Abstract
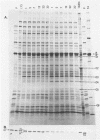
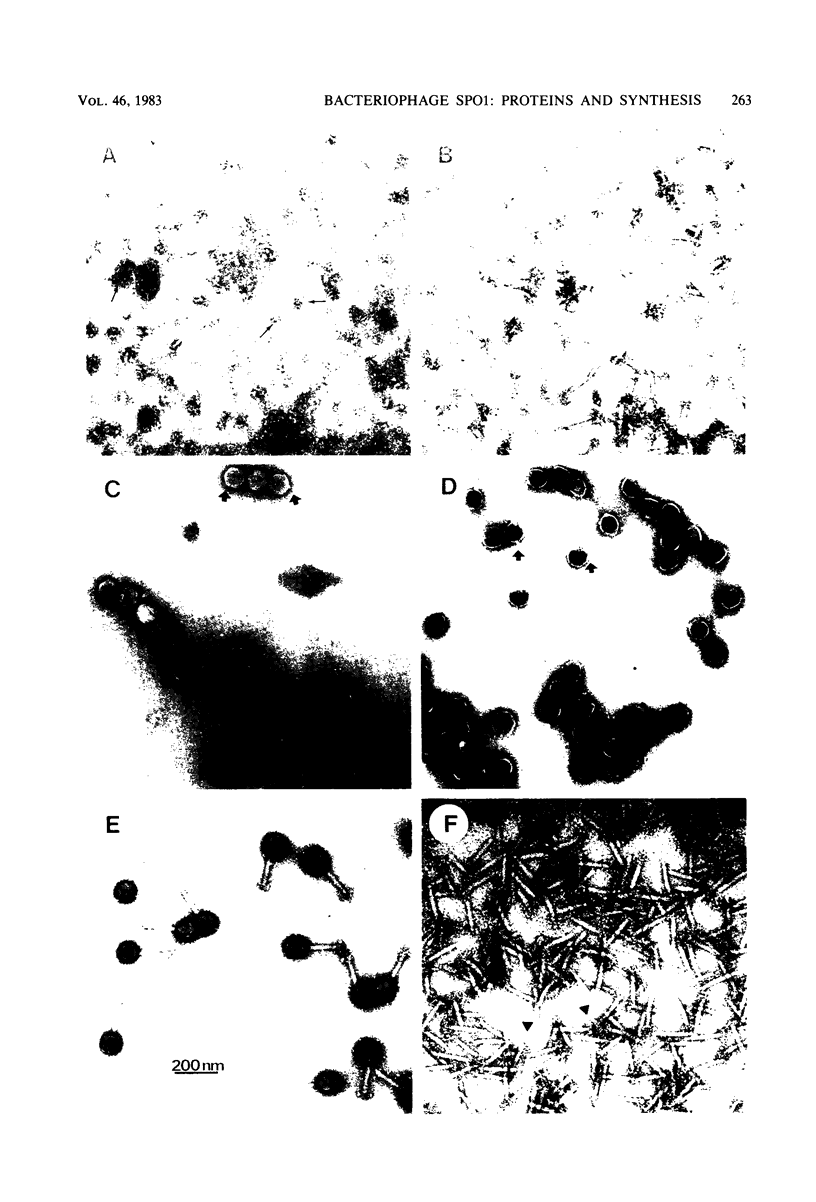
The virion proteins of SPO1 have been determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis methods on purified phage components and on phage lysates. The phage head contains 16 proteins, and the connector or neck structure has an additional 3 proteins not found in the head. The proximal part of the tail, composed of sheath, tube and connecting components, contains six proteins. The distal baseplate is the most complex structure, with 28 proteins identifiable on sodium dodecyl sulfate gels. The maximum number of proteins found in phage subassemblies is 53, which would account for nearly half the coding capacity of the SPO1 genome.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bijlenga R. K., Ishii T., Tsugita A. Complete primary structure of the small outer capsid (soc) protein of bacteriophage T4. J Mol Biol. 1978 Apr 5;120(2):249–263. doi: 10.1016/0022-2836(78)90066-9. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Eiserling F. A. T4 gene 40 mutants. II. Phenotypic properties. Virology. 1979 Aug;97(1):77–89. doi: 10.1016/0042-6822(79)90374-x. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs D. H., Eiserling F. A. Studies on the structure, protein composition and aseembly of the neck of bacteriophage T4. J Mol Biol. 1977 Nov 5;116(3):375–405. doi: 10.1016/0022-2836(77)90076-6. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Fujita D. J. Effect of nalidixic acid on deoxyribonucleic acid synthesis in bacteriophage SPO1-infected Bacillus subtilis. J Bacteriol. 1969 Apr;98(1):96–103. doi: 10.1128/jb.98.1.96-103.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriphage SPO1 development. II. Some modulations and prerequisites of the transcription program. Virology. 1971 Apr;44(1):200–210. doi: 10.1016/0042-6822(71)90165-6. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Protein cleavage in bacteriophage lambda tail assembly. Virology. 1974 Sep;61(1):156–159. doi: 10.1016/0042-6822(74)90250-5. [DOI] [PubMed] [Google Scholar]
- Hershko A., Fry M. Post-translational cleavage of polypeptide chains: role in assembly. Annu Rev Biochem. 1975;44:775–797. doi: 10.1146/annurev.bi.44.070175.004015. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Bacteriophage T4 mutants deficient in alteration and modification of the Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):739–750. doi: 10.1016/0022-2836(74)90537-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrie J. M., Downard J. S., Whiteley H. R. Bacillus subtilis bacteriophages SP82, SPO1, and phie: a comparison of DNAs and of peptides synthesized during infection. J Virol. 1978 Sep;27(3):725–737. doi: 10.1128/jvi.27.3.725-737.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel J. A., Goldstein R. N., Marsh M., Sunshine M. G., Calendar R. Bacteriophage P2 head morphogenesis: cleavage of the major capsid protein. Virology. 1973 May;53(1):1–23. doi: 10.1016/0042-6822(73)90461-3. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Siminovitch L. The morphogenesis of bacteriophage lambda. IV. Identification of gene products and control of the expression of the morphogenetic information. Virology. 1972 Jun;48(3):785–823. doi: 10.1016/0042-6822(72)90162-6. [DOI] [PubMed] [Google Scholar]
- Parker M. L., Eiserling F. A. Bacteriophage SPO1 structure and morphogenesis. I. Tail structure and length regulation. J Virol. 1983 Apr;46(1):239–249. doi: 10.1128/jvi.46.1.239-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero J., Hannett N. M., Talkington C. Restriction cleavage map of SP01 DNA: general location of early, middle, and late genes. J Virol. 1979 Jul;31(1):156–171. doi: 10.1128/jvi.31.1.156-171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock T. J., Tessman E. S., Tessman I. Suppression of polar effects of nonsense mutations by ultraviolet irradiation. J Bacteriol. 1979 Apr;138(1):122–125. doi: 10.1128/jb.138.1.122-125.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine F. M., Steinberg W. Bacillus subtilis bacteriophage SP01, SP82, and phi e require host lysyl- and tryptophanyl-tRNA synthetases for phage development. J Virol. 1974 Aug;14(2):402–406. doi: 10.1128/jvi.14.2.402-406.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Mertens G., Amann E. Early development of bacteriophages SP01 and SP82G in minicells of Bacillus subtilis. J Mol Biol. 1978 Apr 5;120(2):183–207. doi: 10.1016/0022-2836(78)90064-5. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Black L. W. Assembly core of bacteriophage T4: an intermediate in head formation. Nat New Biol. 1973 Mar 21;242(116):70–75. doi: 10.1038/newbio242070a0. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Isobe E., Onorato L. Bacteriophage T4 prehead proteinase. I. Purification and properties of a bacteriophage enzyme which cleaves the capsid precursor proteins. J Mol Biol. 1976 Oct 15;107(1):35–54. doi: 10.1016/s0022-2836(76)80016-2. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Aebi U., Showe M. K. Folding and capsomere morphology of the P23 surface shell of bacteriophage T4 polyheads from mutants in five different head genes. J Mol Biol. 1976 Apr 15;102(3):373–400. [PubMed] [Google Scholar]
- Summers D. F., Shaw E. N., Stewart M. L., Maizel J. V., Jr Inhibition of cleavage of large poliovirus-specific precursor proteins in infected HeLa cells by inhibitors of proteolytic enzymes. J Virol. 1972 Oct;10(4):880–884. doi: 10.1128/jvi.10.4.880-884.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M. E., Reilly B. E., Anderson D. L. Morphogenesis of bacteriophage phi29 of Bacillus subtilis: cleavage and assembly of the neck appendage protein. J Virol. 1975 Nov;16(5):1282–1295. doi: 10.1128/jvi.16.5.1282-1295.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Arisaka F., van Driel R., Engel J. Purification, characterization and reassembly of the bacteriophage T4D tail sheath protein P18. J Mol Biol. 1979 Feb 25;128(2):247–258. doi: 10.1016/0022-2836(79)90128-1. [DOI] [PubMed] [Google Scholar]
- Ward S., Dickson R. C. Assembly of bacteriophage T4 tail fibers. 3. Genetic control of the major tail fiber polypeptides. J Mol Biol. 1971 Dec 28;62(3):479–492. doi: 10.1016/0022-2836(71)90149-5. [DOI] [PubMed] [Google Scholar]
- Wilson D. L., Geiduschek E. P. A template-selective inhibitor of in vitro transcription. Proc Natl Acad Sci U S A. 1969 Feb;62(2):514–520. doi: 10.1073/pnas.62.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]
- van Driel R. Assembly of bacteriophage T4 head-related structures. Assembly of polyheads in vitro. J Mol Biol. 1977 Jul;114(1):61–72. doi: 10.1016/0022-2836(77)90283-2. [DOI] [PubMed] [Google Scholar]