Abstract
OBJECTIVE—Diabetic neuropathy is manifested either by loss of nociception (painless syndrome) or by mechanical hyperalgesia and tactile allodynia (pain in response to nonpainful stimuli). While therapies with vasodilators or neurotrophins reverse some functional and metabolic abnormalities in diabetic nerves, they only partially ameliorate neuropathic pain. The reported link between nociception and targets of the anti-inflammatory drug sulfasalazine prompted us to investigate its effect on neuropathic pain in diabetes.
RESEARCH DESIGN AND METHODS—We examined the effects of sulfasalazine, salicylates, and the poly(ADP-ribose) polymerase-1 inhibitor PJ34 on altered nociception in streptozotocin-induced diabetic rats. We also evaluated the levels of sulfasalazine targets in sciatic nerves and dorsal root ganglia (DRG) of treated animals. Finally, we analyzed the development of tactile allodynia in diabetic mice lacking expression of the sulfasalazine target nuclear factor-κB (NF-κB) p50.
RESULTS—Sulfasalazine completely blocked the development of tactile allodynia in diabetic rats, whereas relatively minor effects were observed with other salicylates and PJ34. Along with the behavioral findings, sciatic nerves and DRG from sulfasalazine-treated diabetic rats displayed a decrease in NF-κB p50 expression compared with untreated diabetic animals. Importantly, the absence of tactile allodynia in diabetic NF-κB p50−/− mice supported a role for NF-κB in diabetic neuropathy. Sulfasalazine treatment also increased inosine levels in sciatic nerves of diabetic rats.
CONCLUSIONS—The complete inhibition of tactile allodynia in experimental diabetes by sulfasalazine may stem from its ability to regulate both NF-κB and inosine. Sulfasalazine might be useful in the treatment of nociceptive alterations in diabetic patients.
Distal symmetric sensory neuropathy is a common complication of diabetes. Diabetic patients can suffer from either a painless syndrome, with loss of sensation to touch, pain, or temperature, or a painful disorder characterized by mechanical hyperalgesia and allodynia (i.e., pain in response to nonpainful stimuli), depending on the type of nerve fibers being affected. Experimental models of the disease exhibit responses similar to those present in patients, including a decrease in mechanical nociceptive thresholds, enhanced action potential, and alterations in second messengers (1).
The mechanisms underlying abnormal nociception in diabetes are unclear. Although therapies with neurotrophins or vasodilators reverse some functional and metabolic abnormalities in diabetic nerves, they only partially ameliorate abnormal pain perception (2,3). These findings suggest that other pathways contribute to enhanced nociception in diabetes.
Sulfasalazine is a sulfonamide and aminosalicylate conjugate long used in the treatment of inflammatory conditions, such as inflammatory bowel disease and rheumatoid arthritis. Approximately one-third of orally administered sulfasalazine is absorbed in the intestine, with the rest being cleaved by enteric bacteria into 5-aminosalicyic acid and sulfapyridine. In vitro and in vivo studies suggest that several mechanisms may contribute to the anti-inflammatory action of sulfasalazine. These include inhibition of nuclear factor-κB (NF-κB) activity (4–6); enhanced release of adenosine (7,8); inhibition of superoxide, leukotrienes, and thromboxane B2 production in leukocytes (9–11); and inhibition of secretory phospholipase A2 release (12).
The reported link between nociception and some of the mediators of the anti-inflammatory action of sulfasalazine, such as NF-κB and adenosine, prompted us to investigate the effects of sulfasalazine on neuropathic pain in experimental diabetes. We report that administration of sulfasalazine completely blocks the development of tactile allodynia in diabetic rats.
RESEARCH DESIGN AND METHODS
Induction of diabetes.
Diabetes was induced in Lewis rats (weighing ∼200 g, fasted overnight) by a single intraperitoneal injection of streptozotocin (60 mg/kg body wt). After 48 h, body weight and blood levels of glucose and GHb were monitored regularly and also at the time of death. Insulin was administered as needed to maintain body weight but without preventing chronic hyperglycemia. Animals exhibiting poor health or blood glucose levels <250 mg/dl were excluded. Diabetic rats were randomly divided in groups of 8–10 animals and received sulfasalazine (0.6 mg/ml drinking water, equivalent to 100 mg · kg body wt−1 · day−1), sodium salicylate or aspirin (0.3 g/kg diet, equivalent to 30 mg · kg body wt−1 · day−1), and the poly(ADP-ribose) polymerase-1 (PARP-1) inhibitor PJ34 (0.3 g/kg diet, equivalent to 25 mg · kg body wt−1 · day−1). The doses of sulfasalazine, salicylates, and PJ34 inhibitor were based on previous studies on inflammation and diabetic retinopathy (6–8,13). Treatment of diabetic rats was initiated 6–10 days after injection of streptozotocin and continued for 3–9 months. A group of normal rats was also treated with the same dose of sulfasalazine for 9 weeks. To determine the amount of drug consumed, drinking water was changed and measured every other day, and food was changed and measured weekly.
Diabetes was also induced in mice lacking expression of NF-κB p105 (precursor of the NF-κB p50 subunit) (The Jackson Laboratories; Bar Harbor, ME), referred to as NF-κB p50−/− in the text, and in their normal wild-type counterparts (B6129PF2/J mice). NF-κB p50−/− mice are viable, appear to develop normally, and exhibit unimpaired development of their immune system (14). Diabetes was induced in NF-κB p50−/− mice by a daily intraperitoneal injection of streptozotocin (60 mg/kg body wt) for 5 consecutive days. At the end of this period, animal weight and serum glucose concentration were monitored regularly.
Behavioral studies
Tactile allodynia.
This test was performed as described previously (15) using a series of six Von Frey filaments (Stoelting, Chicago, IL) with logarithmically increasing stiffness (1.4–15 g for rats and 0.4–6.0 g for mice). The test was considered completed when four measurements were made after the initial change in behavior or after five consecutive negative scores. The resulting sequence of positive and negative scores was used to calculate the force at which there is 50% probability of withdrawal (50% withdrawal threshold) (16). The test was performed on at least six to eight rats per group. Two evaluations per animal (right and left paws) were averaged; the mean ± SE of the individual responses in the six to eight animals was considered the 50% withdrawal threshold for each group. Diabetic rats and mice exhibited tactile allodynia as reflected by 50% withdrawal thresholds <8 and <2.5 g, respectively. The statistical significance of the differences among groups was analyzed by the Kruskal-Wallis nonparametric test. Where significance was observed, multiple comparisons between groups were analyzed by the Dunn's test.
Paw pressure-withdrawal test.
The Randall and Selitto test was performed as described previously (15). The nociceptive flexion reflex was evaluated in four to eight rats per group using a Basile analgesymeter (Stoelting). Four readings per animal were taken at 5-min intervals and subsequently averaged; the mean ± SE of the individual responses was considered the threshold for each group. Significant differences between normal and diabetic rats were analyzed by ANOVA followed by Dunnett's test.
Preparation of fractions and immunoblotting analysis.
Nuclear-enriched and cytosolic fractions were prepared from sciatic nerves and dorsal root ganglia (DRG) (pooled L4-L6) using a commercial kit (Panomics, Redwood City, CA). Briefly, desheathed sciatic nerves and DRG were homogenized at 4°C in a glass-glass homogenizer in 0.4 and 0.15 ml, respectively, of 10 mmol/l HEPES, pH 7.9; 10 mmol/l KCl; 10 mmol/l EDTA; 1 mmol/l dithiothreitol (DTT); and 0.4% IGEPAL supplemented with 1× complete protease and phosphatase inhibitor cocktails (included in the kit). After a 10-min incubation on ice, samples were centrifuged at 15,000 × g for 5 min. The supernatants (cytosolic fractions) were removed, and pellets were resuspended in 100 μl 20 mmol/l HEPES, pH 7.9; 400 mmol/l NaCl; 1 mmol/l EDTA; 10% glycerol; and 1 mmol/l DTT, supplemented with protease and phosphatase inhibitors on a rocking platform for 2 h at 4°C, followed by centrifugation for 5 min at 15,000 × g. The supernatants from this second centrifugation (nuclear-enriched fractions) and the cytosolic fractions were stored at −80°C. Protein concentration was measured by a modified Lowry assay. The purity of nuclear-enriched fractions was confirmed by immunoblotting using antibodies against the cytosolic proteins calpain 2 and phospho-Akt (Cell Signaling, Danvers, MA).
Samples of 10–20 μg protein were subjected to SDS-PAGE (11% gels) and transferred onto polyvinylidene fluoride membranes (NEN, Boston, MA) at 0.3 A for 1 h. Membranes were blocked with 5% milk in Tris-HCl–buffered saline plus 0.05% Tween-20 (blocking solution) and incubated 1–2 h at room temperature with goat anti–NF-κB p50, rabbit anti–NF-κB p65 (Santa Cruz Biotechnology, Santa Cruz, CA), or mouse anti–phospho-p65 NF-κB (Cell Signaling) (1:200, 1:750, and 1:1,000, respectively). After incubation for 45 min at room temperature with horseradish peroxidase–conjugated secondary antibodies, blots were developed with enhanced chemiluminescence substrate (Perkin Elmer, Boston, MA). Immunoreactivity was quantified by densitometry and compared with the extracellular signal–regulated kinase 2 (ERK2) signal in each sample to correct for differences in protein loading.
Assay of adenosine/adenosine metabolites
Reverse-phase high-performance liquid chromatography.
Desheathed sciatic nerves from normal, diabetic, and sulfasalazine-treated diabetic rats were homogenized in cold 5% perchloric acid followed by centrifugation at 14,000 × g for 5 min. Deproteinized supernatants were neutralized with potassium bicarbonate and subjected to reverse-phase high-performance liquid chromatography (HPLC) on Alltech C18 Adsorbosphere columns as described previously (17). Samples were eluted at 1.3 ml/min with a methanol gradient formed by mixing buffer A (0.1 mol/l KH2PO4, pH 6) and buffer B (0.1 mol/l KH2PO4, pH 6, and 15% methanol). The elution protocol was 0–4 min with 100% buffer A, 4–8 min with 30% buffer A and 70% buffer B, 8–25 min with 100% buffer B, and 25–35 min with 100% buffer A. Absorbance was measured at 260 nm. This method resolves ATP, ADP, AMP, adenosine, and inosine standards. Under these conditions, we did not detect an adenosine peak in the sciatic nerve extracts, but we observed two large peaks corresponding to AMP and inosine and two smaller peaks corresponding to ATP and ADP.
Tandem mass spectrometry.
Sciatic nerves were homogenized in 10 mmol/l sodium phosphate, pH 7.2, followed by freeze/thawing and centrifugation at 14,000 × g. Homogenates were filtered thorough 10-kDa cutoff centrifugal filters, and 10 μl ultrafiltrates was injected. The extracts were assayed by liquid chromatography–tandem mass spectrometry using 2695 Separation Module (Atlantis dC-18 column; 2.1 × 50 mm, 3 μm; Waters, Milford, MA) with a Micromass Quattro Micro triple quadrupole mass spectrometer (Waters MicroMass, Manchester, U.K.). Mobile phase was 26 mmol/l ammonium formate, pH 3.8 (solvent A), and 90% acetonitrile in water (solvent B) (Burdick & Jackson, Muskegon, MI) in gradient: 0–10 min with 100–95% A, 10–11 min with 95–40% A, 11–20 min with 40% A, 20–21 min with 40–100% A, and 21–30 min with 100% A. Flow rate was 0.2 ml/min. Adenosine (tR = 6.4 min) and inosine (tR = 3.2 min) were detected by electronspray positive ionization–mass spectrometric multiple reaction monitoring. Adenosine was monitored for m/z 268.08 parent ion and m/z 136.1 fragment ion, and inosine was monitored for m/z 269.08 parent ion and m/z 137.1 fragment ion.
RESULTS
Effect of experimental treatments on physiological parameters.
Streptozotocin-induced diabetic rats, either untreated or subjected to any of the experimental treatments, exhibited significantly lower body weights than their age-matched normal controls (Table 1). Similarly, blood glucose and GHb for diabetic rats and diabetic rats treated with sulfasalazine, other salicylates, or PJ34 were significantly elevated compared with the normal controls throughout the study periods (Table 1).
TABLE 1.
Therapies with sulfasalazine, salicylates, or PJ34 do not normalize the alterations in body weight, blood glucose, and GHb levels in experimentally diabetic rats
| Body wt (g) | Blood glucose (mg/dl) | GHb (%) | |
|---|---|---|---|
| 9 months | |||
| Normal | 609 ± 58 | 76 ± 11 | 4.0 ± 0.2 |
| Diabetic | 304 ± 48* | 325 ± 30* | 9.0 ± 1.1* |
| Diabetic + sulfasalazine | 300 ± 36* | 344 ± 1* | 9.9 ± 0.7* |
| 3 months (experiment 1) | |||
| Normal | 449 ± 20 | 109 ± 12 | 3.7 ± 0.1 |
| Diabetic | 267 ± 31* | 281 ± 43* | 9.1 ± 1.2* |
| Diabetic + sulfasalazine | 263 ± 30* | 314 ± 25* | 7.7 ± 1.3* |
| Diabetic + sodium salicylate | 269 ± 29* | 299 ± 23* | 7.6 ± 0.5* |
| Diabetic + buffered aspirin | 257 ± 24* | 314 ± 33* | 8.1 ± 1.1* |
| 3 months (experiment 2) | |||
| Normal | 458 ± 14 | 122 ± 1 | 3.8 ± 0.2 |
| Diabetic | 276 ± 42* | 291 ± 39* | 9.2 ± 0.9* |
| Diabetic + sulfasalazine | 256 ± 18* | 293 ± 1* | 9.2 ± 2.0* |
| Diabetic + PJ34 | 263 ± 28* | 287 ± 61* | 9.2 ± 1.1* |
Data are means ± SD of 6–10 animals.
Significantly different from normal rats at P < 0.01 (ANOVA followed by Dunnett's test).
Sulfasalazine treatment completely prevents development of tactile allodynia in diabetic rats.
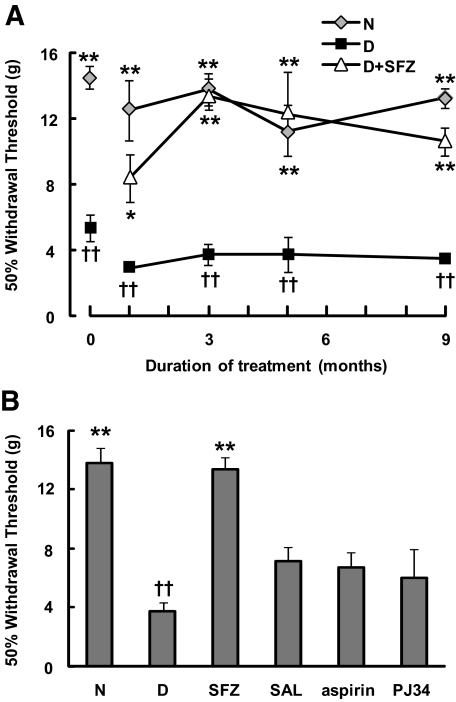
Consistent with previous observations (18), experimentally diabetic rats exhibited signs of tactile allodynia (i.e., pain in response to nonpainful, low-intensity stimuli) 4–7 days after streptozotocin injection, as evidenced by a significant decrease in the 50% withdrawal thresholds to von Frey filaments (0 time in Fig. 1A). The tactile allodynia in the diabetic animals persisted during the 9-month experimental period. Importantly, treatment with sulfasalazine completely blocked the development of tactile allodynia in diabetic rats (Fig. 1A). The effectiveness of sulfasalazine was also evident when the percentage of animals exhibiting a 50% withdrawal threshold higher than a reference value (set at 8 g) was calculated: responsiveness was observed in 50% of the diabetic rats after 1 month and in 100% after 3 months of therapy. We compared the effect of sulfasalazine on diabetic tactile allodynia with those of other salicylates and the PARP-1 inhibitor PJ34. In contrast to sulfasalazine treatment, administration of sodium salicylate, acetylsalicylic acid, or PJ34 only resulted in a partial, statistically nonsignificant, amelioration of tactile allodynia (Fig. 1B). The higher effectiveness of sulfasalazine was also evident when the percentage of animals exhibiting a 50% withdrawal threshold >8 g was calculated: responsiveness was observed in 90% of diabetic rats treated for 3 months with sulfasalazine compared with only 38, 38, and 25% of those treated with sodium salicylate, acetylsalicylic acid, or PJ34, respectively. Interestingly, although daily insulin administration to 4-month diabetic rats for 4 months corrected the rate of body weight gain and GHb levels, it only partially ameliorated tactile allodynia (Table 2).
FIG. 1.
Sulfasalazine prevents the development of tactile allodynia in experimentally diabetic rats. A: Experimental diabetes in Lewis rats was induced by a single injection of streptozotocin as described in research design and methods. Diabetic rats (D) exhibited early signs of allodynia 4–7 days after streptozotocin injection, as indicated by the values shown at 0 time (representative of an independent evaluation of two separate sets comprising a total of 52 diabetic and 16 normal rats). Streptozotocin-induced diabetic rats were either left untreated or subjected to treatment with sulfasalazine (starting 6–10 days after streptozotocin injection) for up to 9 months (D+SFZ). B: Streptozotocin-induced diabetic animals were treated for 3 months with sulfasalazine, sodium salicylate (SAL), acetylsalicylic acid (aspirin), or PJ34. The presence of tactile allodynia was investigated at different time points (A) or after 3 months (B) by comparison with responses in normal animals (N) (n = 6–8 animals per group were used). Results are means ± SE of the averages of the 50% withdrawal thresholds measured on the left and right paw of each animal (see research design and methods). Significantly different from diabetic animals at *P < 0.05 and **P < 0.01. ††Significantly different from normal animals at P < 0.01, as calculated by Kruskal-Wallis’ test followed by Dunn's test.
TABLE 2.
Tight glucose control in 4-month diabetic rats normalizes the rate of body weight gain and GHb levels but only partially ameliorates tactile allodynia
| Body wt (g) | GHb (%) | 50% Withdrawal thresholds (g) | |
|---|---|---|---|
| Normal/8 months | 535 ± 46 | 3.7 ± 0.1 | 13.80 ± 2.03 |
| Poor glucose control–diabetic/8 months | 270 ± 28* | 9.5 ± 0.7* | 3.04 ± 0.9† |
| Poor glucose control–diabetic/4 months (before insulin treatment) | 308 ± 39 | 9.9 ± 1.6 | 2.92 ± 0.89 |
| Tight glucose control–diabetic/4 months (after insulin treatment) | 403 ± 44‡ | 4.9 ± 0.2‡ | 6.76 ± 1.46†§ |
Data are means ± SD of 6–10 animals. Four-month diabetic rats (poor glucose control–diabetic/4 months) were either left untreated for an additional 4 months (poor glucose control–diabetic/8 months) or subjected to tight glucose control for 4 months (tight glucose control–diabetic/4 months). The presence of tactile allodynia was evaluated as indicated in the legend to Fig. 1.
Significantly different from normal rats at P < 0.01 (Student's t test).
Significantly different from poor glucose control–diabetic/4 months at P < 0.01 (paired Student's t test). Significantly different from
normal and
poor glucose control–diabetic/4 months at P < 0.01 (Kruskal-Wallis test, followed by Dunn's test).
Sulfasalazine treatment partially ameliorates mechanical hyperalgesia in diabetic rats.
Experimentally diabetic rats also developed mechanical hyperalgesia (evidenced by a ∼50% decrease in the withdrawal thresholds to mechanical stimuli) as early as 2 weeks after induction of the disease. In contrast to the complete inhibition of tactile allodynia, treatment with sulfasalazine for either 6 or 9 months only resulted in a modest amelioration of mechanical thresholds in diabetic rats that did not reach statistical significance (Table 3). Treatment of diabetic rats with PJ34 for 9 months was ineffective (Table 3).
TABLE 3.
Effects of sulfasalazine and PJ34 on mechanical hyperalgesia in experimentally diabetic rats
| Mechanical hyperalgesia withdrawal threshold (g) |
||
|---|---|---|
| 6 months | 9 months | |
| Normal | 218 ± 50 | 198 ± 4 |
| Diabetic | 71 ± 3* | 87 ± 7* |
| Diabetic + sulfasalazine | 111 ± 14* | 133 ± 34 |
| Diabetic + PJ34 | ND | 63 ± 5* |
Data are means ± SE of four to eight animals.
Significantly different from normal rats at P < 0.01 (ANOVA, followed by Dunnett's test). The statistical analysis showed no significant differences when comparing the diabetic + sulfasalazine or diabetic + PJ34 with the untreated diabetic rats; however, the diabetic + sulfasalazine withdrawal thresholds at 9 months were not significantly different than those in normal rats. ND, not determined.
Cellular targets of sulfasalazine.
We next investigated candidate targets of sulfasalazine that may mediate its beneficial effects on tactile allodynia in experimentally diabetic rats. In particular, we analyzed changes in levels of NF-κB, a transcription factor proposed to mediate the anti-inflammatory actions of sulfasalazine (4–6), and adenosine, a nucleoside known to act as an analgesic agonist. We focused on two initial sites of processing of sensory information in the peripheral nervous system (PNS): sciatic nerves and DRG. The sciatic nerves include axons, Schwann cells, and endothelial cells, whereas the DRGs comprise sensory neurons, satellite, and endothelial cells.
Changes in levels of NF-κB subunits in diabetic rats.
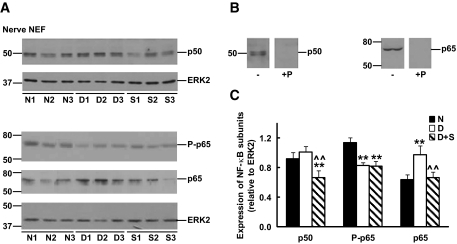
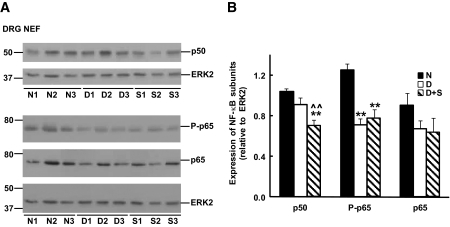
We examined the steady-state levels of NF-κB subunits in nuclear-enriched fractions of sciatic nerves and DRG. Although the expression of NF-κB p50 was similar in sciatic nerves and DRG from normal and diabetic rats, a significant decrease was observed after sulfasalazine treatment (Figs. 2 and 3). A different pattern was observed for NF-κB p65. Sciatic nerve extracts from diabetic rats displayed increased levels of NF-κB p65 when compared with normal samples that were corrected by sulfasalazine treatment (Fig. 2). In contrast, diabetic DRG displayed a ∼30% decrease in NF-κB p65 compared with normal samples that was not significantly altered after treatment with sulfasalazine (Fig. 3). Finally, a similar pattern, decreased levels in diabetic compared with normal samples that were not corrected by sulfasalazine treatment, was observed for NF-κB phospho-p65 (P-p65) in sciatic nerves and DRG nuclear-enriched fractions (Figs. 2 and 3).
FIG. 2.
Expression of NF-κB p50, phospho-p65 (P-p65), and p65 subunits in nuclear-enriched fractions (NEF) of sciatic nerves from normal (N), 3-month diabetic (D), and sulfasalazine-treated 3-month diabetic (S) rats. A: Immunoreactivity of sciatic nerve nuclear-enriched fractions from three individual animals in each group. Blots were also incubated with anti-ERK2 to control for differences in sample loading. Numbers at the left of each panel depict the position of molecular mass markers (in kDa). B: Specificity of the signals was confirmed by immunoblotting of sciatic nerve extracts with dilutions of anti–NF-κB p50 or anti–NF-κB p65 antisera previously incubated in the presence (+P) or absence (−) of immunogenic peptides. C: Densitometry of immunoreactivity of NF-κB subunits relative to ERK2 for all animals analyzed in each group (n = 5–7) (Significantly different from **normal and ∧∧untreated diabetics at P < 0.01 as calculated by ANOVA followed by Dunnett's test).
FIG. 3.
Expression of NF-κB subunits in nuclear-enriched fractions (NEF) of DRG from normal (N), 3-month diabetic (D), and sulfasalazine-treated 3-month diabetic (S) rats. A: Blots show the immunoreactivity in samples from three individual animals in each group. Blots were also incubated with anti-ERK2 to control for differences in sample loading. B: Densitometry of immunoreactivity of NF-κB subunits relative to ERK2 for all the animals analyzed in each group (n = 5–7). (Significantly different from **normal and ∧∧untreated diabetics at P < 0.01 as calculated by ANOVA followed by Dunnett's test).
We also performed a control experiment and subjected normal rats to treatment with sulfasalazine. This treatment did not significantly change levels of NF-κB subunits in nuclear-enriched fractions of sciatic nerves and DRG. Densitometry of sciatic nerve nuclear-enriched fractions showed that NF-κB p50 and p65 levels (relative to ERK2) were 1.00 ± 0.11 and 1.04 ± 0.05 (normal rats) and 1.06 ± 0.18 and 1.01 ± 0.17 (sulfasalazine-treated normal rats), respectively. Levels of NF-κB p50 and p65 in DRG nuclear-enriched fractions were 0.94 ± 0.07 and 1.49 ± 0.04 (normal rats) and 0.81 ± 0.11 and 1.33 ± 0.12 (sulfasalazine-treated normal rats), respectively (means ± SE, n = 4; nonsignificant differences by Student's t test).
Diabetic NF-κB p50−/− mice do not develop tactile allodynia.
We also analyzed the role of NF-κB in the development of enhanced nociception in experimental diabetes using NF-κB p50−/− mice. To this end, we compared the development of tactile allodynia by experimental diabetes in wild-type (B6129PF2/J) and NF-κB p50−/− mice. Blood glucose and GHb levels in diabetic wild-type and NF-κB p50−/− mice were significantly higher than in their normal counterparts (Table 4). As expected, 6-month diabetic wild-type mice developed tactile allodynia compared with the normal controls (Table 5). Significantly, normal and diabetic NF-κB p50−/− mice displayed withdrawal thresholds similar to those of normal wild-type mice (Table 5).
TABLE 4.
Body weight, blood glucose, and GHb levels in normal and diabetic wild-type and NF-κB p50−/− mice
| Body wt (g) | Blood glucose (mg/dl) | GHb (%) | |
|---|---|---|---|
| B6129PF2/J wild type | |||
| Normal | 36.0 ± 12.0 | 126 ± 14 | 3.0 ± 0.2 |
| Diabetic | 30.0 ± 3.0* | 327 ± 41† | 10.9 ± 1.1† |
| NF-κB p50−/− | |||
| Normal | 27.8 ± 5.8 | 135 ± 22 | 3.0 ± 0.2 |
| Diabetic | 26.4 ± 3.8 | 299 ± 24† | 9.4 ± 0.8† |
Data are means ± SD of 8–9 wild-type and 18–19 knockout mice. Measurements were performed 6 months after induction of experimental diabetes. Significantly different from normal rats at
P < 0.05 and
P < 0.01 (Student's t test).
TABLE 5.
Diabetic NF-κB p50−/− mice do not develop tactile allodynia
| 50% Withdrawal threshold (g) |
||
|---|---|---|
| B6129PF2/J wild-type mice | NF-κB p50−/− mice | |
| Normal | 5.93 ± 0.21 | 4.54 ± 1.59 |
| Diabetic | 0.84 ± 0.46* | 5.59 ± 0.66 |
Data are means ± SD of 8–9 wild-type and 18–19 knockout mice. Behavioral measurements were performed 6 months after induction of experimental diabetes.
Significantly different from normal mice at P < 0.01 (Kruskal-Wallis test, followed by Dunn's test).
Sulfasalazine treatment increases inosine levels in sciatic nerves of diabetic rats.
To determine whether the beneficial effects of sulfasalazine might be also mediated through changes in levels of adenosine or its metabolites, we analyzed sciatic nerve extracts from normal, diabetic, and sulfasalazine-treated diabetic rats. Reverse-phase HPLC showed that sciatic nerve extracts contain significant amounts of inosine, an adenosine deamination metabolite, whereas significant levels of the precursor nucleoside were not detected. The presence of low levels of adenosine in sciatic nerve extracts is consistent with its metabolism to AMP and/or with high adenosine deaminase activity causing degradation to inosine and other breakdown products. Although levels of inosine in normal and diabetic nerves were similar, extracts from sulfasalazine-treated diabetic rats displayed a significant increase in this nucleoside (Table 6). The presence of inosine in sciatic nerve extracts, together with relatively minor adenosine peaks (10–40 times smaller than those corresponding to inosine), was also detected by tandem mass spectrometry (not shown). However, the small magnitude of the adenosine peaks detected by liquid chromatography–tandem mass spectrometry prevented quantitative analysis among the different experimental groups.
TABLE 6.
Sulfasalazine treatment increases inosine levels in sciatic nerve extracts of diabetic rats
| Rats | Inosine (nmol/mg protein) |
|---|---|
| Normal | 3.92 ± 0.47 |
| Diabetic | 3.98 ± 0.38 |
| Sulfasalazine-treated diabetic | 5.25 ± 0.64* |
Data are means ± SD of extracts from three to five animals analyzed by reverse-phase HPLC. Assays were performed 9 months after induction of experimental diabetes.
Significantly different from normal and diabetic rats at P < 0.05 (ANOVA, followed by Dunnett's test).
DISCUSSION
Herein, we demonstrate that administration of sulfasalazine to experimentally diabetic rats completely inhibits the development of tactile allodynia observed in untreated diabetic animals. The protective effect of this salicylate conjugate was observed within 1 month after the start of treatment and persisted throughout the entire study period (9 months). The complete inhibition of tactile allodynia by sulfasalazine in diabetic rats contrasted with the partial effects observed with other salicylates or the PARP-1 inhibitor PJ34 (Fig. 1).
Recent studies support the role of oxidative and nitrosative stress in the pathogenesis of some of the alterations that characterize diabetic neuropathy. Treatment with inhibitors of PARP-1, an enzyme sensitive to oxidative and nitrosative stress, normalizes deficits in nerve blood flow, conduction velocity, and energy metabolism in experimental diabetes but only partially ameliorates sensory nerve function (19–21). This is consistent with our observation that the PARP-1 inhibitor PJ34 does not significantly prevent the development of tactile allodynia (Fig. 1B) or mechanical hyperalgesia (Table 3) in experimentally diabetic rats. This suggests that normalizing nerve function with therapies aimed at reducing oxidative stress and its downstream effectors in peripheral nerves might not be sufficient to normalize enhanced pain perception in chronic diabetes. This inference is further supported by the observation that normalizing glycemia in 4-month experimentally diabetic rats by daily insulin injection does not completely reverse tactile allodynia (Table 2). Therefore, factors other than oxidative stress might contribute to the development of this diabetes complication.
We observed that tactile allodynia develops rapidly (∼4–7 days) after induction of experimental diabetes in rats by a single streptozotocin injection, in agreement with previous observations (18). This rapid progression is similar to that observed in the reduction of nerve blood flow and precedes the onset of other alterations, such as reduction in motor nerve conduction velocity and sciatic nerve Na+/K+ ATPase activity, which develop 2 and 4 weeks, respectively, after induction of diabetes (22). The rapid development of tactile allodynia indicates that this complication is independent of long-term structural changes and other metabolic and functional alterations that develop more slowly. The absence of tactile allodynia in experimentally diabetic rats treated with sulfasalazine starting 6–10 days after the streptozotocin injection, when a significant number of diabetic animals already exhibit nociceptive alterations (0 time in Fig. 1), indicates that the therapy not only prevents but reverses these changes. Nonetheless, additional studies will be required to assess whether sulfasalazine treatment can also improve alterations resulting from long-term (3–4 months) experimental diabetes.
The different effectiveness of sulfasalazine treatment on tactile allodynia and mechanical hyperalgesia in diabetic rats (complete prevention versus partial amelioration, respectively) is of interest. As discussed above, tactile allodynia develops quickly after streptozotocin injection and appears to involve a mechanism different to that underlying mechanical hyperalgesia (23). This different effectiveness of sulfasalazine may reflect its specific targeting of sensory fibers mediating tactile allodynia (i.e., large Aβ and small myelinated Aδ nociceptive fibers) and/or additional effects of this drug on other peripheral nerve abnormalities (e.g., decreased blood flow). The different effectiveness of sulfasalazine on tactile allodynia and mechanical hyperalgesia in diabetes is reminiscent of the effect of interleukin (IL)-6 and is consistent with the proposal that mechanical hyperalgesia may require the participation of specific nerve fibers and/or their information processing at the central nervous system (CNS) (24).
We observed that inhibition of tactile allodynia by sulfasalazine treatment in diabetic rats is associated with changes in NF-κB expression. Although NF-κB activation appears to induce hyperalgesia in some, but not all, models of inflammatory and neuropathic pain (8,25), its role in diabetic nociceptive alterations remains controversial. Increased activation of NF-κB in the nerve microvasculature of diabetic patients and mice appears to contribute to thermal hypoalgesia (26). In contrast, decreased NF-κB activation has been observed in DRG of diabetic rats (27). We observed decreased expression of NF-κB P-p65, with no significant changes in NF-κB p50, in diabetic DRG nuclear-enriched fractions (Fig. 3). This finding seems consistent with an overall decrease in the nuclear translocation of activated NF-κB (p50/p65 heterodimers) in diabetic DRG. On the other hand, the increase in NF-κB p65 detected in diabetic sciatic nerves might reflect a deficit in retrograde axonal transport of this subunit in diabetes, similar to observations after nerve crush injury (28), and/or augmented expression in endothelial or Schwann cells. The normalization of NF-κB p65 in sciatic nerve nuclear-enriched fractions by sulfasalazine hints at a possible mechanism underlying the reversal of tactile allodynia by this drug in diabetic rats.
An important observation in this study is the absence of tactile allodynia in experimentally diabetic NF-κB p50−/− mice (Table 5). This suggests that NF-κB p50–containing complexes are necessary for activation of target genes contributing to tactile allodynia in experimentally diabetic mice. The association between prevention of tactile allodynia and decreased levels of NF-κB p50 in sciatic nerve and DRG nuclear-enriched fractions of diabetic rats treated with sulfasalazine supports this link. Contrary to the findings with nerve NF-κB p65, however, we did not observe differences in the overall levels of NF-κB p50 in sciatic nerve or DRG nuclear-enriched fractions of normal and diabetic rats, which argues against this subunit contributing to altered nociception in diabetes. It is possible, however, that opposite changes in the expression of NF-κB p50 in different cells of the PNS (i.e., small, medium, and large neurons and satellite and endothelial cells) may result in overall normal levels of this subunit in diabetic nerves and DRG. Clearly, immunocytochemical studies will be required to address the localization and changes in the expression of NF-κB subunits in the different cells or compartments of diabetic sciatic nerves and DRG.
Activation of NF-κB is critical for neuronal survival, Schwann cell differentiation, and myelination in peripheral nerves during development (29). In contrast, after spinal cord injury, selective inhibition of astroglial NF-κB confers a protective effect through upregulation of IL-6 and downregulation of transforming growth factor-β2, CXCL10, and CCL2 (30). These findings are consistent with the beneficial effects of IL-6 on several parameters of diabetic neuropathy (24). It is then possible that the beneficial effects of sulfasalazine therapy on tactile allodynia in diabetic rats may stem from NF-κB–dependent changes in the expression of cytokines in specific cell types of DRG and nerve. This hypothesis is consistent with the observation that sulfasalazine treatment inhibits both the induction of inflammatory mediators and the formation of retinal vasculature lesions in diabetic rats (6).
We also addressed whether the nociceptive improvement in sulfasalazine-treated diabetic rats could be mediated by changes in adenosine levels. This was suggested by the observation that sulfasalazine increases the release of adenosine both in vivo and in vitro (8) and by the improved nerve function and analgesia observed in experimentally diabetic rats treated with adenosine or adenosine kinase inhibitors (31,32). We observed that sciatic nerves contain significant levels of inosine, a nucleoside derived from adenosine deamination, and low levels of the precursor nucleoside. Both adenosine and inosine are important neuromodulators. Adenosine, which increases after nerve injury, induces analgesia through activation of adenosine A1 receptors (33,34). Inosine, on the other hand, induces neurite outgrowth in retinal ganglion cells and cortical neurons (35,36) and is a potent agonist for A1/A3 adenosine receptors (37). Although inosine levels in normal and diabetic nerve extracts were similar, we observed a significant increase in this nucleoside upon treatment with sulfasalazine (Table 6). We hypothesize that the diabetic derangement impairs the enhanced release of adenosine/inosine, which is normally associated with peripheral nerve injury (34), and that sulfasalazine treatment overcomes this blockade. This suggests that sulfasalazine may also impede tactile allodynia in diabetic rats by releasing nucleosides that cause analgesia.
Investigation of additional targets of sulfasalazine, such as NMDA receptors (38), may provide a more complete understanding of the bases for the effectiveness of sulfasalazine in diabetic neuropathy. Importantly, our experiments focused on sciatic nerves and DRG, initial sites of sensory information processing in the PNS. Further studies should examine changes brought about by sulfasalazine treatment in the spinal cord, the first level of sensory processing in the CNS.
In summary, the present study demonstrates that sulfasalazine treatment completely impedes the development of tactile allodynia in experimentally diabetic rats. The effectiveness of sulfasalazine may reflect the combined modulation of levels of NF-κB and inosine and other as yet unidentified targets. The absence of tactile allodynia in diabetic NF-κB p50−/− mice supports a role for NF-κB in the development of nociceptive alterations in experimental diabetes. These findings justify further studies to test the effects of sulfasalazine on altered nociception in diabetic patients.
Acknowledgments
L.N.B.-M. has received National Institutes of Health (NIH) Grant DK-064465. T.S.K. has received NIH Grant EY-000300.
We thank G. Dubyak for advice and materials used in the reverse HPLC experiments and B. Larkin for technical assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 15 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Vinik AI, Park TS, Stansberry KB, Pittenger GL: Diabetic neuropathies. Diabetologia 43:957–973, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Calcutt NA, Freshwater JD, Mizisin AP: Prevention of sensory disorders in diabetic Sprague-Dawley rats by aldose reductase inhibition or treatment with ciliary neurotrophic factor. Diabetologia 47:718–724, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Shutov L, Kruglikov I, Gryshchenko O, Khomula E, Viatchenko-Karpinski V, Belan P, Voitenko N: The effect of nimodipine on calcium homeostasis and pain sensitivity in diabetic rats. Cell Mol Neurobiol 26:1541–1557, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahl C, Liptay S, Adler G, Schmid RM: Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest 101:1163–1174, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber CK, Liptay S, Wirth T, Adler G, Schmid RM: Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology 119:1209–1218, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS: Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes 56:337–345, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Gadangi P, Longaker M, Naime D, Levin RI, Recht PA, Montesinos MC, Buckley MT, Carlin G, Cronstein BN: The anti-inflammatory mechanism of sulfasalazine is related to adenosine release at inflamed sites. J Immunol 156:1933–1941, 1996 [PubMed] [Google Scholar]
- 8.Cronstein BN, Montesinos MC, Weissmann G: Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proc Natl Acad Sci U S A 96:6377–6381, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlin G, Djursater R, Smedegard G: Inhibitory effects of sulfasalazine and related compounds on superoxide production by human polymorphonuclear leukocytes. Pharmacol Toxicol 65:121–127, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Tornhamre S, Edenius C, Smedegård G, Sjöuist B, Lindgren JA: Effects of sulfasalazine and a sulfasalazine analogue on the formation of lipoxygenase and cyclooxygenase products. Eur J Pharmacol 169:225–234, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Bradley SM, le Gallez P, Throughton PR, Gooi HC, Astbury C, Bird HA: The effect of sulphasalazine on neutrophil superoxide generation in rheumatoid arthritis. Br J Rheumatol 36:530–534, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Pruzanski W, Stefanski E, Vadas P, Ramamurthy NS: Inhibition of extracellular release of proinflammatory secretory phospholipase A2 (sPLA2) by sulfasalazine: a novel mechanism of anti-inflammatory activity. Biochem Pharmacol 53:1901–1907, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Zheng L, Szabó C, Kern TS: Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-κB. Diabetes 53:2960–2967, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Sha WC, Liou HC, Tuomanen EI, Baltimore D: Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 80:321–330, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Berti-Mattera LN, Gariepy CE, Burke R, Hall AK: Reduced expression of endothelin B receptors and mechanical hyperalgesia in experimental chronic diabetes. Exp Neurol 201:399–406, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL: Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Joseph SM, Pifer MA, Przybylski RJ, Dubyak, GR: Methylene ATP analogs as modulators of extracellular ATP metabolism and accumulation. Br J Pharmacol 142:1002–1014, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR: Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain 68:293–299, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Obrosova IG, Li F, Abatan OI, Forsell MA, Komjati K, Pacher P, Szabo C, Stevens MJ: Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes 53:711–720, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Li F, Szabó C, Pacher P, Southan GJ, Abatan OI, Charniauskaya T, Stevens MJ, Obrosova IG: Evaluation of orally active poly(ADP-ribose) polymerase inhibitor in streptozotocin-diabetic rat model of early peripheral neuropathy. Diabetologia 47:710–717, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Ilnystka O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtalir N, Pacher P, Yorek MA, Obrosova IG: Poly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes 55:1686–1694, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA: Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int J Exp Diabetes Res 1:131–143, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roytta M, Wei H, Pertovaara A: Spinal nerve ligation-induced neuropathy in the rat: sensory disorders and correlation between histology of the peripheral nerves. Pain 80:161–170, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Cameron NE, Cotter MA: The neurocytokine, interleukin-6, corrects nerve dysfunction in experimental diabetes. Exp Neurol 207:23–29, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Tegeder I, Niederberger E, Schmidt R, Kunz S, Guhring H, Ritzeler O, Michaelis M, Geisslinger G: Specific Inhibition of IkappaB kinase reduces hyperalgesia in inflammatory and neuropathic pain models in rats. J Neurosci 24:1637–1645, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bierhaus A, Haslbeck KM, Humpert PM, Liliensiek B, Dehmer T, Morcos M, Sayed AA, Andrassy M, Schiekofer S, Schneider JG, Schulz JB, Heuss D, Neundörfer B, Dierl S, Huber J, Tritschler H, Schmidt AM, Schwaninger M, Haering HU, Schleicher E, Kasper M, Stern DM, Arnold B, Nawroth PP: Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest 114:1741–1751, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purves TD, Tomlinson DR: Diminished transcription factor survival signals in dorsal root ganglia in rats with streptozotocin-induced diabetes. Ann N Y Acad Sci 973:472–476, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Doyle CA, Hunt SP: Reduced nuclear factor kappaB (p65) expression in rat primary sensory neurons after peripheral nerve injury. Neuroreport 8:2937–2942, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Nickols JC, Valentine W, Kanwal S, Carter BD: Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci 6:161–167, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR: Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202:145–156, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch JJ, Jarvis MF, Kowaluk EA: An adenosine kinase inhibitor attenuates tactile allodynia in a rat model of diabetic neuropathic pain. Eur J Pharmacol 364:141–146, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Saini AK, Arun KH, Kaul CL, Sharma SS: Acute hyperglycemia attenuates nerve conduction velocity and nerve blood flow in male Sprague-Dawley rats: reversal by adenosine. Pharmacol Res 50:593–599, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Cui JG, Sollevi A, Linderoth B, Meyerson BA: Adenosine receptor activation suppresses tactile hypersensitivity and potentiates spinal cord stimulation in mononeuropathic rats. Neurosci Lett 223:173–176, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Sawynok J, Liu XJ: Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol 69:313–340, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Benowitz LI, Jing Y, Tabibiazar R, Jo SA, Petrausch B, Stuermer CA, Rosenberg PA, Irwin N: Axon outgrowth is regulated by an intracellular purine-sensitive mechanism in retinal ganglion cells. J Biol Chem 273:29626–29634, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI: Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A 99:9031–9036, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J: International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552, 2001 [PMC free article] [PubMed] [Google Scholar]
- 38.Gwag BJ, Lee YA, Ko SY, Lee MJ, Im DS, Yun BS, Lim HR, Park SM, Byun HY, Son SJ, Kwon HJ, Lee JY, Cho JY, Won SJ, Kim KW, Ahn YM, Moon HS, Lee HU, Yoon SH, Noh JH, Chung JM, Cho SI: Marked prevention of ischemic brain injury by Neu2000, an NMDA antagonist and antioxidant derived from aspirin and sulfasalazine. J Cereb Blood Flow Metab 27:1142–1151, 2007 [DOI] [PubMed] [Google Scholar]