Abstract
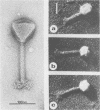
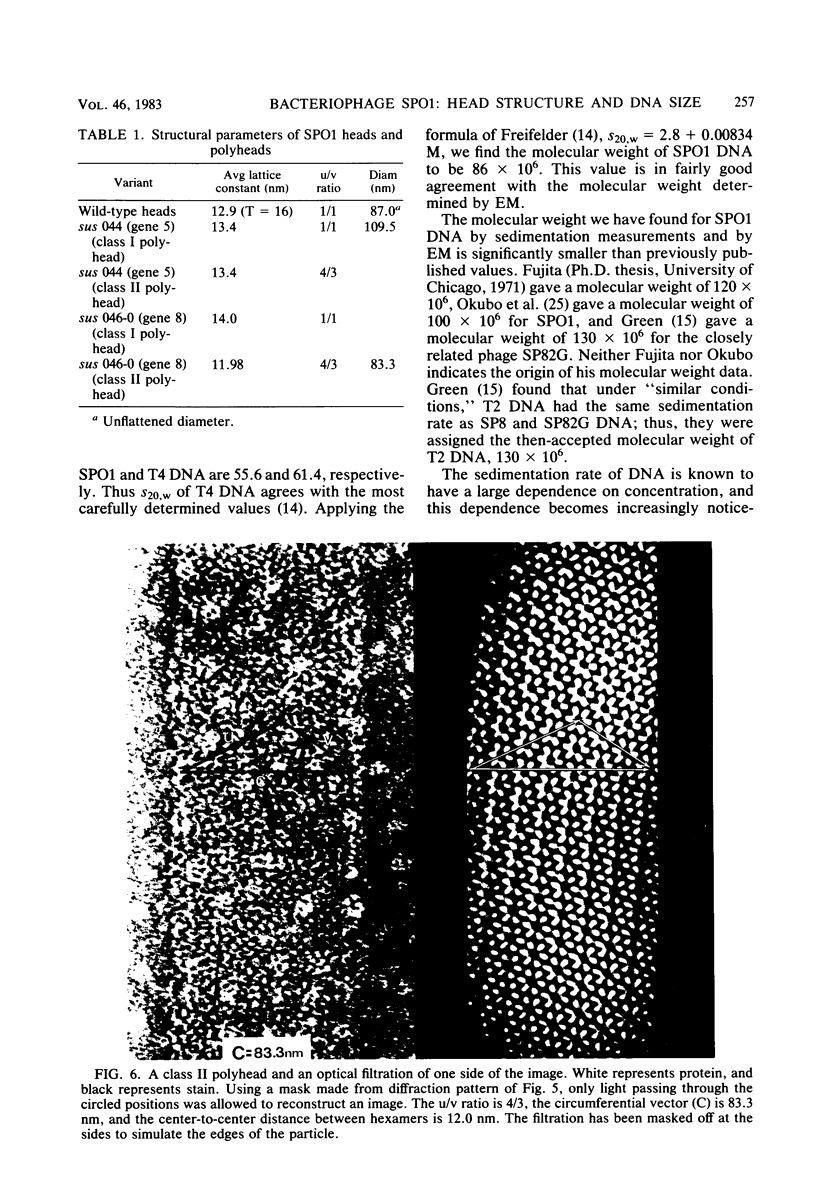
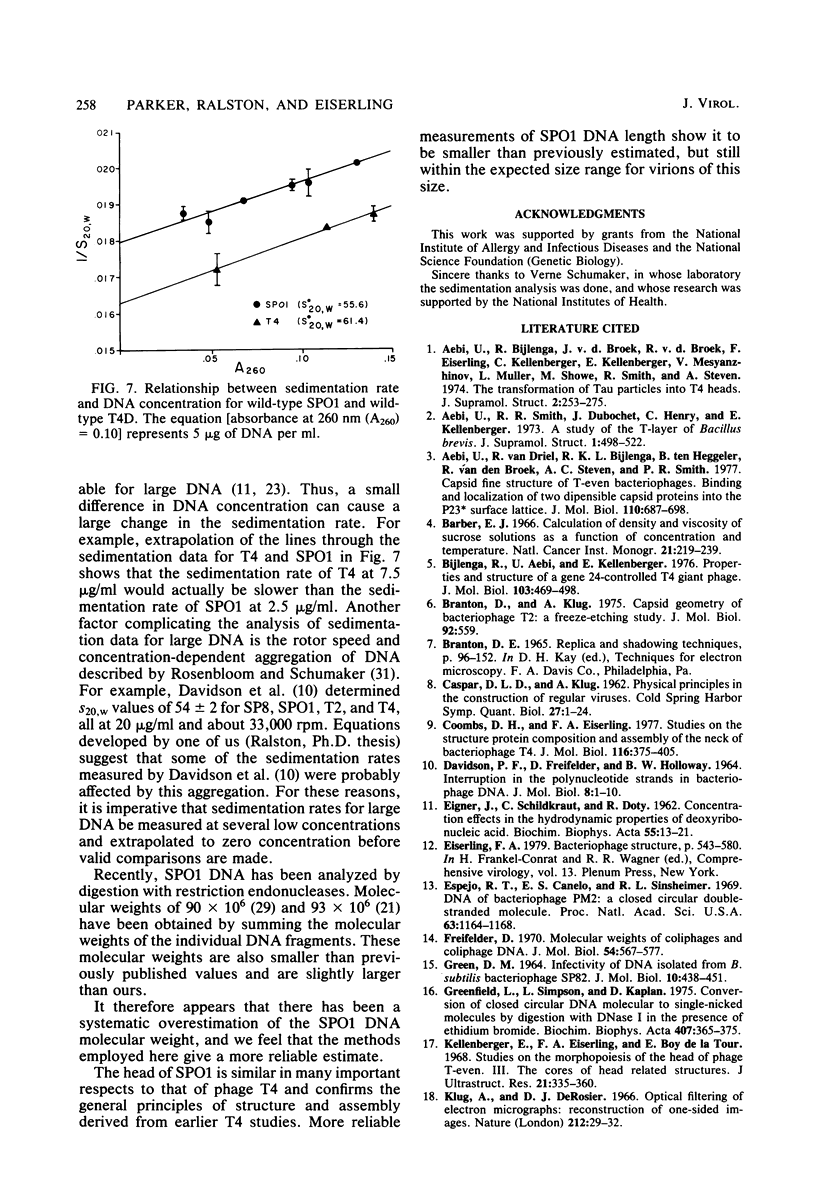
The capsid of bacteriophage SPO1 is icosahedral, and the subunit arrangement on the 87-nm-diameter head suggests the triangulation number T = 16. The major capsid protein (45,700 daltons) is cleaved from a 47,700-dalton precursor. Tubular heads (polyheads) are produced by mutations in genes 5 and 8 and contain cores as well as capped ends. The lattice constant of these structures is 13.4 nm; diameter is 109.5 nm. The size of the double-stranded SPO1 DNA (containing 5' hydroxymethyl uracil in place of thymine) was measured by sedimentation analysis and electron microscopy and has a molecular weight of 86 X 10(6) (about 140 kilobase pairs), which is smaller than several previously reported values.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Bijlenga R., v d Broek J., v d Broek H., Eiserling F., Kellenberger C., Kellenberger E., Mesyanzhinov V., Müller L., Showe M. The transformation of tau particles into T4 heads. II. Transformations of the surface lattice and related observations on form determination. J Supramol Struct. 1974;2(2-4):253–275. doi: 10.1002/jss.400020218. [DOI] [PubMed] [Google Scholar]
- Aebi U., Smith P. R., Dubochet J., Henry C., Kellenberger E. A study of the structure of the T-layer of Bacillus brevis. J Supramol Struct. 1973;1(6):498–522. doi: 10.1002/jss.400010606. [DOI] [PubMed] [Google Scholar]
- Aebi U., van Driel R., Bijlenga R. K., ten Heggeler B., van den Broek R., Steven A. C., Smith P. R. Capsid fine structure of T-even bacteriophages. Binding and localization of two dispensable capsid proteins into the P23* surface lattice. J Mol Biol. 1977 Mar 15;110(4):687–698. doi: 10.1016/s0022-2836(77)80084-3. [DOI] [PubMed] [Google Scholar]
- Barber E. J. Calculation of density and viscosity of sucrose solutions as a function of concentration and temperature. Natl Cancer Inst Monogr. 1966 Jun;21:219–239. [PubMed] [Google Scholar]
- Bijlenga R. K., Aebi U., Kellenberger E. Properties and structure of a gene 24-controlled T4 giant phage. J Mol Biol. 1976 May 25;103(3):469–498. doi: 10.1016/0022-2836(76)90213-8. [DOI] [PubMed] [Google Scholar]
- Branton D., Klug A. Capsid geometry of bacteriophage T2: a freeze-etching study. J Mol Biol. 1975 Mar 15;92(4):559–565. doi: 10.1016/0022-2836(75)90309-5. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Coombs D. H., Eiserling F. A. Studies on the structure, protein composition and aseembly of the neck of bacteriophage T4. J Mol Biol. 1977 Nov 5;116(3):375–405. doi: 10.1016/0022-2836(77)90076-6. [DOI] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D., HOLLOWAY B. W. INTERRUPTIONS IN THE POLYNUCLEOTIDE STRANDS IN BACTERIOPHAGE DNA. J Mol Biol. 1964 Jan;8:1–10. doi: 10.1016/s0022-2836(64)80142-x. [DOI] [PubMed] [Google Scholar]
- EIGNER J., SCHILDKRAUT C., DOTY P. Concentration effects in the hydrodynamic properties of deoxyribonucleic acid. Biochim Biophys Acta. 1962 Jan 22;55:13–21. doi: 10.1016/0006-3002(62)90926-5. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Greenfield L., Simpson L., Kaplan D. Conversion of closed circular DNA molecules to single-nicked molecules by digestion with DNAase I in the presence of ethidium bromide. Biochim Biophys Acta. 1975 Oct 15;407(3):365–375. doi: 10.1016/0005-2787(75)90104-5. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Eiserling F. A., Boy de la Tour E. Studies on the morphopoiesis of the head of phage T-even. 3. The cores of head-related structures. J Ultrastruct Res. 1967 Dec 12;21(3):335–360. doi: 10.1016/s0022-5320(67)80099-6. [DOI] [PubMed] [Google Scholar]
- Klug A., De Rosier D. J. Optical filtering of electron micrographs: reconstruction of one-sided images. Nature. 1966 Oct 1;212(5057):29–32. doi: 10.1038/212029a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Quittner S. F. Maturation of the head of bacteriophage T4. IV. The proteins of the core of the tubular polyheads and in vitro cleavage of the head proteins. Virology. 1974 Dec;62(2):483–499. doi: 10.1016/0042-6822(74)90409-7. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Lawrie J. M., Downard J. S., Whiteley H. R. Bacillus subtilis bacteriophages SP82, SPO1, and phie: a comparison of DNAs and of peptides synthesized during infection. J Virol. 1978 Sep;27(3):725–737. doi: 10.1128/jvi.27.3.725-737.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Salamin L., Onorato L., Showe M. K. Localization of minor protein components of the head of bacteriophage T4. J Virol. 1977 Oct;24(1):121–134. doi: 10.1128/jvi.24.1.121-134.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Okubo S., Yanagida T., Fujita D. J., Olsson-Wilhelm B. M. The genetics of bacteriophage SPO1. Biken J. 1972 Jun;15(2):81–97. [PubMed] [Google Scholar]
- Onorato L., Stirmer B., Showe M. K. Isolation and characterization of bacteriophage T4 mutant preheads. J Virol. 1978 Aug;27(2):409–426. doi: 10.1128/jvi.27.2.409-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. L., Eiserling F. A. Bacteriophage SPO1 structure and morphogenesis. I. Tail structure and length regulation. J Virol. 1983 Apr;46(1):239–249. doi: 10.1128/jvi.46.1.239-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. L., Eiserling F. A. Bacteriophage SPO1 structure and morphogenesis. III. SPO1 proteins and synthesis. J Virol. 1983 Apr;46(1):260–269. doi: 10.1128/jvi.46.1.260-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero J., Hannett N. M., Talkington C. Restriction cleavage map of SP01 DNA: general location of early, middle, and late genes. J Virol. 1979 Jul;31(1):156–171. doi: 10.1128/jvi.31.1.156-171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Mertens G., Amann E. Early development of bacteriophages SP01 and SP82G in minicells of Bacillus subtilis. J Mol Biol. 1978 Apr 5;120(2):183–207. doi: 10.1016/0022-2836(78)90064-5. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J., Schumaker V. Molecular weight dependence of the rotor speed induced aggregation of deoxyribonucleic acid. Biochemistry. 1967 Jan;6(1):276–283. doi: 10.1021/bi00853a043. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Aebi U., Showe M. K. Folding and capsomere morphology of the P23 surface shell of bacteriophage T4 polyheads from mutants in five different head genes. J Mol Biol. 1976 Apr 15;102(3):373–400. [PubMed] [Google Scholar]
- Steven A. C., Couture E., Aebi U., Showe M. K. Structure of T4 polyheads. II. A pathway of polyhead transformation as a model for T4 capsid maturation. J Mol Biol. 1976 Sep 5;106(1):187–221. doi: 10.1016/0022-2836(76)90307-7. [DOI] [PubMed] [Google Scholar]
- Tsui L., Hendrix R. W. Head-tail connector of bacteriophage lambda. J Mol Biol. 1980 Sep 25;142(3):419–438. doi: 10.1016/0022-2836(80)90280-6. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. C., SMITH K. M. The polyhedral form of the Tipula iridescent virus. Biochim Biophys Acta. 1958 Jun;28(3):464–469. doi: 10.1016/0006-3002(58)90507-9. [DOI] [PubMed] [Google Scholar]