Abstract
Nuclear pore complexes (NPCs) are large proteinaceous portals for exchanging macromolecules between the nucleus and the cytoplasm. Revealing how this transport apparatus is assembled will be critical for understanding the nuclear transport mechanism. To address this issue and to identify factors that regulate NPC formation and dynamics, a novel fluorescence-based strategy was used. This approach is based on the functional tagging of NPC proteins with the green fluorescent protein (GFP), and the hypothesis that NPC assembly mutants will have distinct GFP-NPC signals as compared with wild-type (wt) cells. By fluorescence-activated cell sorting for cells with low GFP signal from a population of mutagenized cells expressing GFP-Nup49p, three complementation groups were identified: two correspond to mutant nup120 and gle2 alleles that result in clusters of NPCs. Interestingly, a third group was a novel temperature-sensitive allele of nup57. The lowered GFP-Nup49p incorporation in the nup57-E17 cells resulted in a decreased fluorescence level, which was due in part to a sharply diminished interaction between the carboxy-terminal truncated nup57pE17 and wt Nup49p. Interestingly, the nup57-E17 mutant also affected the incorporation of a specific subset of other nucleoporins into the NPC. Decreased levels of NPC-associated Nsp1p and Nup116p were observed. In contrast, the localizations of Nic96p, Nup82p, Nup159p, Nup145p, and Pom152p were not markedly diminished. Coincidentally, nuclear import capacity was inhibited. Taken together, the identification of such mutants with specific perturbations of NPC structure validates this fluorescence-based strategy as a powerful approach for providing insight into the mechanism of NPC biogenesis.
INTRODUCTION
The exchange of macromolecules between the nuclear and cytoplasmic compartments is mediated by nuclear pore complexes (NPCs)1 embedded in ∼90-nm-diameter pores within the double lipid bilayer of the nuclear envelope (NE). Each NPC is a cylindrical structure with a superficial octagonal symmetry characterized by distinct substructures referred to as spokes, rings, a central plug, cytoplasmic fibrils, and a nuclear basket (Ris, 1991; Hinshaw et al., 1992; Akey and Radermacher, 1993; Akey, 1995; Goldberg and Allen, 1995; Pante and Aebi, 1996). NPCs are believed to be functionally similar in all eukaryotic organisms. Based on the polypeptide complexity of purified yeast complexes (Rout and Blobel, 1993), an NPC may be comprised of at least 50 distinct proteins [called nucleoporins (NUPs)]. To date, at least 25 nucleoporins have been identified in the yeast Saccharomyces cerevisiae with a comparatively smaller fraction identified in higher eukaryotes (reviewed by Corbett and Silver, 1997; Doye and Hurt 1997). While this knowledge of the physical composition of the NPC continues to grow, much less is known about the molecular basis of how nucleoporins are brought together and how this is coordinated with insertion of these macromolecular structures into the NE pore. A precise understanding of NPC transport function will require integrating the location and assembly interactions of nucleoporins into the context of NPC architecture.
Vertebrate cell-free systems have provided excellent models for studying NPC biogenesis (Lohka and Masui, 1983; Miake-Lye and Kirschner, 1985; Burke and Gerace, 1986; Suprynowicz and Gerace, 1986; Newport 1987). NPC assembly and disassembly can be reconstituted in vitro from vertebrate cell extracts, and a general framework for the stages of NPC assembly has been revealed. NPC assembly requires the prior formation of a double nuclear membrane (Macaulay and Forbes, 1996) and is inhibited by the addition of GTPγS, BAPTA (a chelator of Ca++ and Zn++), or wheat germ agglutinin (Newmeyer and Forbes, 1990; Pfaller et al., 1991; Newport and Dunphy, 1992; Boman et al., 1992a,b; Vigers and Lohka, 1992; Sullivan et al., 1993; Macaulay and Forbes, 1996; Goldberg et al., 1997). Depletion of either vesicular or soluble components from the in vitro extracts can also prevent NPC formation (Sheehan et al., 1988; Dabauvalle et al., 1990; Finlay and Forbes, 1990; Finlay et al., 1991; Vigers and Lohka, 1991), yet purification of any assembly factors has not been accomplished.
Based on the models for NPC ultrastructure (Macaulay and Forbes, 1996; Goldberg et al., 1997), interactions between integral membrane proteins are likely required for formation of the pore. Association of individual nucleoporins and integral membrane proteins is presumed essential for anchoring a NPC in the NE pore. Candidates for mediators of NPC assembly and dynamics have been revealed by either molecular analysis of NPC proteins or characterization of yeast mutant phenotypes. Yeast Pom152p and vertebrate Pom121p and gp210 are integral membrane proteins that localize to the pore membrane and may mediate the anchorage of NPCs in the NE (Gerace et al., 1982; Wozniak et al., 1989; Greber et al., 1990; Hallberg et al., 1993; Wozniak et al., 1994). Peripheral membrane proteins with potential roles in NPC biogenesis include those in which NPC and/or NE structure is perturbed in the respective mutant strains (reviewed in Corbett and Silver, 1997; Doye and Hurt, 1997; Wente et al., 1997). In some cases, these mutant phenotypes may reflect direct effects on NPC biogenesis such that assembly is inhibited and/or accumulation of assembly intermediates results. For example, depletion of the essential yeast nucleoporin Nsp1p or NIC96 mutant alleles results in a reduction in NPC density (Mutvei et al., 1992; Zabel et al., 1996). Recently, mammalian and Xenopus homologues of yeast Nic96p have been identified, and immunodepletion of the Xenopus protein from nuclear/NPC assembly extracts further suggests that it plays a role in the biogenesis of wild-type (wt) NPCs (Grandi et al., 1997). Interestingly, clusters of NPCs in aggregated patches of NE are present in yeast cells expressing mutant alleles of nup145, nup133, nup120, nup159, nup84, nup85, and gle2 (Doye et al., 1994; Wente and Blobel, 1994; Aitchison et al., 1995; Gorsch et al., 1995; Heath et al., 1995; Li et al., 1995; Pemberton et al., 1995; Goldstein et al., 1996; Murphy et al., 1996; Siniossoglou et al., 1996). We recently determined that for one yeast mutant (gle2) (Murphy et al., 1996), NPC clustering results from the migration of pre-existing NPCs into aggregates rather than from the assembly of new NPCs into a fixed site on the NE (Bucci and Wente, 1997). All of the known yeast clustering mutants are defective in a specific NPC-associated factor. However, a Drosophila melanogaster mutant for a component of the nuclear lamina has recently been shown to result in NPC clusters (Lenz-Bohme et al., 1997). In addition, a yeast act2 mutant allele was described that causes abnormal NPC morphology and nucleoporin stoichiometry (Yan et al., 1997). These results suggest that both NPC and non-NPC components can contribute to proper NPC assembly and/or maintenance of proper NPC ultrastructure.
To date, direct genetic screens for global mediators of NPC biogenesis have not been conducted. All of the reported NPC structural perturbations in yeast cells have been found as a matter of course during the characterization of the particular genes. We have developed a new strategy specifically directed at isolating NPC biogenesis mutants using an unbiased fluorescence strategy. This approach is based on the functional tagging of nucleoporins with the green fluorescent protein (GFP) and the hypothesis that NPC assembly mutants expressing the GFP-nucleoporin will have distinct fluorescence signals as compared with wt cells. We selected for such mutants by fluorescence-activated cell sorting (FACS) from a mutagenized population of S. cerevisiae cells expressing GFP-Nup49p and identified novel nup120, gle2, and nup57 alleles. The temperature-sensitive nup57-E17 cells have a lowered GFP fluorescence due to decreased NPC incorporation of GFP-Nup49p. The consequences of this perturbation were further characterized and revealed an in vivo network of interactions that mediate NPC structural integrity. Overall, the results exemplify our ability to identify in vivo mediators of NPC structure and assembly.
MATERIALS AND METHODS
Plasmids
All plasmids were made by standard methods (Sambrook et al., 1989), and DH5α was used as the bacterial host. The plasmids were generated as follows:
pSW242 (NUP49/LEU2/2μ): insertion of a 2,415-base pair (bp) BamHI/SalI fragment containing NUP49 locus from pSW40 (Wente et al., 1992) into pRS425 (Christianson et al., 1992).
pSW490 (NUP57/TRP1/CEN): insertion of a SalI/XhoI digested ∼2,960-bp NUP57 fragment made by PCR using oligonucleotide primers 57–1 (GATAACTTTGATGTCGACAGATTCC) and 57–2 (ACGAAAGTTATCCTCTCGAGGACAC), into pRS314 (Sikorski and Hieter, 1989).
pSW626 (GFP-S65T in pBS-SK): insertion of the 756-bp EcoRI fragment from GFP-S65T in pRSETB (Heim and Tsien, 1996) into EcoRI-digested pBS-SK.
pSW634 (GFP-S65T/kanr in pBS-SK): NotI digestion of pSW626 and insertion of the 1,619-bp NotI fragment from pUG6 (Guldener et al., 1996).
pSW636 (GFP-NUP49/LEU2/CEN): insertion of the 1,763-bp XbaI fragment from pSW442 (Bucci and Wente, 1997) into XbaI-digested pSW62 (Wente et al., 1992).
pSW639 (GFP-F64L,S65T/kanr in pBS-SK): insertion of the 756-bp EcoRI fragment from GFP-F64L,S65T in pRSETB (Heim and Tsien, 1996) into EcoRI-digested pSW634.
pSW806 (NUP57/LEU2/CEN): insertion of the ∼2,950-bp XhoI/NotI fragment from pSW490 into pRS315 (Sikorski and Hieter, 1989).
pSW860 (NUP57 in pQE-32) and pSW862 (nup57-E17 in pQE-32): insertion of KpnI fragments generated by PCR using yeast SWY809 and SWY1586, respectively, as genomic template with oligonucleotides QEA (CAAGGTACCACATGTTTGGTTTCA) and QEB (CTCGGTACCCTCCTTGTTGGCTTTGTG) to amplify the entire open reading frames and ∼360 bp of 3′-untranslated region generating a fusion to the His6 tag, in pQE32 (QIAGEN, Chatsworth, CA).
pSW921 (NUP49-C in pACTII): insertion of a ∼848-bp BamHI fragment generated by PCR with oligonucleotide primers 49–2 (AGCGGATCCTGCAGCAACAACCACAA) and 49.B2 (AAAGGATCCTCGGCCTCTAAGACGCC) (encoding for the Nup49p C-terminus (last 253 amino acids of the protein), into pACTII.
pSW924 (NUP57 in pCH432) and pSW925 (nup57-E17 in pCH432): insertion into pCH432 (Hardy, 1996) of BglII fragments generated by PCR with oligonucleotide primers 2HB1 (GACAGATCTATCACATGTTTGGTTT) and 2HB2 (AAGAGATCTGGTGTCTCCTTGTTGG) and pSW860 or pSW682 as templates, respectively, for in-frame fusion to the DNA-binding domain of LexA.
pSW950 (GFP-NIC96/HIS3): insertion of three fragments into the HIS3 integrating vector pRS303 (Christianson et al., 1992). The first fragment was prepared by XhoI/SacI digestion of a PCR product including 528 bp of 5′ NIC96 promoter sequence and the initiation methionine. The second fragment was prepared by XhoI digestion of a PCR product made using oligonucleotide primers GFP-C (GTACTGCAGGATCCTCGAGATGAGTAAAGGAGAAGAA) and GFP-E (TTTCTGCAGGATCCTCGAGGGTTTGTATAGTTCATCCAT) and contains the full open reading frame of GFP-S65T. The final fragment was generated by XhoI/BamHI digestion of a PCR product created using oligonucleotide primers NIC96–6 (TGTGGATCCATTTAAAAGCTGTTCGATAGAC) and NIC96–7 (GGACTCGAGGGCTGCGCGCGGAAATAAGCTGCAT). This product contains two alanine residues followed by 379 bp of the NIC96 coding sequence just downstream of the initiation methionine.
pSW956 (GFP-NSP1/HIS3): insertion of three fragments into the HIS3 integrating vector pRS303. The first fragment was prepared by XhoI/SacI digestion of a PCR product made using oligonucleotide primers NSP1–3 (TCCGAGCTCCACAGGCTCCAATACTTCTAGAA) and NSP1–7 (CGACTCGAGCGCAGCCGTTTTGTTTTGTTGAGG) and includes ∼ 503 bp of NSP1 promoter and intron sequence as well as the first 11 codons of NSP1, followed by two alanine residues. The second fragment containing GFP-S65T was prepared using oligonucleotide primers GFP-C and GFP-E as described above. The last fragment was prepared by XhoI/StuI digestion of a PCR product made using oligonucleotide primers NSP1-C (GTCGAGTCGACTGGTGCGTATTTACTGTC) and NSP1–8 (GGACTCGAGTGCTGCGACGGGGAAGTCAACCGC) and encodes for two alanine residues and amino acids 606–823 of Nsp1p.
Other plasmids used in this study include: pSW406 (GLE2/LEU2/CEN) (Murphy et al., 1996); pCH4 (NUP120/LEU2/CEN) (Heath et al., 1995); pNLS-E1 (NLS-LacZ/URA3/2μ) (Underwood and Fried, 1990); pCH428 (LexA-ORC2/TRP1/2μ) (Hardy, 1996); pSE1111 (GAL4AD-SNF4/LEU2/2μ) (Yang et al., 1992).
Yeast Strains
General yeast manipulations were conducted by standard methods (Sherman et al., 1986) with transformations by the lithium acetate method (Ito et al., 1983). Yeast strains were grown in either rich YPD (yeast extract, peptone, 2% dextrose) or synthetic complete (SC) medium supplemented with 2% sugar (SD, dextrose) when GFP fluorescence was monitored. Mutant strains with temperature-conditional defects were maintained at 23°C unless otherwise noted. The S. cerevisiae strains used in this study are described in Table 1 and as follows. To achieve minimal autofluorescence, all yeast strains used for GFP fluorescence analysis were adenine prototrophs.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Derivation |
|---|---|---|
| CHY104 | Mata ura3-52 his3Δ200 leu2Δ1 NUP120::HIS3 | Heath et al., 1995 |
| L40 | Mata his3Δ200 trp1-901 leu2-3,112 ade2 LYS2::(LexAop)4-HIS3 URA3::(LexAop)8-LacZ | S. Hollenberg |
| nic96-1 | Mata ade2 trp1 leu2 ura3 HIS3::nic96 (pUN100-LEU2-nic96ts [P332L;L260P]) | Zabel et al., 1996 |
| SWY422 | Mata/Matα ade2-1/ade2-1 ura3-1/ura3-1 his3-11,15/his3-11/15 trp1-1/trp1-1 leu2-3,112/leu2-3,112 can1-100/can1-100 nup133Δ::HIS3/nup133 | Chromosomal integration of HIS3 at NUP133 locus |
| SWY423 | Matα ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 nup133Δ::HIS3 | Segregant of SWY422 |
| SWY458 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2-URA3 | Bucci and Wente, 1997 |
| SWY459 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2-URA3 | Bucci and Wente, 1997 |
| SWY518 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 | Bucci and Wente, 1997 |
| SWY519 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 | Bucci and Wente, 1997 |
| SWY595 | Mata/MATα ura3-1/ura3-1 his3-11,15/his3-11,15 trp1-1/trp1-1 leu2-3,112/leu2-3,112 can1-100/can1-100 ade2-1:ADE2/ade2-1:ADE2 | Bucci and Wente, 1997 |
| SWY729 | Matα ade2-1:ADE2-URA3 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 nup133Δ::HIS3 | Transformation of SWY423 with the integrating vector pSW286 (Bucci and Wente, 1997) |
| SWY734 | Mata ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 | Bucci and Wente, 1997 |
| SWY737 | Matα ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 nup49-1::URA3 | Bucci and Wente, 1997 |
| SWY760 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2-URA3 pSW242 (LEU2) | SWY459 transformed with pSW242 |
| SWY809 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 | Bucci and Wente, 1997 |
| SWY811 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 | Segregant of diploid created by crossing SWY737 with SWY518 |
| SWY828 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2-URA3 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 nup133Δ::HIS3 | Segregant of diploid created by crossing SWY729 with SWY734 |
| SWY894 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 pRS315 (LEU2) | SWY809 transformed with pRS315 |
| SWY1186 | Mata ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 lys2 gle1-4 | Murphy and Wente, 1996 |
| SWY1226 | Matα ade2-1 ura3-1 his3-11,15 leu2-3,112 trp1-1 can1-100 GLE2Δ::HIS3 | Murphy et al., 1996 |
| SWY1427 | Mata/Matα ura3-1/ura3-1 his3-11,15/his3-11,15 trp1-1/trp1-1 leu2-3,112/leu2-3,112 can1-100/can1-100 ade2-1:ADE2/ade2-1:ADE2 NUP82-GFP-F64L, S65T-kanR/NUP82 | SWY595 transformed with the PCR product created with oligos NUP82G and NUP82K using pSW639 as template |
| SWY1441 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 NUP82-GFP-F64L,S65T-kanR | Segregant of SWY1427 |
| SWY1511 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 NUP82-GFP-F64L,S65T-kanR pRS425 (LEU2) | SWY1441 transformed with pRS425 |
| SWY1586 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup57-E17 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 | Segregant of a backcross between SWY811 and the nup57-E17 clone obtained from mutagenesis of SWY809 |
| SWY1587 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup57-E17 | Segregant of a backcross between SWY760 and the nup57-E17 clone obtained from mutagenesis of SWY809 |
| SWY1601 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2-URA3 pRS315 (LEU2) | SWY458 transformed with pRS315 |
| SWY1667 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2-URA3 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 nup133Δ::HIS3 pRS315 (LEU2) | SWY828 transformed with pRS315 |
| SWY1693 | Mata/Matα ura3-1/ura3-1 his3-11,15/his3-11,15 trp1-1/trp1-1 leu2-3,112/leu2-3,112 can1-100/can1-100 ade2-1:ADE2/ade2-1:ADE2 GFP-S65T-NIC96-HIS3/NIC96 | SWY595 transformed with AFlII-digested pSW950 |
| SWY1695 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 GFP-S65T-NIC96-HIS3 | Segregant of SWY1693 |
| SWY1705 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 gle2-C18 pRS315 (LEU2) | Mutagenesis of SWY809 and transformation with pRS315 |
| SWY1706 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 gle2-C18 pSW406 (LEU2) | Mutagenesis of SWY809 and transformation with pSW406 |
| SWY1707 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-D66-TRP1 pRS315 (LEU2) | Mutagenesis of SWY811, one backcross to SWY809, and transformation with pRS315 |
| SWY1708 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup57-E17 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 pRS315 (LEU2) | SWY1586 transformed with pRS315 |
| SWY1709 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup57-E17 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 pSW806 (LEU2) | SWY1586 transformed with pSW806 |
| SWY1710 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 nup120-C36 pRS315 (LEU2) | Mutagenesis of SWY811 and transformation with pRS315 |
| SWY1711 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 nup120-C36 pCH4 (LEU2) | Mutagenesis of SWY811 and transformation with pCH4 |
| SWY1717 | Mata/Matα ura3-1/ura3-1 his3-11,15/his3-11,15 trp1-1/trp1-1 leu2-3,112/leu2-3,112 can1-100/can1-100 ade2-1:ADE2/ade2-1:ADE2 nsp1ΔN::GFP-S65T-HIS3/NSP1 | SWY595 transformed with SpeI-digested pSW956 |
| SWY1722 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup57-E17 GFP-S65T-NIC96-HIS3 | Segregant of a diploid created by crossing SWY1695 and SWY1586 |
| SWY1728 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nsp1ΔN:GFP-S65T-HIS3 | Segregant of SWY1717 |
| SWY1729 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nsp1ΔN::GFP-S65T-HIS3 | Segregant of SWY1717 |
| SWY1736 | Matα ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup57-E17 nsp1ΔN::GFP-S65T-HIS3 | Segregant of a diploid created by crossing SWY1729 and SWY1586 |
| SWY1744 | Matx ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup57-E17 NUP82-GFP-F64L,S65T-kanR pRS425 (LEU2) | Segregant of a diploid created by crossing SWY1511 and SWY1586 |
| SWY1824 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2-URA3 nup133Δ::HIS3 GFP-S65T-NIC96-HIS3 | Segregant of a diploid created by crossing SWY729 and SWY1695 |
| SWY1860 | Mata ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ade2-1:ADE2 nup49-1::URA3 nup49ΔGLFG::GFP-S65T-TRP1 HIS3::nic96 (pUN100-LEU2-nic96ts [P332L;L26P]) | Segregant of a diploid created by crossing SWY811 and nic96-1 |
The nup133Δ::HIS3 strains was made by the gene deletion method of Baudin et al. (1993) using oligonucleotides 133D1 (TATGAAGAGGAAAGCCAGGCCTCTCTAATGGACATTTCCATGGAGGGCCTCCTCTAGTACACTC) and 133D2 (GTATTCTACAGTGTTGGTTTCATAGTTGATGGTATAGTTTTTTTCGCGCGCCTCGTTCAGAATG), which anneal at their 3′-ends to the HIS3 locus in pBM2815 (generous gift of Linda Riles, Washington University, St. Louis, MO). PCR generated a 1100-bp fragment flanked on the 5′-end with sequences complementary to NUP133 bp 1042–1086, including the initiation methionine and on the 3′-end with sequences complimentary to NUP133 bp 4378–4422 just before the TAA stop codon. W303 a/α was transformed with the PCR product to create SWY422. Genomic colony PCR confirmed correct chromosomal integration.
C-terminal tagging of Nup82p with GFP was achieved by the gene integration method of Baudin et al. (1993). wt SWY595 cells were transformed with a PCR product generated with oligonucleotides NUP82-G (TTGTTACAAGTTTCTCAGGAATTTACTACTAAAACTCAAGCTGCGATGAGTAAAGGAGAAGAA) and NUP82-K (CCGAGAGACACGATCTGTAGCGGTGATATGAACGTATTCCTCACCCAGCTGAAGCTTCGTACGC) using pSW639 as template. This template contained the GFP-F64L, S65T coding sequence and the kanr cassette. Sequences flanking the PCR product are complementary to nucleotides of the NUP82 locus and placed two alanine residues, and GFP-F64L, S65T, in frame with the NUP82 open reading frame. G418-resistant transformants were selected, and integration at the NUP82 locus was confirmed by PCR.
N-terminal tagging of Nic96p with GFP was completed by chromosomal integration of AflII-digested pSW950. This creates an in-frame fusion of GFP, two alanine residues and the chromosomal copy of NIC96 separated from a truncated NIC96 gene by sequences corresponding to the integrating vector pRS303. Replacement of the N terminus of Nsp1p with GFP was completed by chromosomal integration of SpeI-digested pSW956. This created an in-frame fusion of the first 11 codons of NSP1 with two alanine residues and GFP followed by codons encoding two alanine residues and Nsp1p residues 607 to the end.
Flow Cytometry and FACS Screening
Logarithmically grown yeast cells expressing GFP-Nup49p were analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). For enrichment of mutants, ∼4 × 107 SWY809 and SWY811 cells were mutagenized in 3% EMS and 100 mM potassium phosphate, pH 7.0, to ∼60% viability. After 24 h of incubation in liquid culture at 23°C, the cells were briefly sonicated and then selected by FACS with an Epics 753 flow cytometer (Coulter Electronics, Hialeah, FL). The cells were excited at 488 nm by an argon ion laser (Coherent, Santa Clara, CA), and the emitted light was passed through a 530/30 bandpass filter. A forward scatter threshold was used to select events for acquisition, and forward and side scatter gates were used to restrict analysis to single events. Approximately 1% of the total cells were sorted from those cells having the lowest GFP fluorescence, and ∼10% of the sorted cells formed colonies on YPD plates at 23°C. Approximately 1000 cells from each mating type were screened by direct fluorescence microscopy at 23°C for NPC defects including clustering and low NPC-associated GFP-Nup49p. Eight mutants (2 from the MATα strain, 6 from the MATa strain) found in the visual screen were tested for sensitivity to various temperatures and for complementation by a plasmid encoding GFP-Nup49p and a panel of plasmids encoding NPC-associated components.
Microscopic Characterization of Mutants
GFP fluorescence was visualized directly after fixation of cells for 10 min in 3.7% formaldehyde and 10% methanol. Indirect immunofluorescence staining of yeast cells was performed essentially as described (Wente et al., 1992). Cells were fixed for 10 min in 3.7% formaldehyde and 10% methanol before or after shifts to 37°C. Primary antibodies used were graciously provided from colleagues and used at the following dilutions for 16-h incubations at 4°C: monoclonal anti-Pom152p (mAb 118C3 tissue culture supernatant, undiluted) (Strambio-de-Castillia et al., 1995), monoclonal anti-Nup159p (mAb 165C10, tissue culture supernatant, undiluted) (Kraemer et al., 1995), affinity-purified rabbit polyclonal WU956 raised against the GLFG region of Nup116p (anti-GLFG, 1:200) (provided by J. Watkins, Washington University, St. Louis MO), affinity-purified rabbit polyclonal WU598 raised against the C-terminal region of Nup145p (1:20) (Emtage et al., 1997), affinity-purified rabbit polyclonal WU600 raised against the C-terminal region of Nup116p (1:10) (Iovine et al., 1995), and rabbit polyclonal WU1079 raised against the C-terminal region of Nup57p (1:200) (provided by J. Watkins, Washington University, St. Louis, MO). FITC-conjugated donkey anti-rabbit and goat anti-mouse antibodies (Cappel, Durham, NC) were used to detect the primary antibodies (1:200 dilutions). Photographs were taken using Kodak T-MAX 400 film on an Olympus microscope through a UPlanFL 100× 1.3 NA objective.
Electron microscopy was conducted essentially as described (Wente and Blobel, 1993). Briefly, cells were grown to early logarithmic phase in YPD at 23°C before shifting to 37°C for 4 h. Cells were immediately fixed with 2% glutaraldehyde, 2% formaldehyde (incubation on ice overnight). After cell wall digestion and osmium postfixation, the samples were embedded in EPON. Thin sections collected on nickel grids coated with formvar, stabilized with carbon, were contrasted with uranyl acetate and Reynold’s lead. Specimens were visualized with a Zeiss-902 electron microscope (Carl Zeiss, Thornwood, NY), and photographs were recorded with Kodak electron microscopy film.
Characterization of nup57pE17
The nup57-E17 mutation was sequenced by the dideoxy chain termination method (Sanger et al., 1977) using the Sequenase kit (Version 2, United States Biochemical, Cleveland, OH) and oligonucleotide primers 57–4 (AGCAGATCTCAGTACCGTTGCAACAAACGCAAGC) and 57–7 (CAATTGCAGCATCTTTC), annealing within the nup57-E17 gene of pSW862. To compare expression levels of Nup57p and nup57pE17, total yeast cell extracts and immunoblotting were conducted as described (Iovine et al., 1995) after shifts for various times at 37°C in YPD. The affinity-purified rabbit polyclonal anti-GLFG antibodies (raised against the GLFG region of Nup116p, WU956) were used at a dilution of 1:1000. The GFP-Nup49p was recognized by a commercial polyclonal antibody recognizing GFP (1:500, CLONTECH, Palo Alto, CA). Bands were visualized by developing with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-1-phosphate (Promega, Madison, WI). For biochemical analysis of soluble versus insoluble/nuclear pools of given nucleoporins in wt and nup57-E17 cells, yeast spheroplasts were osmotically lysed, fractionated by low-speed centrifugation, and extracted with 1 M NaCl as described by Bogerd et al. (1994). Protein samples were separated by electrophoresis in SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Immunoblotting was conducted with the polyclonal anti-GFP antibody (CLONTECH) for GFP-Nup49p and GFP-Nsp1p, the polyclonal anti-GLFG antibody for Nup116p (WU956), guinea pig polyclonal antibody recognizing Nup159p (1:10,000) (Gorsch et al., 1995) with a rabbit anti-guinea pig secondary (1:1000, Cappel), an affinity-purified rabbit polyclonal antibody recognizing the C-terminal region of Nup145p (Nup145-Cp) (1:100, WU599, Emtage et al. 1997), and an affinity-purified rabbit polyclonal antibody recognizing the C-terminal region of the integral membrane protein Snl1p (1:1000, WU975, Ho et al., 1998). Bands were visualized by the ECL system (Amersham, Arlington Heights, IL) according to manufacturer’s directions, and the autoradiographs were digitized and quantified using NIH Image (developed at the United States National Institutes of Health and available from the Internet by anonymous FTP from zippy.nimh.gov).
For the two-hybrid interaction analysis, yeast strain L40 was cotransformed with plasmids encoding Gal4p transcriptional activation domain fusions (GAL4AD) and LexAp DNA-binding domain fusions (LexABD). Quantitative analysis of the β-galactosidase activity was determined as described previously (Guarente, 1983).
Nuclear Transport Assays
Analysis of nuclear import and export was completed essentially as described (Iovine et al., 1995). Briefly, nuclear import capacity was assessed by monitoring the localization of a GAL10-induced nuclear localization sequence (NLS) β-galactosidase fusion protein (pNLS-E1) (Underwood and Fried, 1990). Yeast strains transformed with pNLS-E1 were grown in SC media lacking uracil with 2% raffinose for 2.5 h at 37°C. The cells were then shifted into SC media lacking uracil with 2% galactose for an additional 3.5 h at 37°C. The cells were processed for indirect immunofluorescence microscopy as described above with a primary mouse monoclonal anti-β-galactosidase antibody (ascites at 1:100, Sigma, St. Louis, MO), and a secondary rhodamine-conjugated donkey anti-mouse (Cappel). Nuclear export capacity was monitored in strains grown in YPD by the localization of poly(A)+ RNA as previously described (Wente and Blobel, 1993). In situ hybridization was conducted with an oligonucleotide poly(dT)30 probe end labeled with digoxigenin-11-dUTP (Boehringer Mannheim, Mannheim, Germany) by terminal transferase (GIBCO BRL, Gaithersburg, MD). Detection of the probe was achieved using anti-digoxigenin-rhodamine FAb fragments (Boehringer Mannheim).
RESULTS
Rationale for a Fluorescence-based Genetic Strategy to Isolate NPC Mutants
To understand the molecular basis of NPC assembly and to identify mediators of NPC biogenesis, we previously developed assays to monitor NPCs in live cells of the yeast S. cerevisiae (Bucci and Wente, 1997). The nucleoporin Nup49p was functionally tagged with the GFP from Aequora victoria, expressed in yeast cells, and GFP-tagged NPC movement and assembly rates were monitored in live cells. We also expressed the GFP-Nup49p in a gle2 temperature-dependent NPC clustering mutant and observed the dynamics of mutant GFP-tagged NPCs. During this analysis, we speculated that NPC assembly mutants may have distinct GFP fluorescence properties compared with wt cells. We predicted that if a wt strain expressing a GFP-tagged nucleoporin was mutagenized, at least three different classes of mutants might be distinguishable by their fluorescence properties. Mutants that result in NPC clustering (Class I) would have a markedly distinct GFP staining pattern around the nuclear rim. In addition, it has also been observed that during the division of mutants with NPC clusters, the clusters are not necessarily divided equally between the resulting mother and daughter cells (Doye et al., 1994; Wente and Blobel, 1994; Heath et al., 1995; Pemberton et al., 1995). This should result in one cell having a substantially lower total fluorescence signal until it forms a new cluster. Mutants that result in fewer total NPCs per nucleus (Class II) would have a reduced total GFP fluorescence signal as compared with wt cells. Such Class II mutants may be in genes encoding global mediators of NPC assembly, as has been proposed for Nic96p (Zabel et al., 1996; Grandi et al., 1997). Finally, mutants that have wt NPC number but with each NPC having a decreased amount of GFP-Nup incorporated (Class III) would also exhibit a decrease in total GFP fluorescence signal.
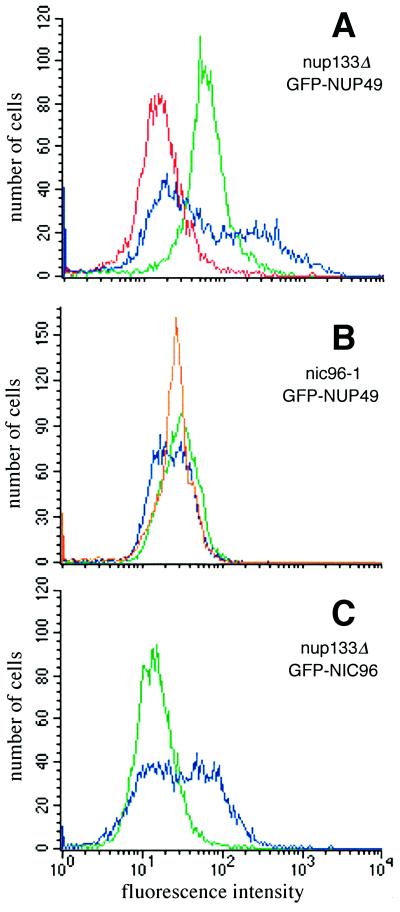
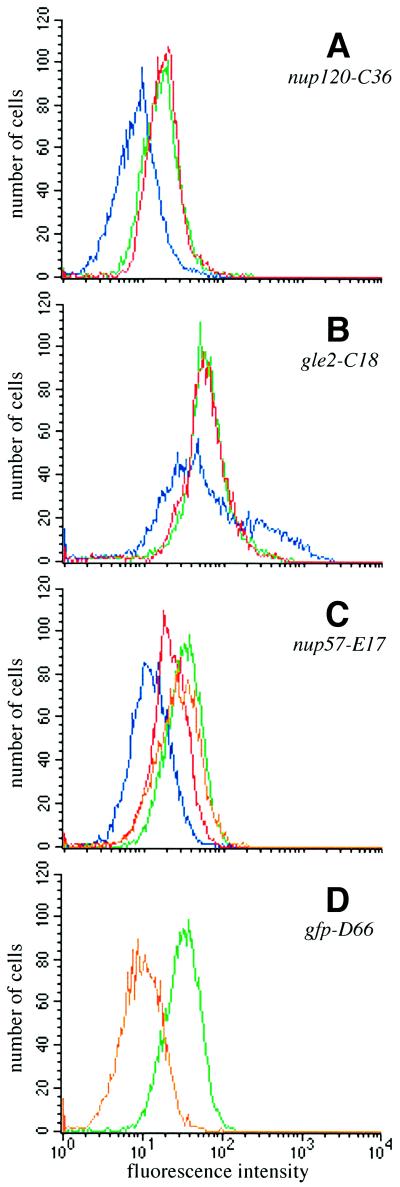
Because of these potential GFP fluorescence signal differences between wt and the three proposed mutant classes, we predicted that such NPC assembly mutant cells could be physically separated from wt cells using fluorescence-activated cell sorting (FACS). To test the feasibility of FACS selection in mutant isolation, we analyzed by flow cytometry wt, nup133Δ, and nic96–1 mutant cells that expressed GFP-Nup49p. As shown in Figure 1A (red), wt cells not expressing GFP protein have an endogenous low fluorescence emission level (488 nm ex, 530 nm em). In contrast, wt cells expressing GFP-Nup49p have a mean fluorescence emission peak nearly five times greater than the endogenous autofluorescence (Figure 1A, green). Strains bearing a null allele (Δ) of NUP133 have constitutive NPC clusters at all growth temperatures (Doye et al., 1994; Li et al., 1995; Pemberton et al., 1995). When analyzed by flow cytometry (Figure 1A, blue profile) after growth at 23°C, the nup133Δ mutant expressing GFP-Nup49p displayed a strikingly different fluorescence profile from either of the two wt strains. The wt GFP-Nup49p profile was markedly symmetrical with one sharp peak, whereas the nup133Δ mutant GFP-Nup49p profile was broader with two distinct peaks. The left-shifted peak was indicative of a fraction of cells with a low GFP signal. The nup133Δ cells with low fluorescence may reflect a population of newly divided cells that lack large NPC clusters. The right-shifted peak represented a nup133Δ cell population with a greater fluorescence intensity and may therefore reflect cells with large NPC clusters.
Figure 1.
NPC mutants expressing GFP-Nup49p have distinct fluorescence properties compared with wt cells expressing GFP-Nup49p. Flow cytometry analysis was performed on cells grown to logarithmic phase in SD lacking leucine (488 nm ex, 530 nm em). wt strains expressing GFP-Nup49p (SWY894, green profiles in panels A and B) have a fluorescence emission level nearly five times as great as the endogenous fluorescence of cells not expressing GFP (SWY1601, red profile in panel A). (A) nup133Δ cells expressing GFP-Nup49p (SWY1667, blue) display a bimodal distribution reflecting two populations of GFP-labeled cells with different fluorescence levels. (B) nic96–1 cells expressing GFP-Nup49p (SWY1860) show a broad, left-shifted peak after growth at 23°C (blue) and a sharp symmetrical peak after growth for 5 h at 37°C (orange). (C) nup133Δ cells expressing GFP-Nic96p (SWY1824, blue) have a distinct profile compared with wt cells expressing GFP-Nic96p (green).
Previous studies by Zabel et al. (1996) have reported a decreased NPC density in temperature-sensitive nic96–1 mutant cells. FACS analysis of the temperature-sensitive nic96–1 mutant expressing GFP-Nup49p also showed perturbations in the flow cytometry pattern compared with wt cells (Figure 1B). When grown at 23°C, the peak for the GFP-Nup49p in nic96–1 cells (Figure 1B, blue) was shifted to the left and was broader than the profile for wt GFP-Nup49p cells (green). After shifting to growth at 37°C for 5 h, the peak for the nic96–1 cells remained left-shifted (Figure 1B, orange) compared with wt although it was markedly sharper than the profile for mutant cells at 23°C. Therefore, on the basis of GFP-Nup49p expression, cells harboring known mutant alleles related to NPC assembly have distinct flow cytometric properties as compared with wt cells.
To assess whether the distinct FACS patterns obtained were dependent on using GFP-Nup49p, the sequence encoding GFP was fused in frame with the sequence encoding the N terminus of full-length Nic96p. The GFP-NIC96 gene was integrated in place of the respective chromosomal NIC96 allele in both wt and nup133Δ strains. The FACS peak for wt cells expressing GFP-Nic96p was symmetrical (Figure 1C, green). Interestingly, the profile for GFP-Nic96p nup133Δ cells was distinct from wt (Figure 1C, blue) and notably broad as was the case for the GFP-Nup49p nup133Δ cells. However, in contrast to the GFP-Nup49p nup133Δ profile, the majority of the GFP-Nic96p nup133Δ peak was not shifted to the low fluorescence intensity region but rather toward a higher fluorescence level. Therefore, the particular GFP-tagged nucleoporin employed may influence the perturbations observed in a mutant cell’s FACS profile. In some cases this may be reflected by functional or physical interactions between the particular nucleoporins. For instance, the established genetic interaction between nup49 and nup133 mutant alleles may suggest that there is a physical/functional association between the two nucleoporins (Doye et al., 1994). The FACS results in Figure 1 further suggested that the types of mutants obtained in a FACS-based genetic screen may be dependent on the particular GFP-tagged nucleoporin expressed.
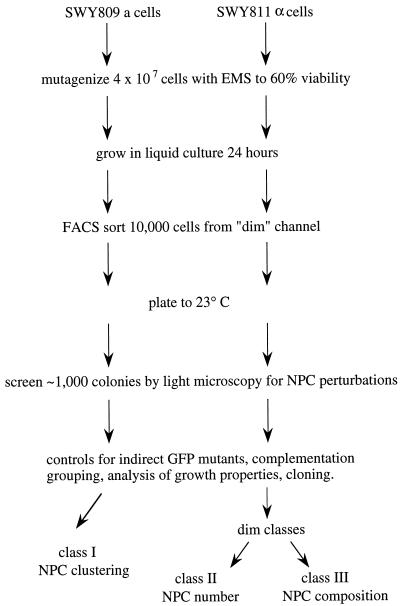
Based on the above rationale and the FACS results, we designed a genetic strategy to identify novel factors required for proper NPC assembly. A flowchart of the strategy is detailed in Figure 2. We focused the strategy on isolating mutants that exhibited a constitutively lowered GFP-Nup49p fluorescence level during growth at 23°C. The choice of GFP-Nup49p was due to our extensive previous analysis of this GFP-tagged nucleoporin (Bucci and Wente, 1997), and the observed FACS shifts to lower fluorescence levels in the known mutant profiles at 23°C (Figure 1). After a primary FACS selection from a chemically mutagenized population of cells, a secondary screen was also included whereby individual colonies resulting from growth at 23°C would be further tested by direct fluorescence microscopy. The microscopic analysis would allow confirmation of a fluorescence perturbation, and the mutants could be coincidentally classed as either NPC clustering mutants (I) or mutants with an overall decrease in GFP-NPC fluorescence intensity (Classes II or III). After identification of mutants, two control experiments were designed to eliminate mutant strains resulting from an indirect loss of GFP signal. A lowered fluorescence level (dim) phenotype could be due to mutations within the GFP portion of the GFP-Nup49p fusion itself, and/or nonspecific defects in GFP-Nup49p transcription, translation, or folding. To eliminate mutants due to indirect GFP perturbations, the strains could be tested for complementation of the dim phenotype by 1) transformation and expression of a plasmid-borne copy of GFP-NUP49 in the haploid mutant, and 2) mating the mutant to a wt strain that does not express GFP-Nup49p. Only dim mutants not rescued by the plasmid transformation but complemented by the mating test would be selected for further analysis. We predicted that such a strategy would result in a focus on the isolation of novel, recessive mutants harboring defects in NPC assembly.
Figure 2.
Schematic diagram for a fluorescence-based strategy to isolate NPC assembly mutants. Strains expressing GFP-Nup49p were selected by FACS and screened by microscopic inspection of GFP-NPC fluorescence patterns. Candidate mutants were tested for plasmid-dependent complementation and for growth phenotypes. We predicted that at least three different classes of mutants would be identified: Class I, NPC clustering; Class II, fewer total NPCs per nucleus, and Class III, wt NPC number, but each NPC with a decreased amount of GFP-Nup incorporated.
Identification of NPC Assembly Mutants
Following the strategy outlined in Figure 2, a FACS screen to isolate novel mutants with lowered GFP-Nup49p fluorescence was conducted. Isogenic MATa and MATα GFP-NUP49 strains were mutagenized with EMS and allowed to recover in liquid culture before a subpopulation of the cells was subjected to FACS sorting. One percent of the cells with the lowest relative GFP fluorescence were collected and plated for colony growth on rich media at 23°C. Approximately 10% of the estimated 10,000 sorted cells formed colonies, and this correlated well with the results of similar FACS strategies in yeast (Wendland et al., 1996). These strains were each individually screened by fluorescence microscopy for visual perturbations in NPC distribution (clustering phenotypes) or fluorescence intensity levels. Eight mutants were isolated with distinctly perturbed GFP-NPC staining as compared with wt: two (designated C36 and C18) had NPC clusters, and six others exhibited an overall dim (low fluorescence level) phenotype.
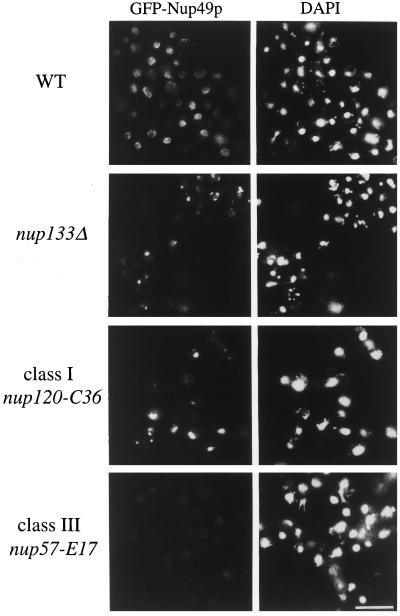
Since the strains with a dim phenotype could be due to mutations within GFP itself rather than defects in NPC assembly, a plasmid-borne copy of GFP-NUP49 (pSW636) was transformed into the six dim strains. The dim phenotype in strains defective for NPC assembly should not be complemented by this plasmid. Five of the dim strains were rescued to nearly wt GFP-NPC staining with plasmid-borne GFP-Nup49p (our unpublished observations) and were likely due to a direct mutation in GFP. These strains were not further analyzed. The remaining dim mutant (E17) was not complemented by plasmid-expressed GFP-Nup49p and was possibly due to a direct defect in NPC assembly. Fluorescence microscopy analysis of wt cells and the E17 and C36 mutants is shown in Figure 3. The fields were exposed and printed for identical times to accurately reflect the intensity and distribution differences in the GFP-Nup49p signal. The signal for the wt parental cells (upper panel) is localized over the entire nuclear rim in a punctate pattern typical for nucleoporin localization. The middle panels show the clustering phenotype of the nup133Δ and the C36 mutant, where the majority of the GFP-Nup49p signal is concentrated in discrete foci representing brightly labeled NPC clusters. The dim phenotype of the E17 mutant strain (Figure 3, lower panel) reflects an overall lower GFP intensity in the NE. The dim phenotype is completely penetrant, with all cells in the population showing similarly low GFP intensities.
Figure 3.
Two classes of NPC mutants were isolated in the GFP-Nup49p fluorescence-based strategy: NPC clustering mutants and dim mutants. Direct fluorescence microscopy analysis of GFP-Nup49p localization in wt, nup133Δ, and two representative mutant strains isolated in the FACS-based screen. The strains were grown in SD media lacking leucine: wt (SWY894), nup133Δ clustering (SWY1667), clustering-Class I (nup120-C36 mutant, SWY1710), Dim-Class III (nup57-E17 mutant, SWY1708). Within each column, photographs were exposed and printed for identical times. DAPI staining for each respective field is shown on the left. Bar, 10 μm.
Complementation Analysis Reveals Mutant Alleles of nup120, gle2, and nup57
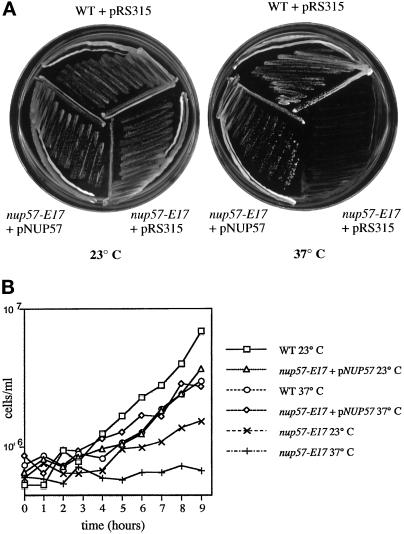
The E17 dim mutant and the C36 and C18 clustering mutants were chosen for further characterization. All three represented alleles in distinct complementation groups, as determined by analyzing the phenotype of diploids formed from pairwise crosses of the respective mutants in MATa or MATα backgrounds. In addition, the C36, C18, and E17 mutants were all temperature-sensitive for growth at 37°C (Figure 4, and our unpublished observations). The temperature sensitivity was also genetically linked with the GFP phenotypes (as determined by backcrossing to the wt parental strains).
Figure 4.
Expression of NUP57 from a plasmid complements the temperature sensitivity of the nup57-E17 mutant. (A) Strains were grown on SD plates lacking leucine for 4 d at 23 or 37°C: wt (SWY894), nup57-E17 with pRS315 (SWY1708), nup57-E17 + pSW806/NUP57 (SWY1709). NUP57 expression restores growth to the nup57-E17 strain at 37°C. (B) Growth analysis of wt, nup57-E17, and nup57-E17 + NUP57 cells at 23 and 37°C. Aliquots of cells growing at early logarithmic phase were counted at various time points after shift to 37°C or continued growth at 23°C. Cell counts are expressed as cell number per ml of culture.
To test whether the C36, C18, and E17 mutants were due to mutations in genes encoding known NPC-associated factors, the strains were each transformed with a bank of CEN/LEU2 plasmids harboring known genes and tested for complementation of the mutant phenotype. Both the temperature sensitivity and the clustering phenotypes of the C36 mutant were complemented by a wt NUP120 gene. Moreover, the temperature sensitivity and the NPC clustering of C36 cells were not complemented after mating to a strain harboring a null allele of NUP120 (CHY104). Similarly, the C18 mutant also represented a mutation in a gene encoding a known NPC-associated factor. The temperature sensitivity and clustering phenotypes of the C18 mutant were complemented by a plasmid harboring GLE2. In addition, the C18 phenotypes were not complemented by mating to a gle2Δ strain (SWY1226). Since the NUP120 and GLE2 plasmids complemented the GFP phenotypes of the C36 and C18 mutants, respectively, we compared mutant strains with or without a complementing plasmid to wt strains in flow cytometry experiments. Figure 5, A and B, shows the fluorescence histograms for these two mutants transformed with either the complementing gene (red) or an empty vector (blue). The nup120-C36 mutant histogram (Figure 5A) is remarkably symmetrical compared with that for the nup133Δ strain shown in Figure 1A; one major peak corresponding to an overall decrease in GFP intensity was observed (blue). When the NUP120 plasmid was expressed in the nup120-C36 cells, the FACS peak was shifted to the right overlapping the wt peak and reflected greater GFP intensity (Figure 5A, red). The gle2-C18 mutant profile was very similar of that observed with nup133Δ cells as reflected by the two broad peaks (Figure 5B, blue). When the gle2-C18 strain harbored the GLE2 plasmid, the profile shifted to a single peak with a fluorescence intensity comparable to wt (Figure 5B, red). Mutant alleles of nup120 (Aitchison et al., 1995; Heath et al., 1995) and gle2 (Murphy et al., 1996) that result in NPC clusters have been previously reported. The differences we observed between the GFP-Nup49p FACS profiles in different clustering mutants may reflect true distinctions between the clustering types. Indeed, the fluorescence microscopy analysis of the mutants (Figure 3) shows the majority of the nup120-C36 cells with clusters have a single large foci of GFP-NPC staining, whereas the clusters in nup133Δ cells are smaller and are often numerous. Alternatively, the distinct profile may also be related to the expression of GFP-Nup49p and perturbations of possible interactions with the given mutant nucleoporin Nup133p, Nup120p, or Gle2p.
Figure 5.
FACS analysis of mutant strains shows that expression of NUP120, GLE2, or NUP57 rescues the GFP phenotypes of the respective mutants. The mutant strains transformed with CEN/LEU plasmids were analyzed by flow cytometry as in Figure 1. In all panels, the GFP-Nup49p profile in wt cells is shown in green, the mutant transformed with an empty CEN/LEU vector is shown in blue, and the mutant transformed with a complementing plasmid is shown in red. (A) nup120-C36 at 23°C (blue, SWY1710; red, SWY1711). (B) gle2-C18 at 23°C (blue, SWY1705; red, SWY1706). (C) nup57-E17 at 23°C (blue, SWY1708; red, SWY1709) and nup57-E17 + NUP57 at 37°C (orange, SWY1709). (D) gfp-D66 (orange, SWY1707).
Interestingly, the temperature-sensitive E17 dim mutant was complemented by a plasmid harboring NUP57 (Figures 4A and 5C). Figure 5, C and D, shows the analysis of two dim mutants: a mutant (D66) representing the group due to mutations in GFP (Figure 5D, orange) and the nup57-E17 dim mutant (Figure 5C, blue). For both, a single broad peak of lowered fluorescence intensity was observed compared with wt cells (green profiles). When the plasmid encoding NUP57 was expressed in the nup57-E17 mutant and cells grown at 23°C were analyzed, the main peak shifted from a low fluorescence intensity (Figure 5C, blue) (comparable to cells expressing no GFP protein) to a higher fluorescence intensity (Figure 5C, red). However, the profile for the nup57-E17 cells expressing NUP57 did not match the wt profile (green). In addition, when the cells were analyzed for relative growth rates, full complementation was not observed at 23°C (Figure 4B). These results suggested that at 23°C the mutant nup57pE17 protein was competing with the wt Nup57p for incorporation into the NPC. Interestingly, when the nup57-E17 cells harboring the NUP57 plasmid were shifted to growth at 37°C, full complementation was observed as reflected by the overlapping growth curves for both wt and nup57-E17 + NUP57 cells at 37°C (Figure 4B). Moreover, the FACS profile at 37°C for nup57-E17 + NUP57 cells (Figure 5C, orange) was also coincident with that for wt (green). Overall, the isolation of known clustering and nucleoporin mutants validates our approach of mutagenizing a wt culture and using a FACS and fluorescence screening strategy to identify genes related to NPC structure.
NPC Incorporation of GFP-Nup49p Is Inhibited in the nup57-E17 Mutant
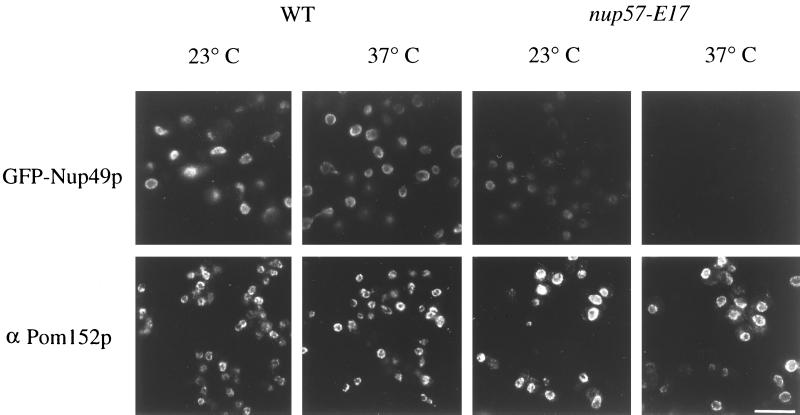
We predicted that further analysis of the nup57-E17 mutant would provide a unique opportunity to analyze the role of Nup57p in vivo. Nup57p belongs to the GLFG family of nucleoporins (Wente et al., 1992; Grandi et al., 1995b), in which each member is characterized by amino-terminal regions with multiple GLFG tetrapeptide repeats separated by uncharged spacer sequences. Studies by others have documented that Nup57p forms a heterotrimeric complex with Nup49p and Nsp1p both in vitro and in immunoprecipitates from yeast cell lysates (Grandi et al., 1995b; Schlaich et al., 1997). Since Nup49p and Nup57p directly interact in vitro (Schlaich et al., 1997), the nup57-E17 phenotype was possibly due to fewer GFP-Nup49p molecules incorporated per NPC instead of a decrease in total NPC number per nucleus. If total NPC number had been substantially changed, we predicted that NPC staining for Pom152p would be coincidentally decreased. Pom152p is an integral membrane protein localized to the pore membrane (Wozniak et al., 1994). Indirect immunofluorescence microscopy was performed with a monoclonal antibody (mAb) recognizing Pom152p (Strambio et al., 1995). The staining level and pattern of anti-Pom152p was identical in wt and nup57-E17 cells grown at either 23 or 37°C (Figure 6, bottom panel). In comparison, when GFP-Nup49p was expressed in wt and nup57-E17 mutant strains, the NPC-associated GFP-Nup49p was greatly diminished in mutant cells as compared with wt (Figure 6, top panel). This suggested that the lack of GFP-Nup49p incorporation in the nup57-E17 mutant did not reflect a total inhibition of NPC and nuclear pore assembly. Thus, the nup57-E17 allele likely represented a Class III mutant with approximately wt NPC number but with each NPC having a decreased level of GFP-Nup49p incorporated.
Figure 6.
Localization of GFP-Nup49p at the NPC is decreased in nup57-E17 cells, whereas Pom152p localization is not perturbed. Within each row, images were photographed and printed for identical times for direct comparison of fluorescence intensities. Top row, direct fluorescence microscopy was performed on wt and nup57-E17 cells expressing GFP-Nup49p (SWY809 and SWY1586, respectively), grown in SD lacking tryptophan. At 23°C, the nup57-E17 strain has less GFP fluorescence signal localized at the NE than wt cells. After 4 h of growth at 37°C, the nup57-E17 cells lack any detectable GFP-Nup49p staining. Bottom row, Indirect immunofluorescence microscopy was performed with monoclonal anti-Pom152p antibodies (mAb 118C3) on wt (SWY519) and nup57-E17 (SWY1587) strains grown in YPD. The anti-Pom152p staining is localized at the NE/NPC, and the intensity level is not notably altered in nup57-E17 cells at either 23 or 37°C compared with wt. Bar, 10 μm.
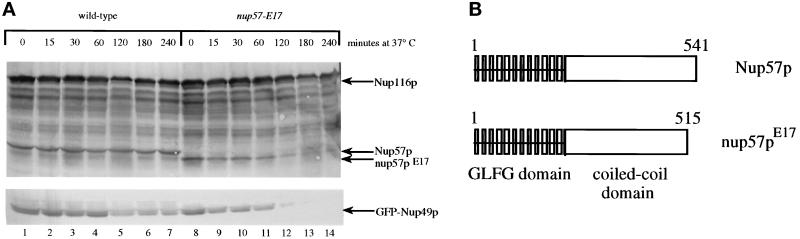
To test whether the diminished GFP-Nup49p level in nup57-E17 cells was due to degradation of GFP-Nup49p, whole yeast cell lysates from wt and mutant cells were immunoblotted with an anti-GFP antibody (Figure 7A, lower panel). When the cells were grown at the permissive temperature, the GFP-Nup49p levels were similar in wt and nup57-E17 strains (lanes 1 and 8, respectively). Considering the lowered total fluorescence level measured by FACS, the high levels of GFP-Nup49p in nup57-E17 cells seemed paradoxical. The level of GFP-Nup49p fluorescence signal was not sensitive to fixation with formaldehyde as similar results were obtained by FACS and microscopy regardless of whether or not the nup57-E17 cells were fixed (our unpublished observations). This suggested that at 23°C unincorporated GFP-Nup49p was stable and that the lack of assembly and concentration at the NPC (and presumed cytoplasmic localization, see below and Figure 8) did not contribute to the total FACS-analyzed GFP fluorescence levels of nup57-E17 cells. GFP-Nup49p that is not properly assembled into the NPC may not fold correctly for detectable fluorescence by either FACS or direct fluorescence microscopy.
Figure 7.
The nup57-E17 allele results in a C-terminal truncation of nup57pE17 and temperature-dependent degradation of nup57pE17 and GFP-Nup49p. (A) Immunoblot analysis reveals the truncated nup57pE17 protein is unstable at 37°C. Total cell lysates prepared from wt (SWY809) and nup57-E17 (SWY1586) cells were separated on 8% SDS-polyacrylamide gels, transferred to nitrocellulose, and analyzed by immunoblotting with the indicated antibody (upper, affinity-purified polyclonal anti-GLFG; lower, polyclonal anti-GFP). The samples were prepared from cells grown at 23°C (lanes 1 and 8) or shifted to growth at 37°C for the indicated times (lanes 2–7 and 9–14). For each sample, identical cell number equivalents were loaded in each well making the protein levels directly comparable. Lysates from wt cells have similar levels of Nup116p, Nup57p, and GFP-Nup49p at all time points. In nup57-E17 cells, the levels of nup57pE17 and GFP-Nup49p decrease with increasing time at 37°C. (B) Diagram of wt Nup57p and mutated nup57pE17. Sequencing of the nup57-E17 allele revealed a single point mutation resulting in a stop codon after the codon for residue 515 and truncation of 26 residues from the C terminus. Numbers reflect amino acid positions within Nup57p. Vertical boxes designate individual GLFG repeats.
Figure 8.
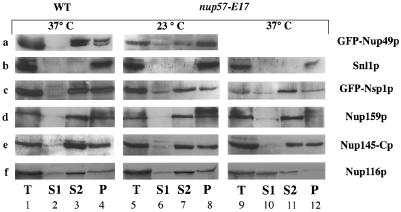
Subcellular fractionation of wt and nup57-E17 cells. Spheroplasts of the respective strains were osmotically lysed and fractionated into soluble (S1) and pellet (P1) fractions. The P1 fraction was further extracted with 1 M NaCl and separated into a supernatant (S2) and insoluble pellet (P2) fractions. Samples of each fraction and an aliquot of total spheroplast lysate (T) were separated by SDS-PAGE and analyzed by immunoblotting with the designated antibodies. For each antibody, only the full-length protein product is shown. In cases where some degradation of the full-length product was observed, the levels of degradation between the wt and mutant cells were equivalent (e.g., Nup116p and Nup159p). For each individual strain under the given growth conditions, identical cell number equivalents were analyzed for each S1, S2, and P2 fraction. The amount of each given protein in the S1, S2, and P2 fractions was quantified as described in the MATERIALS AND METHODS. (A) GFP-Nup49p (SWY809, SWY1586). (B) Snl1p (SWY809, SWY1586). (C) GFP-Nsp1p (SWY1728, SWY1736). (D) Nup159p (SWY809, SWY1586). (E) Nup145-Cp (SWY809, SWY1586). (F) Nup116p (SWY809, SWY1586).
Identical cell lysate samples from the wt and nup57-E17 cells were also probed with an affinity-purified polyclonal antibody raised against the GLFG region of Nup116p that preferentially detects both Nup116p and Nup57p. In general, the relative levels of Nup116p and Nup57p or nup57pE17 appeared similar between wt and mutant cells at 23°C (Figure 7A, upper panel). Interestingly, the nup57pE17 protein migrated faster than the wt Nup57p. Since the nup57-E17 mutant was lethal at 37°C, cell lysates were also analyzed after growth at the nonpermissive temperature for various times (Figure 7A, compare lanes 9–14 with lanes 2–7). At the early time points of 15, 30, or 60 min, nup57pE17 was present. However, after 2 h at 37°C, the nup57pE17 signal was virtually undetectable. Levels of GFP-Nup49p decreased in a similar manner coincident with the loss of nup57pE17 at 37°C. To address the reversibility of the temperature-induced growth defect, after growth at 37°C the nup57-E17 cells were plated and grown at 23°C (Table 2). Viability of the mutant decreased substantially between the 2- and 3-h time points, indicating the growth defect was irreversible once GFP-Nup49p and nup57pE17 were degraded. These results further support a model wherein at 23°C the nup57pE17 protein is incorporated into NPCs even in the presence of wt Nup57p. At 37°C, the degradation of nup57pE17 would result in only wt Nup57p incorporation into NPCs and full complementation of wt function and growth.
Table 2.
Viability of nup57-E17 mutant strains
| Time at 37°C (h) | % Viable cells |
|---|---|
| 0 | 100 |
| 1 | 92 |
| 2 | 80 |
| 3 | 32 |
| 4 | 15 |
| 5 | 5 |
wt (SWY519) and mutant (SWY1587) cells were grown at 23°C or shifted to 37°C for the indicated times. ∼300 cells were plated to YPD at 23°C after each time point. % viable cells reflects the number of mutant cells that formed colonies relative to the wt strain.
The nup57-E17 Mutation Abolishes Binding of nup57pE17 to Nup49p
The faster migration of nup57pE17 compared with Nup57p suggested that the mutation induced by EMS resulted in a truncation of the NUP57 gene product. Furthermore, since the N-terminal GLFG region is still recognized by the anti-GLFG antibody, we predicted that the protein was prematurely truncated at the C terminus. To delineate the structural basis for the mutant phenotype, the nup57-E17 allele was sequenced (see MATERIALS AND METHODS). A single mutation was found in the codon for the glutamine residue at position 516 resulting in this CAA codon being converted to the ochre stop codon TAA. We confirmed that this truncation mutation was sufficient to confer the dim and temperature-sensitive phenotypes of the nup57-E17 mutant. Moreover, the predicted 26-amino acid difference in the protein resulting from the translation of the nup57-E17 transcript corresponds with the ∼3-kDa shift in molecular mass observed by immunoblotting (Figure 7B).
Given the established in vitro interaction between Nup57p and Nup49p (Schlaich et al., 1997), there were at least two alternative models for the lack of NPC incorporation of GFP-Nup49p in the nup57-E17 cells. The nup57pE17 may interact with GFP-Nup49p, but the nup57pE17-Nup49p dimer may not be efficiently assembled into NPCs. In contrast, the nup57pE17 may be incorporated into NPCs, but the interaction site for GFP-Nup49p may be removed, thereby inhibiting GFP-Nup49p NPC assembly. To determine whether the nup57-E17 mutation had a direct effect on the interaction with Nup49p, the yeast two-hybrid assay was used. The C-terminal region of Nup49p was fused to the LexA-DNA-binding domain (BD), and full-length Nup57p and nup57pE17 were each fused to the Gal4p transcriptional activation domain (AD). When LexA-Nup57p and GAL4AD-Nup49p were coexpressed in the reporter strain, β-galactosidase activity was very high, reflecting a strong interaction between the two wt proteins (Table 3). The GAL4AD-Nup49p fusion protein failed to activate transcription of the reporter when coexpressed with an unrelated fusion to the LexABD (Orc2p). Likewise, LexABD-Nup57p was unable to mediate interaction with an unrelated GAL4AD fusion (Snf4p) or with the GAL4AD alone. These results suggested strong and specific binding between the wt Nup49p C terminus and Nup57p. In contrast, the interaction level of LexABD-nup57pE17 with GAL4AD-Nup49p was at least 60-fold lower with virtually undetectable reporter activity (Table 3). The results were similar regardless of whether the strains were grown and assayed at 23 or 30°C, temperatures that support the growth of the nup57-E17 mutant strain. In addition, these strains express fusion proteins with the predicted molecular masses when analyzed by immunoblotting with mAbs against the LexABD or the GAL4AD (our unpublished observations). These results suggested that truncation of the Nup57p C-terminal region results in a decreased interaction with Nup49p and may therefore inhibit NPC assembly of GFP-Nup49p.
Table 3.
C-terminal truncation of Nup57p results in a decreased two-hybrid interaction with Nup49p.
| Lex ABD | GAL4AD | β-Galactosidase activity |
|---|---|---|
| Nup57p | Nup49p (242-493) | 58.2 ± 2.8 |
| Nup57p (1-515) | Nup49p (242-493) | 1.0 ± 0.6 |
| Orc2p | Nup49p (242-493) | 0.1 ± 0.2 |
| Nup57p | Snf4p | 0.6 ± 0.6 |
| Nup57p | — | 0.9 ± 1.2 |
L40 was cotransformed with plasmids containing the indicated activation domain (pSW921, pSE1111) and DNA-binding domain constructs (pSW924, pSW925, pCH428) and grown in SD lacking tryptophan and leucine. Each β-galactosidase assay was completed in triplicate. Plasmids expressing Gal4pAD-Snf4p (Yang et al., 1992) and LexABD-Orc2p (Hardy, 1996) were used to determine the specificity of the Nup49p-Nup57p interaction.
To confirm the correlation between truncation of Nup57p and a decreased GFP-Nup49p association with NPCs, wt and mutant cells were analyzed by crude subcellular fractionation into a soluble (S1) fraction and an organelle-containing pellet (P1) fraction. The P1 crude pellet was further analyzed by extraction with 1 M NaCl yielding a solubilized fraction (S2) and insoluble pellet (P2). The level of GFP-Nup49p in these fractions was determined by quantitative immunoblot analysis of S1, S2, and P2 samples with an anti-GFP antibody (Figure 8A). As a control, the fractionation of the ER/NE integral membrane protein Snl1p (Ho et al., 1998) was coincidentally monitored (Figure 8B). In wt and mutant cells, the Snl1p fractionated exclusively with the P2 pellet. In wt cells grown at either 23°C (our unpublished observations) or 37°C, the fractionation pattern showed that a minimal (4%) amount of GFP-Nup49p was in the S1 fraction, with the vast majority of GFP-Nup49p in wt cells either extracted in the S2 fraction (45%) or in the insoluble P2 pellet (51%) (Figure 8A). These results are consistent with previous reports of FG repeat nucleoporin fractionation (Bogerd et al., 1994; Kenna et al., 1996). In contrast, the S1 pool of GFP-Nup49p in nup57-E17 cells grown at 23°C was significantly increased (17% S1, 30% S2, 53% P2). The increase in the S1 pool may reflect the accumulation of soluble unincorporated GFP-Nup49p in the cytoplasm. Thus, nup57pE17 results in a decreased two-hybrid interaction with Nup49p and an increased soluble pool of the GFP-Nup49p in mutant cells.
To test whether NPC association of nup57pE17 was also perturbed, polyclonal antibodies were raised against the C-terminal region of Nup57p. In immunoblotting, the anti-C Nup57p antibodies recognized both full-length Nup57p and the mutant nup57pE17 with equal efficiency (our unpublished observations). Indirect immunofluorescence microscopy was conducted on wt and nup57-E17 cells grown at the permissive and nonpermissive growth temperatures (Figure 9, left column). At 23°C, the nup57-E17 cells exhibited a markedly diminished staining intensity compared with wt cells. This suggested that the mutant protein was not incorporated into NPCs at wt levels. The signal was completely absent from nup57-E17 cells shifted to growth at 37°C, corresponding to the degradation of the nup57pE17 protein (Figure 7A). Taken together, the dim GFP-Nup49p phenotype in nup57-E17 cells likely reflects a combinatorial phenomenon: the truncation of Nup57p diminishes both NPC association of the mutant nup57pE17 protein and interaction with Nup49p.
Figure 9.
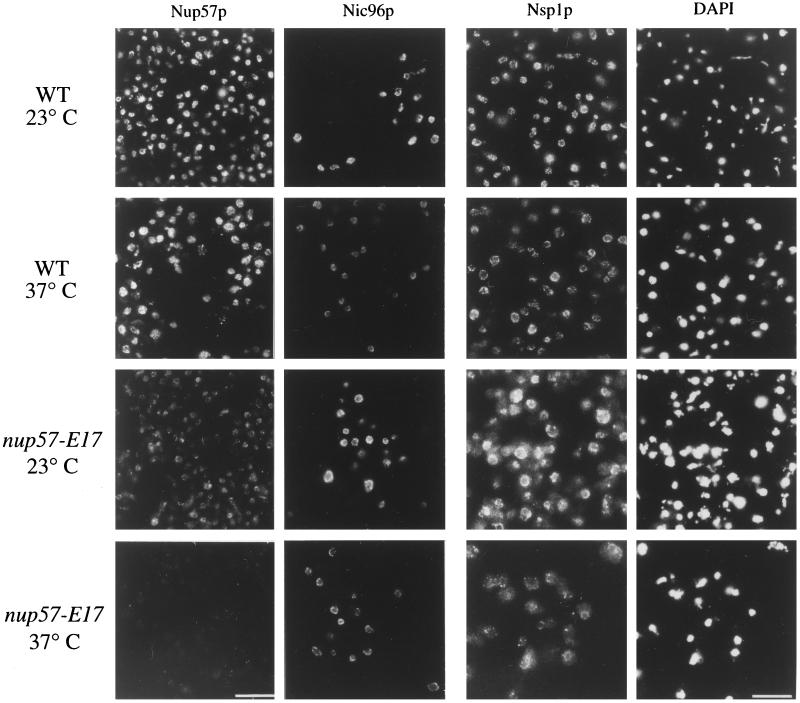
Microscopic analysis of NPC/NE association of nup57pE17, Nic96p, and Nsp1p in nup57-E17 cells reveals decreased staining for nup57pE17 and Nsp1p. Indirect immunofluorescence microscopy was performed on wt (SWY519) and nup57-E17 (SWY1587) strains using antibodies recognizing the C-terminal region of Nup57p (left column). Direct fluorescence microscopy was performed on wt (SWY1695, SWY1728) and nup57-E17 (SWY1722, SWY1736) strains expressing GFP-tagged Nic96p or Nsp1p, respectively. Cells were shifted to growth at 37°C for 4 h. Within each column, images were photographed and printed for identical times for direct comparison of fluorescence intensities. The DAPI photographs in the right column correspond to the fields of Nsp1p cells. Bars, 10 μm, with Nup57p and Nic96p images at identical magnifications.
NPC Composition Is Perturbed in nup57-E17 Cells
To analyze the extent of the nup57-E17 perturbation on overall NPC structure, we conducted a series of in situ localization and crude subcellular fractionation studies to monitor the NPC association of other nucleoporins in nup57-E17 cells. In addition to Nup49p, Nup57p also directly interacts with Nsp1p in vitro (Schlaich et al., 1997). Moreover, Nup57p copurifies in a complex containing Nup49p, Nsp1p, and the nucleoporin Nic96p (Grandi et al., 1995b). To test Nsp1p and Nic96p localization, the GFP-Nic96p described above was used and the sequence encoding GFP was fused in frame with the sequence encoding the essential C terminus of Nsp1p. The GFP-NSP1 and GFP-NIC96 genes were integrated in place of the respective chromosomal NSP1 and NIC96 alleles in both wt and nup57-E17 strains. The GFP-tagged proteins were functional as reflected by their ability to replace the essential wt proteins. At all growth temperatures, GFP-Nic96p localization and relative NPC incorporation levels in nup57-E17 cells appeared identical to that in wt cells (Figure 9). At 23°C, GFP-Nsp1p in nup57-E17 cells was also similar to wt cells in some cases, although the cytoplasmic background was increased (Figure 9). Interestingly, the NE staining intensity for GFP-Nsp1p was markedly decreased after growth of the nup57-E17 cells at 37°C. When analyzed by crude subcellular fractionation, the soluble S1 pool of GFP-Nsp1p in nup57-E17 cells increased two to threefold over wt levels (8% in the S1 of wt, 24% of the mutant at 23°C and 19% at 37°C) (Figure 8C). In addition, the insoluble P2 pool of GFP-Nsp1p decreased in the mutant cells (44% in wt vs. ∼28% in mutant cells at 23 and 37°C). Therefore, the microscopic perturbations of GFP-Nsp1p localization in nup57-E17 cells correlated with alterations in GFP-Nsp1p fractionation.
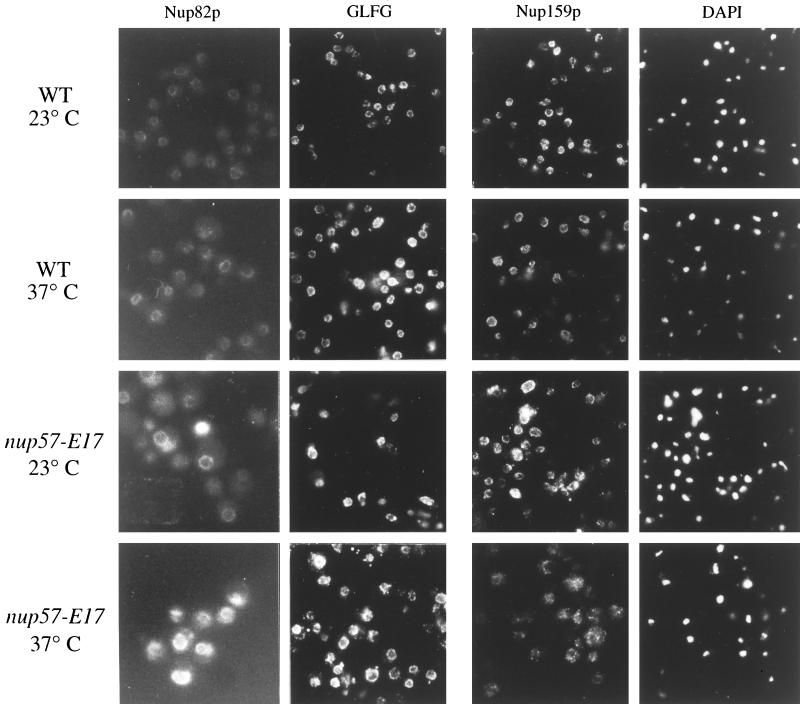
Recent copurification studies have suggested that Nsp1p is also a component of a distinct NPC complex that contains Nup82p and Nup159p (Grandi et al., 1995a; C. Cole, personal communication). To test whether the proposed Nsp1p-Nup82p-Nup159p and the Nsp1p-Nup57p-Nup49p complexes are distinct entities in vivo, we analyzed the localization of Nup82p and Nup159p in nup57-E17 cells. The chromosomal copy of NUP82 was tagged at the C terminus with sequence encoding GFP in both wt and nup57-E17 cells. The majority of the Nup82-GFP fluorescence signal was concentrated at the NE/NPC in both wt and mutant cells. However, the Nup82-GFP fluorescence intensity was noticeably increased in nup57-E17 cells grown at 23 or 37°C (Figure 10, left column). To localize Nup159p, indirect immunofluorescence microscopy was conducted on wt and nup57-E17 cells with a mAb raised against Nup159p (Kraemer et al., 1995). Overall, the anti-Nup159p staining is similar in wt and nup57-E17 mutant cells (Figure 10). In addition, the majority of the Nup159p fractionated in the extracted S2 pool from both wt and mutant cells (Figure 8D). Overall, these results suggested that loss of Nsp1p with the Nsp1p-nup57pE17-Nup49p complex did not grossly inhibit the NPC incorporation of Nup82p and Nup159p.
Figure 10.
NPC/NE association of Nup82p, a GLFG nucleoporin(s), and Nup159p are not markedly diminished in nup57-E17 cells. Direct fluorescence microscopy was performed on wt (SWY1411) and nup57-E17 (SWY1744) strains expressing GFP-tagged Nup82p (left column). Indirect immunofluorescence microscopy was performed on wt (SWY519) and nup57-E17 (SWY1587) strains using antibodies recognizing either the GLFG family of nucleoporins (middle column) or Nup159p (right column), with DAPI staining shown for the anti-Nup159p fields. Cells were shifted to growth at 37°C for 4 h. Within each column, images were photographed and printed for identical times for direct comparison of fluorescence intensities.
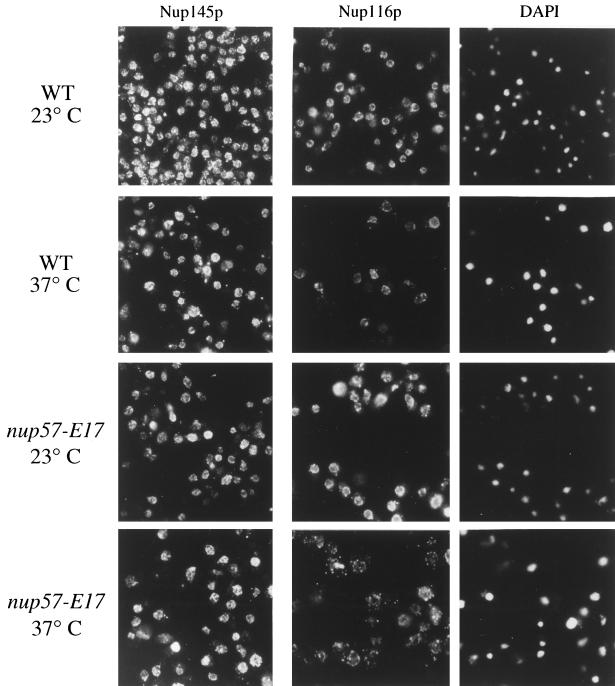
As described, previous studies have shown that Nsp1p is directly associated with two GLFG nucleoporins, Nup49p and Nup57p. Although physical connections have not been reported, NSP1 is also genetically connected to the genes encoding two other GLFG nucleoporins, Nup116p and Nup145p (Wimmer et al., 1992; Fabre et al., 1994). Indirect immunofluorescence was performed using a polyclonal antibody that recognizes the GLFG family of nucleoporins (Figure 10). In both wt and mutant nup57-E17 strains grown at either 23 or 37°C, the anti-GLFG staining reflected normal nucleoporin localization. As Nup49p and nup57pE17 are no longer present at 37°C (Figures 6, 7A, and 9), this suggested that at least one of the other GLFG nucleoporins remains NPC associated in arrested nup57-E17 cells. To directly test the localization of Nup116p and Nup145p, monospecific polyclonal antibodies recognizing their respective unique C-terminal regions were used. The anti-Nup145-Cp staining was not altered in nup57-E17 cells (Figure 11, left column), and subcellular fractionation of Nup145-Cp was not changed (Figure 8E). However, the staining for Nup116p was perturbed at 37°C (Figure 11, middle column). The anti-Nup116p signal in many of the nup57-E17 cells was less intense at the nuclear rim, but the NE-associated staining was not completely absent. The cytoplasmic staining level was also increased. Immunoblot analysis of the crude subcellular fractions confirmed a shift of Nup116p to the S1 soluble pool in nup57-E17 cells grown at 37°C (Figure 8F; 50% of the S1 in mutant cells, and 25% in wt). Taken together, these experiments suggested that the NPC associations of Nup49p, nup57pE17, Nsp1p, and Nup116p are altered in nup57-E17 cells at the nonpermissive growth temperature.
Figure 11.
Localization of the GLFG nucleoporin Nup116p at the NPC/NE is perturbed in temperature-arrested nup57-E17 cells, whereas Nup145-Cp remains predominantly at the NPC/NE. Indirect immunofluorescence microscopy was performed on wt (SWY519) and nup57-E17 (SWY1587) strains using antibodies recognizing either the C-terminal region of Nup145p (left column) or the C-terminal region of Nup116p (middle column). Coincident DAPI staining for the anti-Nup116p field is shown. Cells were shifted to growth at 37°C for 4 h. Within each column, images were photographed and printed for identical times for direct comparison of fluorescence intensities.
Nuclear Protein Import Is Inhibited in Temperature-arrested nup57-E17 Cells
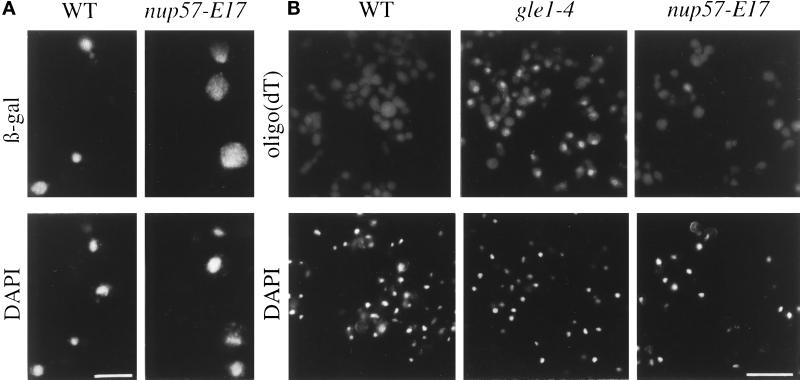
Although mutant nup57 alleles have been previously isolated (Grandi et al., 1995b), the nuclear transport capacity of these cells has not been reported. In situ assays were used to test the nup57-E17 cells for temperature-dependent nuclear protein import and poly(A)+ RNA export defects. The nup57-E17 strain was transformed with plasmids expressing a NLS-β-galactosidase fusion protein, under control of an inducible GAL promoter. If import is diminished during growth at 37°C, the reporter protein will not accumulate in the nucleus. Localization of the fusion protein was determined by indirect immunofluorescence microscopy with anti-β-galactosidase antibodies (Figure 12A). When grown at 37°C, wt cells imported the reporter protein as reflected by bright nuclear staining (Figure 12A, left). In contrast, the nup57-E17 cells at 37°C showed significantly enhanced cytoplasmic staining (Figure 12A, right). To measure poly(A)+ RNA export capacity, samples of wt and mutant cells were processed for in situ hybridization with a digoxigenin-labeled oligo(dT) (Figure 12B). The staining with a rhodamine-conjugated anti-digoxigenin antibody was diffuse and cytoplasmic at 37°C in wt cells and the majority of the mutant cells. A small percentage of the mutant cells showed a slight increase in nuclear poly(A)+ RNA localization, but in comparison to the RNA export mutant gle1–4 (Murphy and Wente, 1996), the nup57-E17 export phenotype was not strong or penetrant. These results suggested that the nup57-E17 cells had a primary defect in nuclear protein import. Given our observed coincident perturbation of Nsp1p in the nup57-E17 cells, this correlates well with the reported specific inhibition of import in nsp1 temperature-sensitive mutants (Mutvei et al., 1992; Nehrbass et al., 1990, 1993).
Figure 12.
Nuclear import capacity is decreased in temperature-arrested nup57-E17 cells. (A) To assay nuclear import, wt (SWY519) and nup57-E17 (SWY1587) cells transformed with a plasmid expressing NLS-β-galactosidase under GAL10 control (pNLS-E1) were analyzed. Cells grown in 2% raffinose media at 37°C for 2.5 h were induced for NLS-β-galactosidase expression by addition of 2% galactose, and growth at 37°C was continued for 3.5 h. Cells were fixed and processed for indirect immunofluorescence microscopy. Localization of the reporter was determined using mAbs against β-galactosidase as described in MATERIALS AND METHODS. (B) Export of poly(A)+ RNA was not strongly inhibited. wt (SWY809), gle1–4 (SWY1186), and nup57-E17 (SWY1586) cells were grown at 23°C, shifted to 37°C for 4 h, and processed for in situ hybridization with a digoxigenin-oligo(dT)30 probe. Rhodamine-conjugated anti-digoxigenin antibodies were used to localize probe binding. Exposure and printing times are identical for wt and mutant cells in a given experiment. Coincident DAPI staining is shown. Bar, 5 μm (A); 15 μm (B).
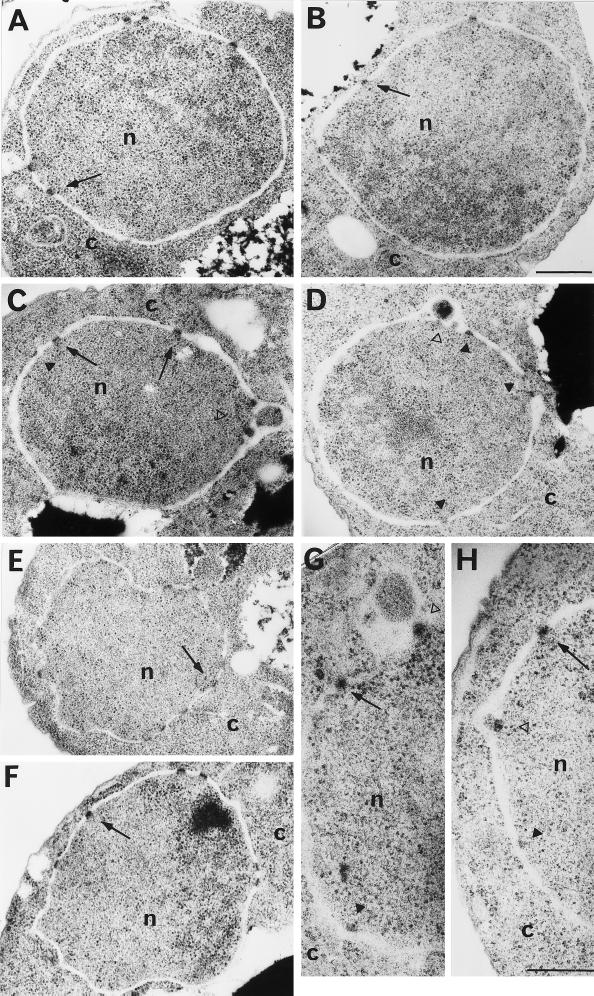
To determine the consequences of nucleoporin mislocalization on NPC and NE structure, we performed thin-section electron microscopy on the nup57-E17 cells. At 23°C, the NPC and NE structure in nup57-E17 cells (Figure 13A) was identical to that of wt cells (Figure 13E). The NPC structure is embedded in a pore formed where the inner and outer number membranes fuse and is represented as an electron dense mass ∼100 nm in diameter (e.g., at the arrows in Figure 13, A and E). After growth at 37°C for 4 h, wt NPC/NE structure was not altered (Figure 13F). However, in nup57-E17 arrested cells (Figure 13, B–D, G, H), some perturbations of NPC/NE structure were observed. The open arrowheads in Figure 13, C, D, and H, highlight possible NE herniations with the mutant NPC structures still anchored to the inner nuclear membrane. These herniations are similar to the perturbations found in temperature-arrested nup116 cells (Wente and Blobel, 1993) and may reflect the alteration in Nup116p association with the nup57-E17 NPCs (Figures 8 and 11). After 4 h at 37°C, some of the nup57-E17 NPCs still appeared similar to those in wt sections (arrows, Figure 13, B, C, G, and H), although sometimes the mass did not appear to span the entire lumenal space between the inner and outer membranes (at black arrowheads in Figure 13, C, D, G, and H). These structures may be similar to those recently reported in act2 mutants (Yan et al., 1997). Overall, the perturbations were clearly not penetrant, and gross perturbations of NE/NPC structure were not coincident with the nup57-E17 phenotype.
Figure 13.
Examination by electron microscopy of NE and NPC morphology in wt and nup57-E17 cells. NUP57 (SWY809) and nup57-E17 (SWY1586) cells were grown at either 23°C or shifted to growth at 37°C for 4 h before processing for thin section electron microscopy. Arrows point to NPCs with apparently wt structure; black arrowheads point to NPC-like electron dense masses that may not span the NE lumen between the inner and outer membranes; open arrowheads point to NE herniations. (A) nup57-E17 at 23°C. (B–D, G, H) nup57-E17 cells at 37°C. (E) wt at 23°C. (F) wt at 37°C. n, nucleus; c, cytoplasm. Bars, 500 nm (A–F); 250 nm (G and H).
DISCUSSION
Understanding the nearest-neighbor and assembly interactions of different nucleoporins in the context of NPC architecture will provide important clues for understanding NPC transport function. In this report we describe a novel genetic strategy to identify mediators of NPC assembly in S. cerevisiae. This approach is based on our ability to tag nucleoporins with GFP and the hypothesis that NPC assembly mutants will have distinct GFP fluorescence properties compared with wt cells. To date, such screens for NPC biogenesis mutants have not been possible. By using FACS to select for cells with low GFP-Nup49p levels, we identified three mutant complementation groups representing alleles of known NPC-associated proteins: NUP120, GLE2, and NUP57. Our studies analyzing NPC structure in the nup57-E17 cells have revealed a network of in vivo interactions between the nucleoporins Nup57p, Nup49p, Nsp1p, and Nup116p. We predict that such fluorescence-based screening will prove a powerful approach for identification and characterization of factors that mediate NPC biogenesis and structural integrity.
Characterization of the temperature-sensitive nup57-E17 mutant provided a unique opportunity to evaluate in vivo the functional consequences of the Nup57p-Nup49p interaction. We determined that the NPC association of Nup49p was diminished in nup57-E17 cells due in part to a decreased interaction of Nup49p with nup57pE17. The nup57pE17 protein is a C-terminal truncation missing the last 26 amino acid residues, and thus most of the third predicted heptad repeat in the C-terminal region of Nup57p is deleted (Grandi et al., 1995b). This suggests that this region of Nup57p contains the primary determinants for the assembly of Nup49p into the NPC.
The NPC assembly of only GFP-Nup49p and nup57pE17 were notably altered at 23°C in nup57-E17 cells. However, at the nonpermissive growth temperature of 37°C, the NPC associations of Nsp1p and Nup116p were also perturbed. In particular, levels of GFP-Nsp1p detectable at the NPC were sharply decreased. These results confirm a hypothesis based on in vitro studies that Nup49p and Nsp1p individually bind Nup57p (Schlaich et al., 1997). Moreover, the stability of the Nsp1p-Nup57p-Nup49p heterotrimer appears key for the NPC association of each individual component. Studies from others have shown that wt Nup57p copurifies from yeast cells in a complex with Nsp1p, Nup49p, and Nic96p (Grandi et al., 1995b). In addition, NPC association of the Nsp1p-Nup57p-Nup49p heterotrimer requires interaction with the amino-terminal coiled-coil domain of Nic96p. Here we further show that the NPC association of Nic96p is not dependent on the presence of Nsp1p-Nup57p-Nup49p. The fact that Nic96p was not perturbed in nup57-E17 cells supports the hypothesis that Nic96p is a core component of the NPC, which anchors the Nsp1p-Nup57p-Nup49p heterotrimer in the intact NPC.
Interestingly, we also observed a perturbation of Nup116p localization at 37°C in nup57-E17 cells. A physical (or genetic) connection between Nup57p and Nup116p has not been previously reported, and a physical connection between Nup116p and the Nsp1p-Nup57p-Nup49p complex has also not been established. However, nup116 mutant alleles are synthetically lethal in combination with nsp1 mutants (Wimmer et al. 1992), suggesting Nup116p may be indirectly perturbed due to the loss of Nsp1p. It is also possible that Nup116p is associated with an as-yet-uncharacterized Nsp1p complex and our results would then demonstrate in vivo a relationship between complexes that potentially behave differently biochemically.
Recent studies have suggested that at least two biochemically distinct Nsp1p complexes exist in the NPC. Subcellular fractionation analysis has shown that Nup82p and Nup159p copurify with Nsp1p in a complex distinct from the Nsp1p-Nup57p-Nup49p heterotrimer (Grandi et al., 1995a; Cole, personal communication). The NPC associations of Nup82p and Nup159p were not notably diminished in nup57-E17 cells. This suggests that at least these two biochemically distinct Nsp1p complexes are also functionally and/or physically distinct in vivo.
The perturbations of NPC structure in nup57-E17 cells coincidentally resulted in a specific inhibition of nuclear protein import capacity. A penetrant and strong block of poly(A)+ RNA export was not observed, despite the fact that nup116 mutants have RNA export defects (Wente and Blobel, 1993). The RNA export defects in the nup116 null mutant are likely the indirect consequence of membranous seals and herniations forming over the majority of the NPCs. Such a penetrant structural defect was not observed in the nup57-E17 cells. It is also possible that Nup57p has a role in export but that in nup57-E17 cells; a rapid import defect coincident with the loss of nup57pE17, Nup49p, and Nsp1p kills the cells and prevents any decrease in export capacity to manifest itself. Alternatively, enough NPCs may remain that contain the functional complement of nucleoporins required to maintain NPC structure and support poly(A)+ RNA export. The fluorescence analysis does not necessarily reflect that all the NPCs in a cell are of the same physical composition, and we suspect that the NPCs in nup57-E17 cells are falling apart at differential rates.
Overall, we conclude that Nup57p plays a role in maintaining NPC structure. This study also reinforces the premise that each nucleoporin may have multiple interactions within the context of the NPC. Extensive interactions among nucleoporins have been highlighted by studies characterizing synthetic lethal genetic interactions between mutant alleles in genes encoding different nucleoporins (reviewed by Davis, 1995; Corbett and Silver, 1997; Doye and Hurt, 1997; Wente et al. 1997). NPC function is quite sensitive to the combined loss/mutation of multiple components. Moreover, as illustrated in nup57-E17 cells, a single mutant can perturb the NPC association of multiple wt nucleoporins. However, individual nucleoporins also behave differently within the NPC structure, and mutations that affect one set of protein–protein interactions do not necessarily affect all of them nor do they necessarily destabilize the overall NPC structure. For example, the NPC localizations of both Nup145p and Pom152p were not apparently perturbed in the nup57-E17 cells.
From the GFP-NPC screen, we expected to obtain at least three different classes of mutants defective in NPC biogenesis: Class I) NPC clustering mutants, Class II) mutants with fewer total NPCs per nucleus, and Class III) mutants with wt NPC number but each NPC having a decreased amount of the particular GFP-protein incorporated. Here we report the isolation of both Class I and Class III mutants from a screen with cells expressing GFP-Nup49p. The temperature-sensitive nup57-E17 mutant was isolated based on its decreased incorporation of GFP-Nup49p into the NPCs (a Class III mutant). Based on these results with GFP-Nup49p and nup57-E17, we predict that similar strategies using different GFP-tagged nucleoporins could be used to identify factors that directly interact with the given GFP-Nup and to map the entire network of interactions critical for a given protein’s incorporation into the NPC.
The identification of known nup120 and gle2 clustering mutants (Class I) further validates our GFP fluorescence-based approach for identifying genes related to NPC biogenesis. Interestingly, the previously characterized gle2–1 mutant displays temperature-conditional NPC clustering (Murphy et al., 1996; Bucci and Wente, 1997), whereas the gle2-C18 allele isolated in this study forms clusters constitutively. Understanding the differences between these alleles may be informative for delineating the role of Gle2p in NPC dynamics. As all three complementation groups isolated were represented by only single alleles and mutations in the other five genes known to be related to NPC clustering defects were not identified, the GFP-Nup49p screen to date has not saturated the entire yeast genome. Moreover, we did not obtain any Class II mutants wherein the total NPC number was constitutively lowered. We are currently conducting a fluorescence-based GFP-NPC screen on a larger scale in which several refinements are being incorporated to achieve greater genomic saturation and to identify global mediators of NPC assembly. The identification and characterization of yeast mutants with a constitutively lowered NPC density will make important contributions toward understanding the mechanism of NPC assembly.
ACKNOWLEDGMENTS
We are indebted to Dr. Lois Weisman for continuously sharing her expertise, providing encouragement during the entire development of this project, and inviting us to conduct the FACS isolations in her laboratory. We thank J. Fishbaugh and the University of Iowa FACS facility for excellent technical assistance; J. Watkins for technical assistance and production of anti-GLFG and anti-57C nucleoporin antibodies; C. Price for technical assistance; M. Levy and L. LaRose for electron microscopy assistance; F. Stutz, C. Cole, R. Wozniak, R. Tsien, L. Riles, H. Fried, C. Hardy, J. Watkins, and K. Iovine for sharing yeast strains and plasmids; C. Strambio-de -Castillia, G. Blobel, and M. Rout for the anti-Nup159p and Pom152p monoclonals; C. Cole for the polyclonal anti-Nup159p; and S. Dowdy for use of his FACScan. K. Iovine, A. Ho, C. Cole, and L. Weisman provided valuable comments on the article and/or critical discussions. This work was supported by a Beckman Young Investigator Award from the Arnold and Mabel Beckman Foundation and an American Cancer Society Junior Faculty Research Award to S.R.W.
Abbreviations used:
- GFP
green fluorescent protein
- NPC
nuclear pore complex
- NE
nuclear envelope
- wt
wild-type
REFERENCES
- Aitchison JD, Blobel G, Rout MP. Nup120p: a yeast nucleoporin required for NPC distribution and mRNA transport. J Cell Biol. 1995;131:1659–1675. doi: 10.1083/jcb.131.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey CW. Structural plasticity of the nuclear pore complex. J Mol Biol. 1995;248:273–293. doi: 10.1016/s0022-2836(95)80050-6. [DOI] [PubMed] [Google Scholar]
- Akey CW, Radermacher M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J Cell Biol. 1993;122:1–19. doi: 10.1083/jcb.122.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin A, Ozier KO, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd AM, Hoffman JA, Amberg DC, Fink GR, Davis LI. nup1 mutants exhibit pleiotropic defects in nuclear pore complex function. J Cell Biol. 1994;127:319–332. doi: 10.1083/jcb.127.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman AL, Delannoy MR, Wilson KL. GTP hydrolysis is required for vesicle fusion during nuclear envelope assembly in vitro. J Cell Biol. 1992a;116:281–294. doi: 10.1083/jcb.116.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman AL, Taylor TC, Melancon P, Wilson KL. A role for ADP-ribosylation factor in nuclear vesicle dynamics. Nature. 1992b;358:512–514. doi: 10.1038/358512a0. [DOI] [PubMed] [Google Scholar]
- Bucci M, Wente SR. In vivo dynamics of nuclear pore complexes in yeast. J Cell Biol. 1997;136:1185–1199. doi: 10.1083/jcb.136.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B, Gerace L. A cell free system to study reassembly of the nuclear envelope at the end of mitosis. Cell. 1986;44:639–652. doi: 10.1016/0092-8674(86)90273-4. [DOI] [PubMed] [Google Scholar]
- Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Corbett AH, Silver PA. Nucleocytoplasmic transport of macromolecules. Microbiol Mol Biol Rev. 1997;61:193–211. doi: 10.1128/mmbr.61.2.193-211.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabauvalle MC, Loos K, Scheer U. Identification of a soluble precursor complex essential for nuclear pore assembly in vitro. Chromosoma. 1990;100:56–66. doi: 10.1007/BF00337603. [DOI] [PubMed] [Google Scholar]
- Davis LI. The nuclear pore complex. Annu Rev Biochem. 1995;64:865–896. doi: 10.1146/annurev.bi.64.070195.004245. [DOI] [PubMed] [Google Scholar]
- Doye V, Hurt E. From nucleoporins to nuclear pore complexes. Curr Opin Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- Doye V, Wepf R, Hurt EC. A novel nuclear pore protein Nup133p with distinct roles in poly(A)+ RNA transport and nuclear pore distribution. EMBO J. 1994;13:6062–6075. doi: 10.1002/j.1460-2075.1994.tb06953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtage JLT, Bucci M, Watkins JL, Wente SR. Defining the essential structural regions of the nucleoporin Nup145p. J Cell Sci. 1997;110:911–925. doi: 10.1242/jcs.110.7.911. [DOI] [PubMed] [Google Scholar]
- Fabre E, Boelens WC, Wimmer C, Mattaj IW, Hurt EC. Nup145p is required for nuclear export of mRNA and binds homopolymeric RNA in vitro via a novel conserved motif. Cell. 1994;78:275–289. doi: 10.1016/0092-8674(94)90297-6. [DOI] [PubMed] [Google Scholar]
- Finlay DR, Forbes DJ. Reconstitution of biochemically altered nuclear pores: transport can be eliminated and restored. Cell. 1990;60:17–29. doi: 10.1016/0092-8674(90)90712-n. [DOI] [PubMed] [Google Scholar]
- Finlay DR, Meier E, Bradley P, Horecka J, Forbes DJ. A complex of nuclear pore proteins required for pore function. J Cell Biol. 1991;114:169–183. doi: 10.1083/jcb.114.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L, Ottaviano Y, Kondor-Koch C. Identification of a major polypeptide of the nuclear pore complex. J Cell Biol. 1982;95:826–837. doi: 10.1083/jcb.95.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MW, Allen TD. Structural and functional organization of the nuclear envelope. Curr Opin Cell Biol. 1995;7:301–309. doi: 10.1016/0955-0674(95)80083-2. [DOI] [PubMed] [Google Scholar]
- Goldberg MW, Wiese C, Allen TD, Wilson KL. Dimples, pores, star-rings, and thin rings on growing nuclear envelopes: evidence for structural intermediates in nuclear pore complex assembly. J Cell Sci. 1997;110:409–420. doi: 10.1242/jcs.110.4.409. [DOI] [PubMed] [Google Scholar]
- Goldstein AL, Snay CA, Heath CV, Cole CN. Pleiotropic nuclear defects associated with a conditional allele of the novel nucleoporin Rat9p/Nup85p. Mol Biol Cell. 1996;7:917–934. doi: 10.1091/mbc.7.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsch LC, Dockendorff TC, Cole CN. A conditional allele of the novel repeat-containing yeast nucleoporin RAT7/NUP159 causes both rapid cessation of mRNA export and reversible clustering of nuclear pore complexes. J Cell Biol. 1995;129:939–955. doi: 10.1083/jcb.129.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P, Dang T, Pane N, Shevchenko A, Mann M, Forbes D, Hurt E. Nup93, a vertebrate homologue of yeast Nic96p, forms a complex with a novel 205-kDa protein and is required for correct nuclear pore assembly. Mol Biol Cell. 1997;8:2017–2038. doi: 10.1091/mbc.8.10.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P, Emig S, Weise C, Hucho F, Pohl T, Hurt EC. A novel nuclear pore protein Nup82p which specifically binds to a fraction of Nsp1p. J Cell Biol. 1995a;130:1263–1273. doi: 10.1083/jcb.130.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P, Schlaich N, Tekotte H, Hurt EC. Functional interaction of Nic96p with a core nucleoporin complex consisting of Nsp1p, Nup49p and a novel protein Nup57p. EMBO J. 1995b;14:76–87. doi: 10.1002/j.1460-2075.1995.tb06977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber UF, Senior A, Gerace L. A major glycoprotein of the nuclear pore complex is a membrane-spanning polypeptide with a large lumenal domain and a small cytoplasmic tail. EMBO J. 1990;9:1495–502. doi: 10.1002/j.1460-2075.1990.tb08267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Guldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg E, Wozniak RW, Blobel G. An integral membrane protein of the pore membrane domain of the nuclear envelope contains a nucleoporin-like region. J Cell Biol. 1993;122:513–21. doi: 10.1083/jcb.122.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CFJ. OAF1, and essential ORC2 associated factor, plays a role in DNA replication. Mol Cell Biol. 1996;16:1832–1841. doi: 10.1128/mcb.16.4.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CV, Copeland CS, Amberg DC, Delpriore V, Snyder M, Cole CN. Nuclear pore complex clustering and nuclear accumulation of poly(A)+ RNA associated with mutation of the Saccharomyces cerevisiae rat2/nup120 gene. J Cell Biol. 1995;131:1677–1697. doi: 10.1083/jcb.131.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim R, Tsien RY. Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr Biol. 1996;6:178–182. doi: 10.1016/s0960-9822(02)00450-5. [DOI] [PubMed] [Google Scholar]
- Hinshaw JE, Carragher BO, Milligan RA. Architecture and design of the nuclear pore complex. Cell. 1992;69:1133–1141. doi: 10.1016/0092-8674(92)90635-p. [DOI] [PubMed] [Google Scholar]
- Ho AK, Raczniak GA, Ives EB, Wente SR. The integral membrane protein Snl1p is genetically linked to yeast nuclear pore complex function. Mol Biol Cell. 1998;9:355–373. doi: 10.1091/mbc.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovine MK, Watkins JL, Wente SR. The GLFG repetitive region of the nucleoporin Nup116p interacts with Kap95p, an essential yeast nuclear import factor. J Cell Biol. 1995;131:1699–1713. doi: 10.1083/jcb.131.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna MA, Petranka JG, Reilly JL, Davis LI. Yeast Nle3/Nup170p is required for normal stoichiometry of FG nucleoporins within the nuclear pore complex. Mol Cell Biol. 1996;16:2025–2036. doi: 10.1128/mcb.16.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer DM, Strambio-de-Castillia C, Blobel G, Rout MP. The essential yeast nucleoporin NUP159 is located on the cytoplasmic side of the nuclear pore complex and serves in karyopherin-mediated binding of transport substrate. J Biol Chem. 1995;270:19017–19021. doi: 10.1074/jbc.270.32.19017. [DOI] [PubMed] [Google Scholar]
- Lenz-Bohme B, Wismar J, Fuchs S, Reifegerste R, Buchner E, Betz H, Schmitt B. Insertional mutation of the Drosophilia nuclear lamin Dmo gene results in defective nuclear envelopes, clustering of nuclear pore complexes, and accumulation of annulate lamellae. J Cell Biol. 1997;137:1001–1016. doi: 10.1083/jcb.137.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li O, Heath CV, Amberg DC, Dockendorff TC, Copeland CS, Snyder M, Cole CN. Mutation or deletion of the Saccharomyces cerevisiae RAT3/NUP133 gene causes temperature-dependent nuclear accumulation of poly(A)+ RNA and constitutive clustering of nuclear pore complexes. Mol Biol Cell. 1995;6:401–417. doi: 10.1091/mbc.6.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka MJ, Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983;220:719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Macaulay C, Forbes DJ. Assembly of the nuclear pore— biochemically distinct steps revealed with nem, gtp-gamma-s, and bapta. J Cell Biol. 1996;132:5–20. doi: 10.1083/jcb.132.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake-Lye R, Kirschner MW. Induction of early mitotic events in a cell-free system. Cell. 1985;41:165–175. doi: 10.1016/0092-8674(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Murphy R, Watkins JL, Wente SR. GLE2, a S. cerevisiae homologue of the S. pombe export factor RAE1, is required for nuclear pore complex structure and function. Mol Biol Cell. 1996;7:1921–1937. doi: 10.1091/mbc.7.12.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R, Wente S. An RNA export mediator with an essential nuclear export signal. Nature. 1996;383:357–360. doi: 10.1038/383357a0. [DOI] [PubMed] [Google Scholar]
- Mutvei A, Dihlmann S, Herth W, Hurt EC. NSP1 depletion in yeast affects nuclear pore formation and nuclear accumulation. Eur J Cell Biol. 1992;59:280–295. [PubMed] [Google Scholar]
- Nehrbass U, Fabre E, Dihlmann S, Herth W, Hurt EC. Analysis of nucleo-cytoplasmic transport in a thermosensitive mutant of nuclear pore protein NSP1. Eur J Cell Biol. 1993;62:1–12. [PubMed] [Google Scholar]
- Nehrbass U, Kern H, Mutvei A, Horstmann H, Marshallsay B, Hurt EC. NSP1: a yeast nuclear envelope protein localized at the nuclear pores exerts its essential function by its carboxy-terminal domain. Cell. 1990;61:979–89. doi: 10.1016/0092-8674(90)90063-k. [DOI] [PubMed] [Google Scholar]
- Newmeyer DD, Forbes DJ. A N-ethylmaleimide-sensitive cytosolic factor necessary for nuclear protein import: requirement in signal-mediated binding to the nuclear pore. J Cell Biol. 1990;110:547–557. doi: 10.1083/jcb.110.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J. Nuclear reconstitution in vitro: stages of assembly around protein-free DNA. Cell. 1987;48:205–217. doi: 10.1016/0092-8674(87)90424-7. [DOI] [PubMed] [Google Scholar]
- Newport JW, Dunphy W. Characterization of the membrane binding and fusion events during nuclear envelope assembly using purified components. J Cell Biol. 1992;116:295–306. doi: 10.1083/jcb.116.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pante N, Aebi U. Molecular dissection of the nuclear pore complex. Crit Rev Biochem Mol Biol. 1996;31:153–199. doi: 10.3109/10409239609106583. [DOI] [PubMed] [Google Scholar]
- Pemberton LF, Rout MP, Blobel G. Disruption of the nucleoporin gene NUP133 results in clustering of nuclear pore complexes. Proc Natl Acad Sci USA. 1995;92:1187–1191. doi: 10.1073/pnas.92.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller R, Smythe C, Newport JW. Assembly/disassembly of the nuclear envelope membrane: cell cycle-dependent binding of nuclear membrane vesicles to chromatin in vitro. Cell. 1991;65:209–217. doi: 10.1016/0092-8674(91)90155-r. [DOI] [PubMed] [Google Scholar]
- Ris H. The three dimensional structure of the nuclear pore complex as seen by high voltage electron microscopy and high resolution low voltage scanning electron microscopy. EMSA Bull. 1991;21:54–56. [Google Scholar]
- Rout MP, Blobel G. Isolation of the yeast nuclear pore complex. J Cell Biol. 1993;123:771–783. doi: 10.1083/jcb.123.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaich NL, Haner M, Lustig A, Aebi U, Hurt EC. In vitro reconstitution of a heterotrimeric nucleoporin complex consisting of recombinant Nsp1p, Nup49p, and Nup57p. Mol Biol Cell. 1997;8:33–46. doi: 10.1091/mbc.8.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan MA, Mills AD, Sleeman AM, Laskey RA, Blow JJ. Steps in the assembly of replication-competent nuclei in a cell-free system from Xenopus eggs. J Cell Biol. 1988;106:1–12. doi: 10.1083/jcb.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou S, Wimmer C, Rieger M, Doye V, Tekotte H, Weise C, Emig S, Segref A, Hurt EC. A novel complex of nucleoporins, which includes Sec13p and a Sec13p homolog, is essential for normal nuclear pores. Cell. 1996;84:265–275. doi: 10.1016/s0092-8674(00)80981-2. [DOI] [PubMed] [Google Scholar]
- Strambio-de-Castillia C, Blobel G, Rout MP. Isolation and characterization of nuclear envelopes from the yeast Saccharomyces. J Cell Biol. 1995;131:19–31. doi: 10.1083/jcb.131.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KM, Busa WB, Wilson KL. Calcium mobilization is required for nuclear vesicle fusion in vitro: implications for membrane traffic and IP3 receptor function. Cell. 1993;73:1411–1422. doi: 10.1016/0092-8674(93)90366-x. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Gerace L. A fractionated cell-free system for analysis of prophase nuclear disassembly. J Cell Biol. 1986;103:2073–2081. doi: 10.1083/jcb.103.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MR, Fried HM. Characterization of nuclear localizing sequences derived from yeast ribosomal protein L29. EMBO J. 1990;9:91–99. doi: 10.1002/j.1460-2075.1990.tb08084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers GP, Lohka MJ. A distinct vesicle population targets membranes and pore complexes to the nuclear envelope in Xenopus eggs. J Cell Biol. 1991;112:545–556. doi: 10.1083/jcb.112.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers GP, Lohka MJ. Regulation of nuclear envelope precursor functions during cell division. J Cell Sci. 1992;102:273–284. doi: 10.1242/jcs.102.2.273. [DOI] [PubMed] [Google Scholar]
- Wendland B, McCaffery JM, Xiao Q, Emr SD. A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J Cell Biol. 1996;135:1485–1500. doi: 10.1083/jcb.135.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente SR, Blobel G. A temperature-sensitive NUP116 null mutant forms a nuclear envelope seal over the yeast nuclear pore complex thereby blocking nucleocytoplasmic traffic. J Cell Biol. 1993;123:275–284. doi: 10.1083/jcb.123.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente SR, Blobel G. NUP145 encodes a novel yeast glycine-leucine-phenylalanine-glycine (GLFG) nucleoporin required for nuclear envelope structure. J Cell Biol. 1994;125:955–969. doi: 10.1083/jcb.125.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente SR, Gasser SM, Caplan AJ. The nucleus and nucleocytoplasmic transport in Saccharomyces cerevisiae. In: Pringle J, Broach J, Jones E, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 471–546. [Google Scholar]
- Wente SR, Rout MP, Blobel G. A new family of yeast nuclear pore complex proteins. J Cell Biol. 1992;119:705–723. doi: 10.1083/jcb.119.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer C, Doye V, Grandi P, Nehrbass U, Hurt EC. A new subclass of nucleoporins that functionally interact with nuclear pore protein NSP1. EMBO J. 1992;11:5051–5061. doi: 10.1002/j.1460-2075.1992.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak RW, Bartnik E, Blobel G. Primary structure analysis of an integral membrane glycoprotein of the nuclear pore. J Cell Biol. 1989;108:2083–2092. doi: 10.1083/jcb.108.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak RW, Blobel G, Rout MP. POM152 is an integral protein of the pore membrane domain of the yeast nuclear envelope. J Cell Biol. 1994;125:31–42. doi: 10.1083/jcb.125.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Leibowitz N, Melese T. A role for the divergent actin gene, ACT2, in nuclear pore complex structure and function. EMBO J. 1997;16:3572–3586. doi: 10.1093/emboj/16.12.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hubbard EJ, Carlson M. A protein kinase substrate identified by the two-hybrid system. Science. 1992;257:680–682. doi: 10.1126/science.1496382. [DOI] [PubMed] [Google Scholar]
- Zabel U, Doye V, Tekotte H, Wepf R, Grandi P, Hurt EC. Nic96p is required for nuclear pore formation and functionally interacts with a novel nucleoporin, Nup188p. J Cell Biol. 1996;133:1141–1152. doi: 10.1083/jcb.133.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]