2836(73)90200-3. [DOI] [PubMed] [Google Scholar]
- Moody M. F. Structure of the sheath of bacteriophage T4. I. Structure of the contracted sheath and polysheath. J Mol Biol. 1967 Apr 28;25(2):167–200. doi: 10.1016/0022-2836(67)90136-2. [DOI] [PubMed] [Google Scholar]
- Moody M. F. Structure of the sheath of bacteriophage T4. II. Rearrangement of the sheath subunits during contraction. J Mol Biol. 1967 Apr 28;25(2):201–208. doi: 10.1016/0022-2836(67)90137-4. [DOI] [PubMed] [Google Scholar]
- Okubo S., Yanagida T., Fujita D. J., Olsson-Wilhelm B. M. The genetics of bacteriophage SPO1. Biken J. 1972 Jun;15(2):81–97. [PubMed] [Google Scholar]
- Okubo S., Yanagida T. Isolation of a suppressor mutant in Bacillus subtilis. J Bacteriol. 1968 Mar;95(3):1187–1188. doi: 10.1128/jb.95.3.1187-1188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. II. Structure and function of the baseplate. Virology. 1967 Jun;32(2):298–305. doi: 10.1016/0042-6822(67)90278-4. [DOI] [PubMed] [Google Scholar]
- Smith P. R., Aebi U. Studies of the structure of the T4 bacteriophage tail sheath. I. The recovery of three-dimensional structural information from the extended sheath. J Mol Biol. 1976 Sep 15;106(2):243–271. doi: 10.1016/0022-2836(76)90083-8. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germination. VI. Origin of spore core and coat proteins. J Biol Chem. 1968 Sep 10;243(17):4588–4599. [PubMed] [Google Scholar]
- Tschopp J., Arisaka F., van Driel R., Engel J. Purification, characterization and reassembly of the bacteriophage T4D tail sheath protein P18. J Mol Biol. 1979 Feb 25;128(2):247–258. doi: 10.1016/0022-2836(79)90128-1. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Smith P. R. Extra-long bacteriophage T4 tails produced under in vitro conditions. J Mol Biol. 1977 Aug 5;114(2):281–286. doi: 10.1016/0022-2836(77)90211-x. [DOI] [PubMed] [Google Scholar]
- Venyaminov S. Y., Rodikova L. P., Metlina A. L., Poglazov B. F. Secondary structure change of bacteriophage T4 sheath protein during sheath contraction. J Mol Biol. 1975 Nov 15;98(4):657–664. doi: 10.1016/s0022-2836(75)80001-5. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Bloomfield V. A. In vitro polymerization of bacteriophage T4D tail core subunits. J Mol Biol. 1977 Nov 5;116(3):347–359. doi: 10.1016/0022-2836(77)90074-2. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Fisher H. W. Electron microscopy of tobacco mosaic virus under conditions of minimal beam exposure. J Mol Biol. 1970 Aug 28;52(1):121–123. doi: 10.1016/0022-2836(70)90181-6. [DOI] [PubMed] [Google Scholar]