Abstract
OBJECTIVE—Transforming growth factor-β (TGF-β) can exhibit strong immune suppression but has also been shown to promote T-cell growth. We investigated the differential effect of this cytokine on CD8+ T-cells in autoimmunity and antiviral immunity.
RESEARCH DESIGN AND METHODS—We used mouse models for virally induced type 1 diabetes in conjunction with transgenic systems enabling manipulation of TGF-β expression or signaling in vivo.
RESULTS—Surprisingly, when expressed selectively in the pancreas, TGF-β reduced apoptosis of differentiated autoreactive CD8+ T-cells, favoring their expansion and infiltration of the islets. These results pointed to drastically opposite roles of TGF-β on naïve compared with antigen-experienced/memory CD8+ T-cells. Indeed, in the absence of functional TGF-β signaling in T-cells, fast-onset type 1 diabetes caused by activation of naïve CD8+ T-cells occurred faster, whereas slow-onset disease depending on accumulation and activation of antigen-experienced/memory CD8+ T-cells was decreased. TGF-β receptor–deficient CD8+ T-cells showed enhanced activation and expansion after lymphocytic choriomeningitis virus infection in vivo but were more prone to apoptosis once antigen experienced and failed to survive as functional memory cells. In vitro, TGF-β suppressed naïve CD8+ T-cell activation and γ-interferon production, whereas memory CD8+ T-cells stimulated in the presence of TGF-β showed enhanced survival and increased production of interleukin-17 in conjunction with γ-interferon.
CONCLUSIONS—The effect of TGF-β on CD8+ T-cells is dependent on their differentiation status and activation history. These results highlight a novel aspect of the pleiotropic nature of TGF-β and have implications for the design of immune therapies involving this cytokine.
Transforming growth factor β1 (TGF-β) is a multifunctional cytokine that acts on a wide assortment of cell types regulating both cell growth and differentiation. TGF-β can have potent inhibitory effects on T-cell proliferation, cytokine production, and cytolytic functions (1). In addition, the development of mice lacking functional TGF-β receptor signaling specifically in T-cells has illustrated the important role of TGF-β in maintaining normal immune homeostasis (2,3). However, despite the clear inhibitory effects of TGF-β on T-cells, several studies also demonstrate that this cytokine can in certain circumstances enhance the growth of T-cells (4–6). For example, TGF-β, along with interleukin (IL)-2, enables prolonged CD4+ T-cell effector expansion (4). The enhanced accumulation of T-cells cultured in TGF-β may be at least in part caused by the antiapoptotic effect of this cytokine (4,5,7).
The diverse effects of TGF-β are dependent on T-cell type (Th1, Th2, CD4+, CD8+), differentiation status (naïve versus memory), and microenvironment (cytokine and cellular milieu) (4,6,8). Because of its ability to inhibit T-cell responses, many studies have evaluated TGF-β as a potential therapeutic agent for treating autoimmune diseases like type 1 diabetes. Indeed, systemic administration of TGF-β was shown to protect from this disease (9). In addition, TGF-β is produced on therapy or induction of regulatory T-cells that protect from type 1 diabetes (10,11). Transgenic expression of TGF-β in pancreatic β-cells was also found to prevent diabetes in the NOD model for type 1 diabetes (12–14). However, in other diabetes models, TGF-β expressed in the islets either had no effect (15) or resulted in spontaneous disease when expressed along with tumor necrosis factor-α (16). A superimposed problem in some of these previous studies was that constitutive expression of TGF-β led to significant fibrosis in the islets, which made interpretation of the findings more difficult (15). Overall, little is known about the effect of TGF-β on CD8+ T-cells in type 1 diabetes.
Here, we examined the role of TGF-β on CD8+ T-cell effector functions in the pathogenesis of virally induced type 1 diabetes by using transgenic mouse models allowing for precise tracking of the autoreactive CD8+ T-cell response, selective expression of TGF-β in the pancreatic islets, or elimination of functional TGF-β receptor expression on T-cells. The RIP-LCMV mouse model for virally induced type 1 diabetes (17,18) is well suited to address the role played by TGF-β in autoimmunity while at the same time evaluating the effect of this cytokine on antiviral immunity. RIP-LCMV mice express viral antigens from LCMV under the control of RIP selectively on pancreatic β-cells and, in some lines, in the thymus (19). In C57BL/6 RIP-LCMV–glycoprotein (RIP-GP) and BALB/c RIP-LCMV–nucleoprotein (RIP-NP) mice, which do not express the viral antigen in the thymus, infection with LCMV induces rapid CD8+ T-cell–dependent diabetes. In contrast, disease develops more slowly in C57BL/6 RIP-NP mice as a consequence of expression of the LCMV nucleoprotein not only in the pancreas but also the thymus. In C57BL/6 RIP-NP mice, LCMV infection is cleared before insulitis and autoimmunity fully develop. In this model, the CD8+ T-cells infiltrating the islets are of the memory effector phenotype, whereas in the more rapid diabetes models, primary activation of naïve CD8+ T-cells in lymphoid organs in the periphery on viral infection is responsible for β-cell destruction.
In the current study, we crossed RIP-LCMV mice to different mouse trains which enabled control over TGF-β expression or signaling. First, we used the tetracycline-dependent TGF-β gene transcription system (TTA/TGF-β) (14), thereby allowing for TGF-β expression selectively in the islets to be turned on and off at will. This strategy appeared necessary in light of earlier studies showing that constitutive expression of TGF-β in islets leads to profound fibrosis, making immunologic interpretation difficult (12,13). Second, we used dnTGFβR transgenic mice whose T-cells lack expression of the receptor for TGF-β as a result of transgenic expression of a dominant-negative form of TGF-β receptor selectively in T-cells (2). The double-transgenic mice generated using the RIP-LCMV and TTA/TGF-β or dnTGFβR strains constituted ideal models to address the role of TGF-β in autoreactive and antiviral CD8+ T-cell responses in vivo. Our results highlight a differential effect of TGF-β on naïve compared with antigen-experienced/memory CD8+ T-cells and their function in virally induced type 1 diabetes.
RESEARCH DESIGN AND METHODS
TTA/TGF-β mice expressing TGF-β selectively on β-cells on removal of doxycycline from the diet were generated on the NOD background (14) and crossed with BALB/c mice. TTA/TGF-β mice on the BALB/c background were crossed with BALB/c RIP-NP mice. dnTGFβR mice on the C57BL/6 background (2) were crossed onto the C57BL/6 RIP-GP and C57BL/6 RIP-NP lines (18,19). Naïve T-cell receptor transgenic P14 mice on the C57BL/6 background (20) were used to obtain GP33-specific CD8+ T-cells. All mice were 6–9 weeks old when used in experiments. In all experiments using transgenic RIP-LCMV mice for diabetes studies, transgene-positive and -negative RIP-LCMV littermates were used. Blood glucose was monitored using the OneTouch Ultra system (LifeScan) at weekly intervals. Diabetes was defined as blood glucose values >300 mg/dl (21).
Virus and peptides.
LCMV Armstrong clone 53b (22) was used throughout all experiments. Virus was plaque-purified three times on Vero cells, and stocks were prepared by a single passage on BHK-21 cells. Mice were infected with a single dose of 105 plaque-forming units intraperitoneally. The dominant H2-ld–restricted LCMV epitope NP118–126 (NP118) or the dominant H2-Db–restricted LCMV epitope GP33–41 (GP33) (LIAI Peptide Core Facility) were used for viral and diabetes studies (23).
Immunohistochemistry.
For all stainings, 6- to 10-μm tissue sections were cut using a cryomicrotome and sialin-coated Superfrost Plus slides (Fisher Scientific). For TGF-β staining, tissues were fixed in 4% formalin and paraffin embedded. Sections were then rehydrated, quenched with H2O2 to block endogenous peroxidase, and treated in a microwave oven in the presence of citrate buffer (10 mmol/l, pH 6.0) for 10 min (two cycles of 5 min each). TGF-β was stained using rabbit anti-mouse TGF-β1 (Santa Cruz Biotechnology) and biotinylated anti-rabbit secondary antibody (Vector Laboratories). For CD8 staining, tissues were immersed in Tissue-Tek O.C.T. (Bayer) and snap-frozen on dry ice. Sections were then fixed with 90% ethanol at −20°C and washed with PBS, and avidin/biotin was blocked using a commercial kit (Vector Laboratories). CD8 was stained using rat anti-mouse CD8α (BD Biosciences) and biotinylated anti-rat secondary antibody (Vector Laboratories) for 60 min each. For both TGF-β and CD8 staining, the color reaction was performed by sequential incubation with avidin-peroxidase conjugate (Vector Laboratories) and diaminobenzidine-hydrogen peroxide. Terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling (TUNEL) was performed using an ApopTag kit (Intergen Company).
Isolation of lymphocytes from the pancreas.
Pancreata were collected and pancreatic draining lymph nodes removed. Pancreata were mechanically disrupted using glass slides. Material was pooled from three to four mice, and lymphocytes were isolated using a lympholyte sucrose gradient (CedarLane).
Flow cytometry.
Fluorescently labeled monoclonal antibodies were obtained from BD Biosciences, eBioscience, BioLegend, or Caltag. H2-ld/NP118 or H2-Db/GP33 tetramers were produced as previously described (24). Cells were processed on a LSRII or FACScalibur (BD Biosciences) and results analyzed using FlowJo (Tree Star). For intracellular staining, single-cell suspensions were stimulated as described, stained for surface molecules, fixed in 2% paraformaldehyde (Sigma-Aldrich), permeabilized, and stained for intracellular cytokines in buffer containing 0.05% saponin (Sigma-Aldrich). Annexin V staining was performed using a commercial kit (BD Biosciences).
Cell culture.
For dendritic cell purification, spleens and mesenteric lymph nodes were pooled from naïve C57BL/6 mice and disrupted using collagenase D (Roche) in Hanks’ balanced salt solution media (Invitrogen). Dendritic cells were then purified by magnetic separation using anti-CD11c MACS microbeads, washed, and loaded for 2 h at 37°C with 3 μg/ml GP33 peptide. Alternatively, cells were infected with LCMV at multiplicity of infection (MOI) of 0.1 on start of culture. Naïve or memory CD8+ T-cells were purified by magnetic separation using anti-CD8 MACS microbeads. T-cells were labeled for 10 min with 5 μmol/l 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE; Sigma-Aldrich), washed, and cultured with GP33-loaded dendritic cells (at a ratio of 1 dendritic cell to 3 T-cells) in RPMI 1640 (Invitrogen) supplemented with 10% FCS (Hyclone), 2 mmol/l l-glutamine (Sigma-Aldrich), and 50 μmol/l 2-mercaptoethanol (Sigma-Aldrich), in the presence or absence of 3 ng/ml recombinant human TGF-β1 (R&D systems). Then, 3 days later, Brefeldin A was added to the wells for 4 h, and cells were stained for intracellular cytokines.
RNase protection assay.
Total RNA was isolated from CD8+ T-cells with chloroform using TRI reagent (Molecular Research Center), precipitated with isopropanol, and washed with ethanol. We used 5 μg of total RNA for hybridization with a 32P-UTP–labeled multitemplate obtained from a commercial kit (RiboQuant, mCYC-1; BD Biosciences). RNase protection assay was carried out according to the manufacturer's guidelines. The resulting analytical acrylamide gel was scanned using a Storm 860 PhosphorImaging system (Molecular Dynamics), and the intensity of bands corresponding to protected mRNAs was quantified with ImageQuant image analysis software (Molecular Dynamics) using L32 as a reference gene.
Statistical analysis.
Data are expressed as the means ± SD. Statistical significance was determined using Student's t test, and differences were considered significant when P < 0.05.
RESULTS
Mice induced to express TGF-β in the islets show increased islet infiltration by autoreactive CD8+ T-cells activated in the periphery.
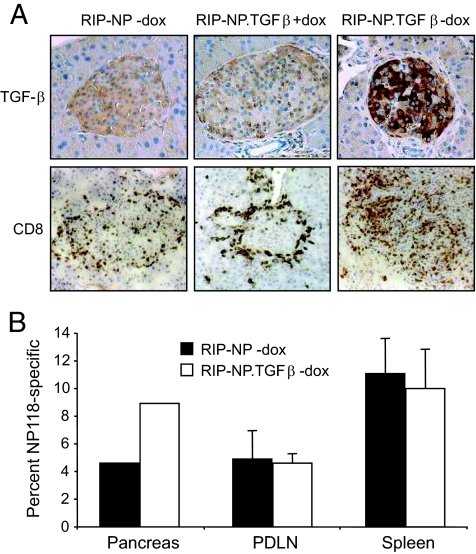
We investigated the effect of TGF-β expression locally in the pancreas of RIP-LCMV mice. BALB/c RIP-NP mice were generated in the tetracycline-dependent TGF-β gene transcription system (TTA/TGF-β), which allows for TGF-β expression in the islets to be turned on and off at will. TGF-β transcription was induced by removing doxycycline from the diet of double-transgenic TTA/TGF-β × BALB/c RIP-NP mice (RIP-NP.TGFβ mice). Then, 1 week later, these mice were infected with LCMV and analyzed 7 days later for TGF-β expression in the islets by immunohistochemistry. Only RIP-NP.TGFβ mice that were fed a doxycycline-free diet expressed the TGF-β protein selectively in the islets, whereas doxycycline-fed RIP-NP.TGFβ mice and their transgene-negative littermates did not (Fig. 1A). The islets of naïve RIP-NP.TGFβ mice fed a doxycycline-free diet showed no sign of infiltration (data not shown). On the other hand and surprisingly, TGF-β expression in the pancreas of LCMV-infected RIP-NP mice induced the accumulation of CD8+ T-cells with diabetogenic properties (15), reflecting their previous activation in the periphery (17,18). These observations indicate an enhancing effect of TGF-β on the survival of previously activated CD8+ T-cells. Furthermore, whereas RIP-NP mice and RIP-NP.TGFβ mice fed doxycycline displayed comparable infiltration, with CD8+ T-cells present mostly around the periphery of the islets, the islets of RIP-NP.TGF-β mice induced to express TGF-β by removal of doxycycline were massively infiltrated with CD8+ T-cells. In addition, tetramer staining of lymphocytes from pooled pancreata of mice fed a doxycycline-free diet revealed the presence of twice as many nucleoprotein-specific CD8+ T-cells in the pancreas of TGF-β–expressing compared with control RIP-NP mice (Fig. 1B). Of note, accumulation of CD8+ T effectors was seen only in the pancreas, where TGF-β was expressed, but not in the spleen and pancreatic draining lymph node. Accordingly, systemic immunity to LCMV was identical in TGF-β–expressing and control mice, which cleared the infection with similar kinetics (data not shown).
FIG. 1.
Mice induced to express TGF-β in the islets show increased islet infiltration by autoreactive CD8+ T-cells activated in the periphery. TTA/TGF-β BALB/c mice were crossed with BALB/c RIP-NP mice to generate double-transgenic RIP-NP.TGFβ offspring. RIP-NP.TGFβ mice and their single-transgenic RIP-NP littermates were fed a diet containing doxycycline (dox) during gestation and after birth. A: Doxycycline was either maintained or removed from the diet 7 days before infection with LCMV, and pancreata were harvested 7 days later (day 14 after doxycycline removal). Tissue sections were analyzed for TGF-β production and CD8+ T-cell infiltration after staining with anti–TGF-β (top panels) or anti-CD8 (bottom panels) and counterstaining with hematoxylin. B: Single-cell suspensions were prepared from the pancreas, spleen, or pancreatic draining lymph nodes (PDLN) of mice fed a doxycycline-free diet. The frequency of NP118-specific/responsive CD8+ T-cells was evaluated in pooled pancreata (three to four) by tetramer staining and in the spleen and pancreatic draining lymph nodes by flow cytometry after stimulation with NP118 ex vivo. (Please see http://dx.doi.org/10.2337/db08-0609 for a high-quality digital representation of this figure.)
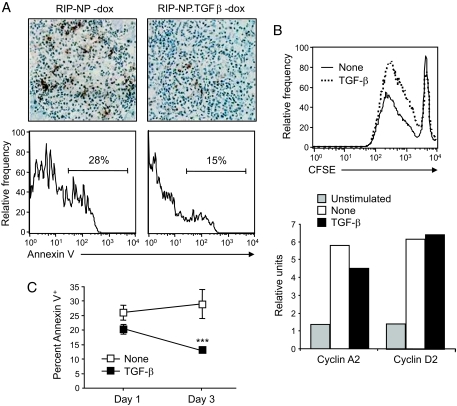
TGF-β promotes the survival of previously activated autoreactive CD8+ T-cells by decreasing their apoptosis.
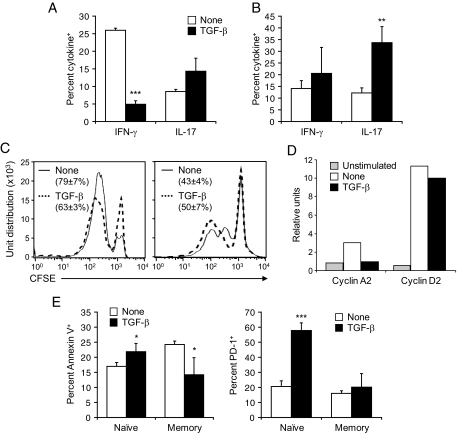
To identify the cause for the increased number of autoreactive effectors in TGF-β–expressing islets, we assessed apoptosis of these cells by TUNEL. At 7 days after infection with LCMV, the number of TUNEL-positive cells appeared smaller in infiltrated islets of RIP-NP.TGFβ mice than in those of their RIP-NP littermates (Fig. 2A), which indicated that TGF-β reduced apoptosis of previously activated CD8+ T-cell effectors. However, it was difficult to compare apoptosis quantitatively in the islets of RIP-NP.TGFβ and RIP-NP mice, which differed in the extent of infiltration. Cell suspensions were thus prepared from pooled pancreata 7 days after infection and stained with H2-ld/NP118 tetramers and annexin V. As shown in Fig. 2A, the frequency of nucleoprotein-specific CD8+ T-cells expressing annexin V was higher in RIP-NP.TGFβ mice induced to express TGF-β in the islets by doxycycline removal compared with control RIP-NP littermates. Therefore, expression of TGF-β in the vicinity of previously activated CD8+ T-cells led to their enhanced survival and subsequent accumulation at the effector site. These results indicate that CD8+ T-cell effectors that have previously responded to antigenic stimulation are enhanced rather than suppressed by TGF-β. Accordingly, TGF-β did not hinder but rather enhanced the capacity of antigen-experienced CD8+ T-cells obtained from LCMV-immune BALB/c mice to proliferate in response to LCMV in vitro and did not prevent upregulation of the cell cycle proteins cyclin D and A2 (Fig. 2B) (25). In fact, binding of annexin V to these cells was significantly reduced in the presence of TGF-β, indicating an antiapoptotic effect of this cytokine on antigen-experienced CD8+ T-cells (Fig. 2C).
FIG. 2.
TGF-β promotes the survival of previously activated autoreactive CD8+ T-cells by decreasing their apoptosis. A: Apoptosis was evaluated by TUNEL in pancreatic tissue sections obtained from mice fed a doxycycline (dox)-free diet (top panels). Cell suspensions were prepared from the pooled pancreatic draining lymph nodes of three to four mice and stained with H2-ld/NP118 tetramers and annexin V (bottom panels). Histograms show the frequency of tetramer-positive CD8+ T-cells stained with annexin V. B: CD8+ T-cells were purified from the spleen of LCMV-immune BALB/c mice, labeled with CFSE, and cultured with LCMV-infected, irradiated, antigen-presenting cells in the presence or absence of 3 ng/ml rhTGF-β1. Cell proliferation was assessed by measuring CFSE dilution by flow cytometry after 6 days in culture (top panel) or cell cycle protein RNA was quantitated by RNase protection assay after 24 h (bottom panel). C: CD8+ T-cells were isolated from the spleen of LCMV-immune BALB/c mice and cultured with LCMV-infected, irradiated, antigen-presenting cells in the presence or absence of 3 ng/ml rhTGF-β1. After 1 and 3 days in culture, T-cells were analyzed for apoptosis by flow cytometry after staining with annexin V. Data represents the average frequency of CD8+ T-cells stained with annexin V ± SD from three independent experiments. (Please see http://dx.doi.org/10.2337/db08-0609 for a high-quality digital representation of this figure.)
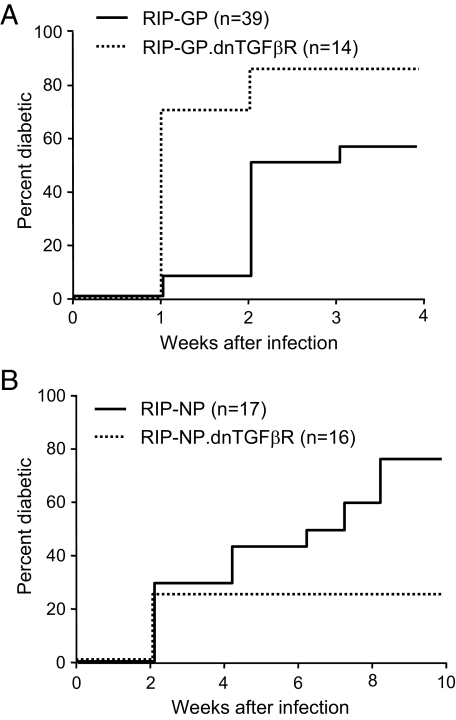
Absence of TGF-β receptor signaling in T-cells has opposing effects on autoimmune diabetes depending on the effector mechanism.
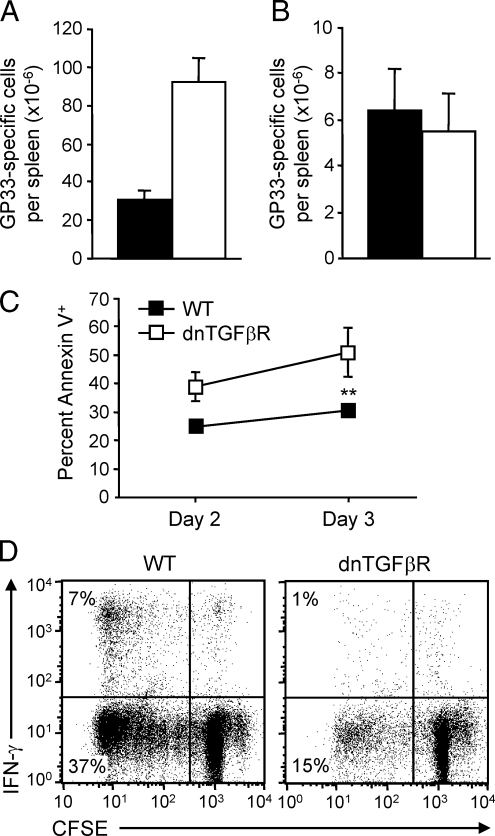
These observations pointed to opposite roles of TGF-β on naïve compared with antigen-experienced/memory CD8+ T-cells. We thus evaluated the impact of TGF-β in two type 1 diabetes models that differed in terms of requirement for CD8+ T-cell activation: the fast-onset C57BL/6 RIP-GP model, which is caused by robust activation of naïve CD8+ T-cells, and the slow-onset C57BL/6 RIP-NP model, which results from accumulation and activation of antigen-experienced/memory CD8+ T-cells. We generated C57BL/6 RIP-GP and C57BL/6 RIP-NP mice in which autoreactive CD8+ T-cells are unable to signal through the TGF-β receptor by crossing these mice with dnTGFβR mice, which express a dominant-negative TGF-β receptor specifically in T-cells. Although “naïve-dependent” fast-onset diabetes was dramatically accelerated in RIP-GP.dnTGFβR compared with RIP-GP mice (Fig. 3A), incidence of “memory-dependent” slow-onset diabetes was reduced more than twofold in RIP-NP.dnTGFβR compared with RIP-NP mice (Fig. 3B). Absence of TGF-β receptor signaling in T-cells resulted in a threefold increase in the total number of LCMV-specific CD8+ T-cells in the spleen 7 days after LCMV infection (Fig. 4A). Thus, impaired responsiveness of naïve CD8+ T-cells to TGF-β enhanced their activation and expansion, which likely accounted for accelerated diabetes in the RIP-GP.dnTGFβR group and in the few RIP-NP.dnTGFβR mice that developed disease (between 1 and 2 weeks after infection in this otherwise slow-onset model). However, by day 30 the overall number of GP33-specific CD8+ T-cells was similar in wild-type and dnTGFβR mice (Fig. 4B), indicating that TGF-β–nonresponsive CD8+ T-cells had undergone increased contraction. Furthermore, TGF-β receptor–deficient memory CD8+ T-cells were more prone to apoptosis (Fig. 4C) and completely defective in terms of secondary expansion (Fig. 4D) when stimulated by LCMV-infected antigen-presenting cells in vitro. Thus, impaired responsiveness of activated CD8+ T-cells to TGF-β diminished their survival and maintenance as memory cells, which likely accounted for reduced slow-onset diabetes in the RIP-NP.dnTGFβR system. This indicates that TGF-β prevents activation of naïve CD8+ T-cells, which cause fast-onset diabetes, but promotes the survival and accumulation of antigen-experienced cells, which cause slow-onset disease.
FIG. 3.
Absence of TGF-β receptor signaling in T-cells has opposite effects on virally induced autoimmune diabetes depending on the effector mechanism. dnTGFβR mice were crossed onto fast-onset diabetes RIP-GP or slow-onset diabetes RIP-NP lines. Groups of RIP-GP.dnTGFβR (A) mice, RIP-NP.dnTGFβR (B) mice, and their single transgenic RIP-GP or RIP-NP littermates were infected with LCMV, and diabetes incidence was monitored by measuring blood glucose. Mice were considered diabetic when blood glucose exceeded 300 mg/dl.
FIG. 4.
Absence of TGF-β receptor signaling in T-cells enhances antiviral CD8+ T-cell responses at the acute phase but reduces memory responses. dnTGFβR mice and their wild-type (WT) C57BL/6 littermates were infected with LCMV. A and B: Mice were killed and spleens harvested on day 7 (peak) (A) or 28 (memory) (B) postinfection. The frequency of IFN-γ–producing cells (measured by flow cytometry after stimulation with GP33) was multiplied by the total number of splenocytes to obtain the number of GP33-responsive CD8+ T-cells per spleen. Data represents the average number of GP33-responsive CD8+ T-cells per spleen ± SD from three individual mice per group. ▪, wild-type; □, dnTGFβR. C: Splenocytes were harvested from dnTGFβR mice 28 days postinfection, labeled with CFSE, and cultured with LCMV-infected, irradiated, antigen-presenting cells. Cells were harvested 2 or 3 days later and stained for expression of CD8 and annexin V. Data represents the average frequency of CD8+ T-cells stained with annexin V ± SD from three mice per group. D: Alternatively, cells were harvested 6 days later and analyzed for IFN-γ production by flow cytometry after stimulation with GP33. Representative fluorescence-activated cell sorting plots are shown.
TGF-β suppresses naïve CD8+ T-cell activation and enhances the survival and effector function of antigen-experienced/memory CD8+ T-cells.
Based on these observations, we investigated in further detail the differential effect of TGF-β on activation, proliferation, and effector function of naïve compared with antigen-experienced/memory CD8+ T-cells. This was addressed by comparing the response mounted in vitro by naïve and memory GP33-specific CD8+ T-cells stimulated with GP33-loaded dendritic cells in the presence or absence of TGF-β. We observed that naïve CD8+ T-cells lost their capacity to produce γ-interferon (IFN-γ) when stimulated in the presence of TGF-β (Fig. 5A and B). Interestingly, IL-17 production by these cells was, on the other hand, increased, though modestly, indicating that TGF-β favored a switch of CD8+ T-cells from a Th1-like (Tc1) to a Th17-like phenotype (Tc17). Furthermore, naïve CD8+ T-cells stimulated in the presence of TGF-β showed impaired proliferation associated with upregulation of cyclin D but not A2 (Fig. 5C and D), which indicated a block in the S phase of the cell cycle (26). In addition, binding of annexin V and expression of the proapoptotic molecule programmed death 1 (PD-1) (27) on stimulated naïve cells was increased by TGF-β (Fig. 5E). In marked contrast, TGF-β did not affect proliferation of stimulated GP33-specific memory CD8+ T-cells, consistent with our observation in Fig. 2C using polyclonal memory CD8+ T-cells (Fig. 5C). Furthermore, although IL-17 production was increased in GP33-specific memory cells (as observed in naïve GP33-specific cells), the overall increase was greater in these compared with naïve cells and not accompanied by a loss in IFN-γ production (Fig. 5A and B). In addition, as opposed to naïve cells, memory cells stimulated in the presence of TGF-β showed decreased apoptosis and did not upregulate PD-1 (Fig. 5E). These observations indicate that TGF-β not only increases the survival of activated antigen-experienced/memory CD8+ T-cells but also enhances their capacity to produce IL-17 in the presence of IFN-γ by inducing an incomplete switch from Tc1 to Tc17.
FIG. 5.
TGF-β suppresses naïve CD8+ T-cell activation and enhances the survival and effector function of antigen-experienced/memory CD8+ T-cells. CD8+ T-cells were purified from the spleen of naïve P14 or LCMV-immune C57BL/6 mice, labeled with CFSE, cultured with GP33-loaded dendritic cells in the presence or absence of 3 ng/ml rhTGF-β1, and harvested 2 days later. A and B: Cells were analyzed for IFN-γ and IL-17 production by flow cytometry after stimulation with GP33. Data represents the average frequency of naïve (A) and memory (B) CD8+ T-cells producing IFN-γ or IL-17 ± SD from three individual mice per group. C: Cell proliferation was assessed by measuring CFSE dilution by flow cytometry. Representative fluorescence-activated cell sorting plots are shown. Values show the percentage of naïve (left panel) or memory (right panel) CD8+ T-cells that have undergone at least one division on stimulation in the presence or absence of TGF-β ± SD from three individual mice per group. D: Naïve GP33-specific CD8+ T-cells were analyzed for cell cycle protein RNA by RNase protection assay as described in Fig. 2B. E: Cells were analyzed by flow cytometry for apoptosis after staining with annexin V or for expression of PD-1. Data represents the average frequency of CD8+ T-cells stained with annexin V (left panel) or expressing PD-1 (right panel) ± SD from three individual mice per group.
DISCUSSION
We observed that TGF-β expression in the vicinity of already activated autoreactive T-cells promotes their survival and accumulation, which highlighted a differential effect of TGF-β on CD8+ T-cell responses derived from naïve versus antigen-experienced/memory progenitors. Our results show that the role of TGF-β in type 1 diabetes is highly dependent on the differentiation state and activation history of CD8+ T-cells. We found that TGF-β inhibits activation and proliferation of naïve but not antigen-experienced/memory effector CD8+ T-cells. Furthermore, TGF-β exerts an antiapoptotic effect on activated antigen-experienced CD8+ T-cells and thus enhances their survival. In addition, TGF-β promotes a switch from a Tc1 to a Tc17 phenotype, which is incomplete in stimulated memory cells, resulting in both IFN-γ and IL-17 production by these cells. These results correlate precisely with our observation that lack of a functional TGF-β receptor on T-cells has enhancing and dampening effects on diabetes induced by activation of naïve and antigen-experienced/memory CD8+ T-cells, respectively. Previous work also indicates divergent effects of TGF-β on naïve versus differentiated CD4+ T-cells in vitro (28,29).
Our results using TTA/TGF-β × BALB/c RIP-NP mice (RIP-NP.TGFβ) stand in some contrast to studies in the NOD mouse, in which constitutive expression of TGF-β in the islets prevented diabetes (12,13,30). Similarly, in studies using TTA/TGF-β NOD mice, where no fibrosis was observed, TGF-β exerted a protective effect on type 1 diabetes (14). An important factor might thus be whether priming of autoreactive T-cells occurs in the periphery or within the islets of Langerhans. Based on our present observations, one would expect TGF-β to have a protective effect at locations where priming of naïve CD8+ T-cells occurs. In NOD mice, it is possible that a significant proportion of priming occurs locally in the islets of Langerhans or in the pancreatic draining lymph nodes, where the earliest signs of immunopathogenesis are seen. Previous observations in NOD mice show that priming in the islets and the lymph nodes are not necessarily linked (31). In contrast, in RIP-LCMV models for fast-onset diabetes (BALB/c RIP-NP and C57BL/6 RIP-GP), activation of autoreactive T-cells occurs in the periphery in lymphoid organs and at sites of viral replication. Furthermore, in these models, as opposed to the slow-onset C57BL/6 RIP-NP model, maintenance of antigen-experienced/memory cells over time is not necessary for attack and destruction of β-cells. Thus, the antiapoptotic effect of TGF-β on effector T-cells will outweigh its suppressive function on naïve precursors locally in the islets. This fits well with the previous observation that TGF-β does not protect against already differentiated diabetogenic T-cells, as evidenced by similar destruction of TGF-β–expressing and control islet transplants in diabetic mice (13,15). Thus, our results suggest that TGF-β will have different effects in vivo depending on where priming of autoreactive T-cells occurs.
We found that in the absence of TGF-β receptor signaling in T-cells, priming and expansion of naïve antiviral/autoreactive CD8+ T-cells are enhanced in vivo. Although this might be caused by a direct effect of TGF-β on naïve CD8+ T-cells, it is also possible that absence of TGF-β receptor signaling decreases the function or induction of regulatory T-cells, which thereby enables enhanced naïve T-cell activation. Previous work has shown that human CD4+CD25+ regulatory T-cells can be expanded in vitro using TGF-β (32). In the mouse, TGF-β was shown to prevent spontaneous type 1 diabetes by promoting the expansion of CD4+CD25+ regulatory T-cells in the islets (14). Furthermore, it was recently shown that expression of TGF-β selectively in β-cells promotes the induction of CD4+CD25+ regulatory T-cells that protect from virally induced type 1 diabetes (30). However, expansion of regulatory T-cells in the mouse in vivo occurred only when TGF-β was expressed early, starting from birth (14), or constitutively in the islets (30). Furthermore, as discussed above, the effect of TGF-β might rely on where T-cell priming occurs in vivo. This further underscores our observation that the effect of TGF-β on T-cells depends on their activation/differentiation state. Of note, the role of TGF-β in the function of CD4+CD25+ regulatory T-cells is not clear, with some work demonstrating an important role for this cytokine (10,33–35), and other no involvement (36). In any case, we observed that naïve CD8+ T-cells isolated from CD4+ T-cells and stimulated in vitro in the presence of TGF-β show impaired activation and proliferation, indicating that CD4+ regulatory T-cells are not involved in the process. Furthermore, we observed no upregulation of Foxp3 or decrease in CD127 expression on naïve CD8+ T-cells stimulated in the presence of TGF-β (data not shown), making it unlikely that TGF-β induced the differentiation of CD8+ regulatory T-cells. Thus, impaired TGF-β receptor signaling in T-cells enhanced activation of naïve T-cells and impaired the survival of memory T-cells in vivo most likely as a direct effect of TGF-β on CD8+ T-cell effectors. As a consequence, fast-onset diabetes, which depends on activation of naïve T-cells, was accelerated, whereas slow-onset diabetes, which requires maintenance of antigen-experienced/memory effectors, was reduced.
Our results indicate that the importance of TGF-β for the survival of antigen-experienced/memory T-cells and their subsequent effect on type 1 diabetes is attributable at least partially to the antiapoptotic properties of this cytokine. We observed that TGF-β receptor-deficient CD8+ T-cells are more prone to apoptosis and harbor impaired capacity of secondary expansion. In this respect, previous work by others indicates that T-cells derived from TGF-β–deficient mice show enhanced apoptosis in vitro (37,38). In vitro, we found that TGF-β diminishes annexin V binding and thus apoptosis of CD8+ T-cell effectors derived from memory but not naïve precursors. Accordingly, TGF-β was reported to have potent antiapoptotic effects in vitro, possibly as a result of inhibition of Fas ligand and c-Myc expression (4,5,7). Although we did not assess Fas ligand or c-Myc expression on T-cells stimulated in vitro, we found that TGF-β induced naïve but not memory CD8+ T-cells to upregulate PD-1, which is a proapoptotic molecule that controls T-cell responses in both LCMV infection and type 1 diabetes (27,39–42). In vivo, we found that expression of TGF-β locally in the islets results in accumulation of autoreactive CD8+ T-cell effectors, which exhibited reduced apoptosis. Importantly, the frequency of antigen-specific CD8+ T-cells was enhanced only in the vicinity of TGF-β in the islets, but not in the spleen or pancreatic draining lymph node. In other words, absence of TGF-β expression in the periphery enabled naïve CD8+ T-cell activation to occur normally in the spleen and pancreatic draining lymph node, whereas TGF-β expressed locally in the islets exerted its antiapoptotic effect on subsequently activated effectors infiltrating the pancreas.
Based on our observations, another important parameter accounting for the differential effect of TGF-β on naïve compared with antigen-experienced/memory CD8+ T-cells might be their capacity to produce IL-17. It was recently reported that TGF-β can induce IL-17 production by CD4+ T-cells when acting in combination with the proinflammatory cytokine IL-6 (43). Such Th17 cells produce IL-17 but not IFN-γ, which actually inhibits their differentiation and function (44,45). Th17 cells and IL-17 production might play a crucial role in mediating pathology in autoimmune diseases such as multiple sclerosis (43). In fact, IL-17 is an important regulator of inflammation and is elevated in a number of inflammatory conditions (46). IL-17 production by CD8+ T-cells (Tc17) has also been described and shown to harbor colitogenic properties (47). Although the role of Th17/Tc17 cells and IL-17 in type 1 diabetes is not understood, a recent report indicates that normoglycemia can be restored but not prevented in NOD mice through inhibition of IL-17 production after antigen administration (48). Therefore, although IL-17 may not initiate β-cell destruction, it may contribute to pathology once autoimmunity has been induced. We do not know whether IL-17 production plays a role in the pathological function of memory CD8+ T-cells stimulated in the presence of TGF-β. However, we observed that these cells, as opposed to naïve cells, are also capable of producing IFN-γ and thus likely to cause β-cell destruction. It is thus possible that TGF-β provides memory CD8+ T-cells with enhanced capacity to exacerbate β-cell destruction once they have initiated it. On the other hand, naïve cells stimulated with TGF-β, despite their capacity to produce IL-17, are not capable of IFN-γ production and might thus lack essential properties to be pathological.
Our results also have important implications for understanding the factors required to optimize antiviral vaccines, where the objective is to generate and maintain an optimal number of memory T-cells rather than inducing a strong primary response to the vaccine. Based on our observations, TGF-β, which is normally thought of as an immune dampening factor, might actually be beneficial for expanding and maintaining such antiviral memory T-cells. On the other hand, blockade of TGF-β might be beneficial in situations where boosting the priming of effector T-cells is desired. For example, mice deficient in TGF-β receptor signaling generate an increased tumor-specific CD8+ T-cell response that leads to tumor eradication and long-term survival (49). Although it is not known whether these mice generate long-term memory, for example after tumor rechallenge, they nonetheless show improved survival compared with control mice after initial tumor vaccination. In our studies, reduced secondary expansion of memory CD8+ T-cells to LCMV was observed in the absence of TGF-β receptor signaling, but viral clearance occurred normally, probably because of the massive expansion of effector CD8+ T-cells that occurred during the primary response. Thus, blockade of TGF-β may still constitute a strong therapeutic strategy for generating antitumor immunity, even if long-term memory generation is impaired.
In conclusion, our studies have implications for the therapeutic use of TGF-β. In autoimmune diseases such as type 1 diabetes, TGF-β may not always be inhibitory and may actually act to enhance the survival of autoreactive T-cells, depending on their differentiation state. Therefore, it is possible that TGF-β therapy initiated during the early stages of autoimmune disease is beneficial by suppressing activation of naïve autoreactive T-cells or inducing regulatory T-cells, whereas late initiation of treatment may be detrimental because most autoreactive T-cells are already fully differentiated at this point. On the other hand, blockade of TGF-β to boost immune responses, for example after tumor cell vaccination, may enhance the priming of T-cells but could have negative implications for the generation of long-lived memory T-cells. Thus, for systemic treatments involving the administration or blockade of TGF-β, one will have to consider the precise balance of naïve and effector/memory T-cells within the targeted population.
Acknowledgments
This work was supported by National Institutes of Health grants AI44451, DK51091, and U-19 AI 51973; a Juvenile Diabetes Research Foundation (JDRF) research grant (to M.G.vH.); grants from the American Diabetes Association and the National Institute of Diabetes and Digestive and Kidney Diseases (to R.A.F.); and two JDRF postdoctoral fellowships (to A.E.J. and C.M.F.). R.A.F. is an investigator of the Howard Hughes Medical Institute.
We thank Dr. Urs Christen and Antje Rhode for the generation of MHC class I tetramers, Malina McClure for mouse colony maintenance, and Priscilla Colby for administrative assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 8 August 2008.
C.M.F. and A.E.J. contributed equally to this study.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Gorelik L, Flavell RA: Transforming growth factor-beta in T-cell biology. Nat Rev Immunol 2:46–53, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Gorelik L, Flavell RA: Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12:171–181, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Letterio JJ, Roberts AB: Regulation of immune responses by TGF-beta. Annu Rev Immunol 16:137–161, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Giangreco L, Broome HE, Dargan CM, Swain SL: Control of CD4 effector fate: transforming growth factor beta 1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. J Exp Med 182:699–709, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genestier L, Kasibhatla S, Brunner T, Green DR: Transforming growth factor beta1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J Exp Med 189:231–239, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HM, Rich S: Differential activation of CD8+ T cells by transforming growth factor-beta 1. J Immunol 151:668–677, 1993 [PubMed] [Google Scholar]
- 7.Cerwenka A, Kovar H, Majdic O, Holter W: Fas- and activation-induced apoptosis are reduced in human T cells preactivated in the presence of TGF-beta 1. J Immunol 156:459–464, 1996 [PubMed] [Google Scholar]
- 8.Ludviksson BR, Seegers D, Resnick AS, Strober W: The effect of TGF-beta1 on immune responses of naive versus memory CD4+ Th1/Th2 T cells. Eur J Immunol 30:2101–2111, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Piccirillo CA, Chang Y, Prud'homme GJ: TGF-beta1 somatic gene therapy prevents autoimmune disease in nonobese diabetic mice. J Immunol 161:3950–3956, 1998 [PubMed] [Google Scholar]
- 10.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L: TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 9:1202–1208, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Polanski M, Melican NS, Zhang J, Weiner HL: Oral administration of the immunodominant B-chain of insulin reduces diabetes in a cotransfer model of diabetes in the NOD mouse and is associated with a switch from Th1 to Th2 cytokines. J Autoimmun 10:339–346, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Grewal IS, Grewal KD, Wong FS, Wang H, Picarella DE, Janeway CA Jr, Flavell RA: Expression of transgene encoded TGF-beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun 19:9–22, 2002 [DOI] [PubMed] [Google Scholar]
- 13.King C, Davies J, Mueller R, Lee MS, Krahl T, Yeung B, O'Connor E, Sarvetnick N: TGF-beta1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity 8:601–613, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA: TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci U S A 101:4572–4577, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MS, Sawyer S, Arnush M, Krahl T, von Herrath M, Oldstone MA, Sarvetnick N: Transforming growth factor-beta fails to inhibit allograft rejection or virus-induced autoimmune diabetes in transgenic mice. Transplantation 61:1112–1115, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Sanvito F, Nichols A, Herrera PL, Huarte J, Wohlwend A, Vassalli JD, Orci L: TGF-beta 1 overexpression in murine pancreas induces chronic pancreatitis and, together with TNF-alpha, triggers insulin-dependent diabetes. Biochem Biophys Res Commun 217:1279–1286, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H: Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65:305–317, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H: Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell 65:319–331, 1991 [DOI] [PubMed] [Google Scholar]
- 19.von Herrath MG, Dockter J, Oldstone MB: How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity 1:231–242, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Brandle D, Burki K, Wallace VA, Rohrer UH, Mak TW, Malissen B, Hengartner H, Pircher H: Involvement of both T cell receptor V alpha and V beta variable region domains and alpha chain junctional region in viral antigen recognition. Eur J Immunol 21:2195–2202, 1991 [DOI] [PubMed] [Google Scholar]
- 21.von Herrath MG, Guerder S, Lewicki H, Flavell RA, Oldstone MB: Coexpression of B7–1 and viral (“self”) transgenes in pancreatic beta cells can break peripheral ignorance and lead to spontaneous autoimmune diabetes. Immunity 3:727–738, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Dutko FJ, Oldstone MB: Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol 64:1689–1698, 1983 [DOI] [PubMed] [Google Scholar]
- 23.Oxenius A, Bachmann MF, Ashton-Rickardt PG, Tonegawa S, Zinkernagel RM, Hengartner H: Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur J Immunol 25:3402–3411, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Busch DH, Pilip IM, Vijh S, Pamer EG: Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8:353–362, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Morgan DO: The dynamics of cyclin dependent kinase structure. Curr Opin Cell Biol 8:767–772, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Blanchard JM: Cyclin A2 transcriptional regulation: modulation of cell cycle control at the G1/S transition by peripheral cues. Biochem Pharmacol 60:1179–1184, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Ishida Y, Agata Y, Shibahara K, Honjo T: Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11:3887–3895, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, Silver PB, Tarrant TK, Chan CC, Caspi RR: Tgf-beta inhibits activation and uveitogenicity of primary but not of fully polarized retinal antigen-specific memory-effector T cells. Invest Ophthalmol Vis Sci 44:4805–4812, 2003 [DOI] [PubMed] [Google Scholar]
- 29.McKarns SC, Schwartz RH: Distinct effects of TGF-beta 1 on CD4+ and CD8+ T cell survival, division, and IL-2 production: a role for T cell intrinsic Smad3. J Immunol 174:2071–2083, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Richer MJ, Straka N, Fang D, Shanina I, Horwitz MS: Regulatory T-cells protect from type 1 diabetes after induction by coxsackievirus infection in the context of transforming growth factor-β. Diabetes 57:1302–1311, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Yamanouchi J, Verdaguer J, Han B, Amrani A, Serra P, Santamaria P: Cross-priming of diabetogenic T cells dissociated from CTL-induced shedding of beta cell autoantigens. J Immunol 171:6900–6909, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA: A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol 166:7282–7289, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K, Kitani A, Strober W: Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 194:629–644, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA: CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci U S A 100:10878–10883, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F: CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med 197:111–119, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM: CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med 196:237–246, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bommireddy R, Saxena V, Ormsby I, Yin M, Boivin GP, Babcock GF, Singh RR, Doetschman T: TGF-beta 1 regulates lymphocyte homeostasis by preventing activation and subsequent apoptosis of peripheral lymphocytes. J Immunol 170:4612–4622, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Chen W, Jin W, Tian H, Sicurello P, Frank M, Orenstein JM, Wahl SM: Requirement for transforming growth factor beta1 in controlling T cell apoptosis. J Exp Med 194:439–453, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H Jr, Sayegh MH: The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 198:63–69, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH: PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A 101:10691–10696, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH: Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 203:883–895, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R: Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK: Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT: Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123–1132, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C: A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolls JK, Linden A: Interleukin-17 family members and inflammation. Immunity 21:467–476, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Tajima M, Wakita D, Noguchi D, Chamoto K, Yue Z, Fugo K, Ishigame H, Iwakura Y, Kitamura H, Nishimura T: IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med 205:1019–1027, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H: Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med 205:207–218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorelik L, Flavell RA: Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 7:1118–1122, 2001 [DOI] [PubMed] [Google Scholar]