Abstract
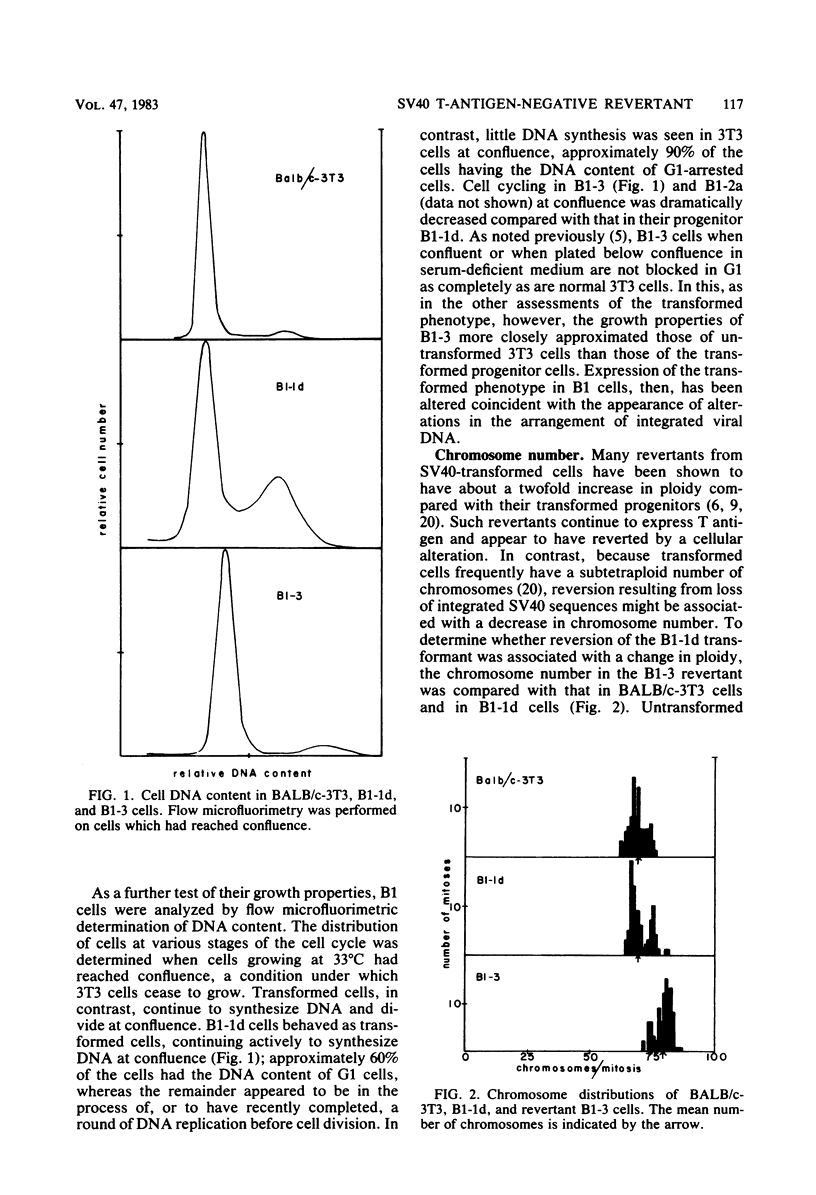
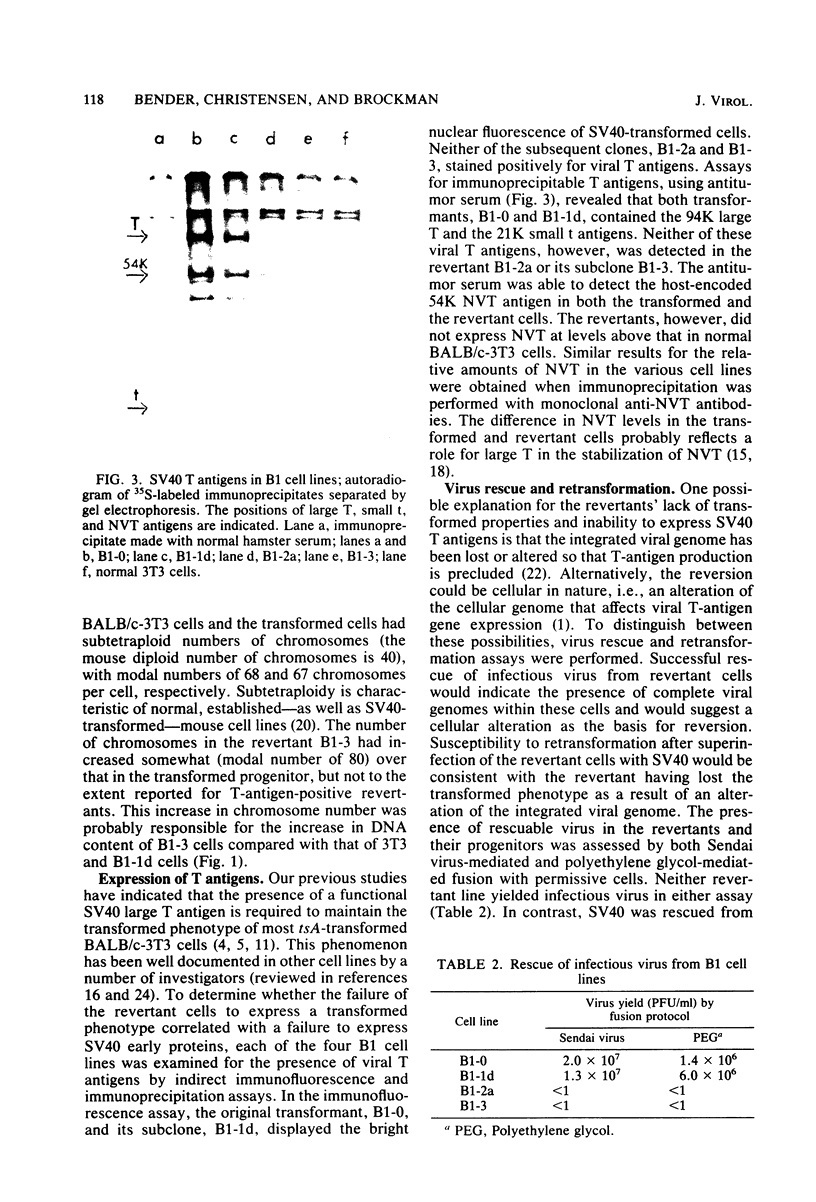
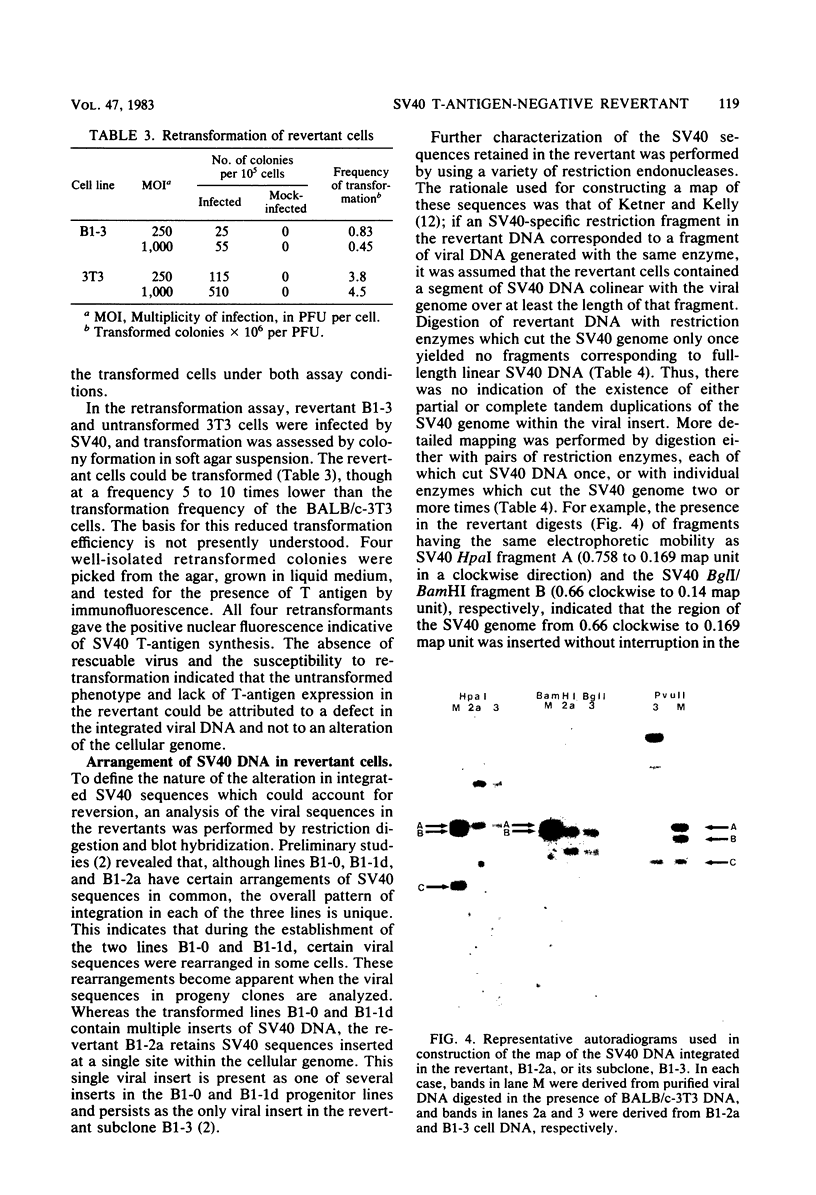
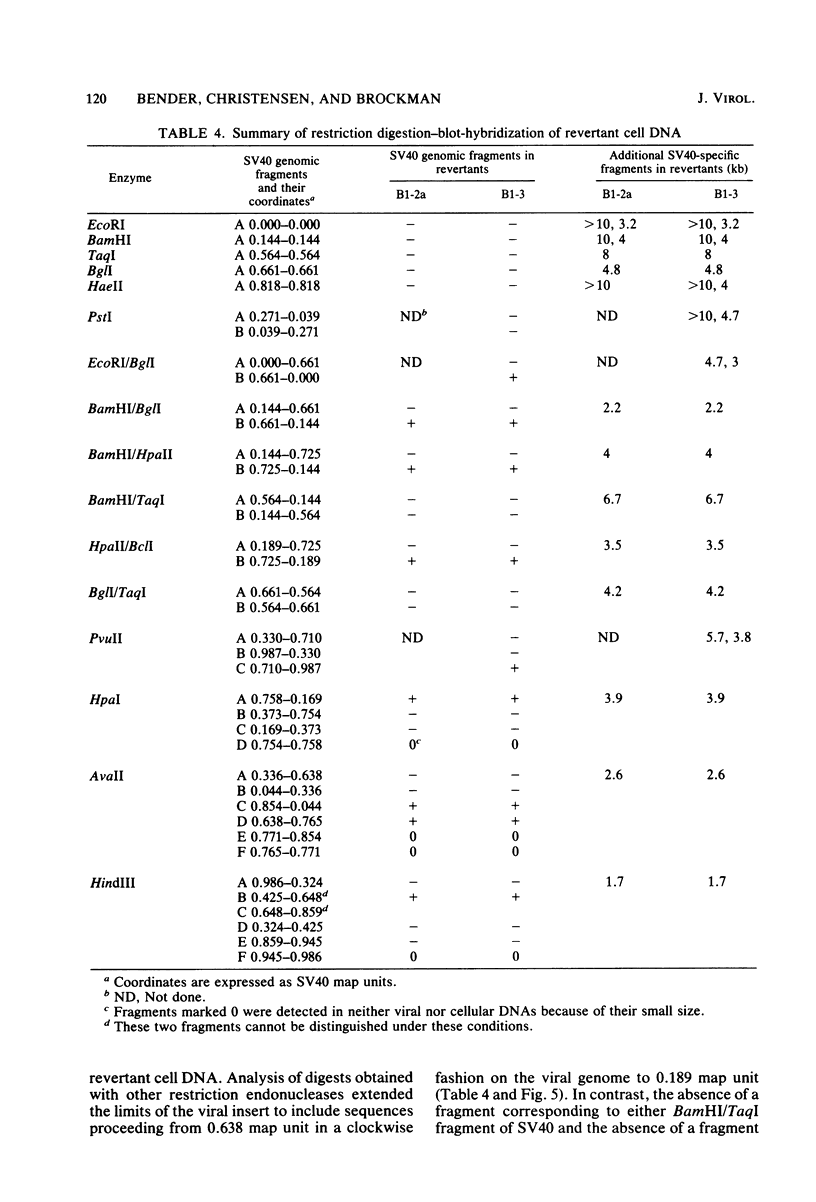
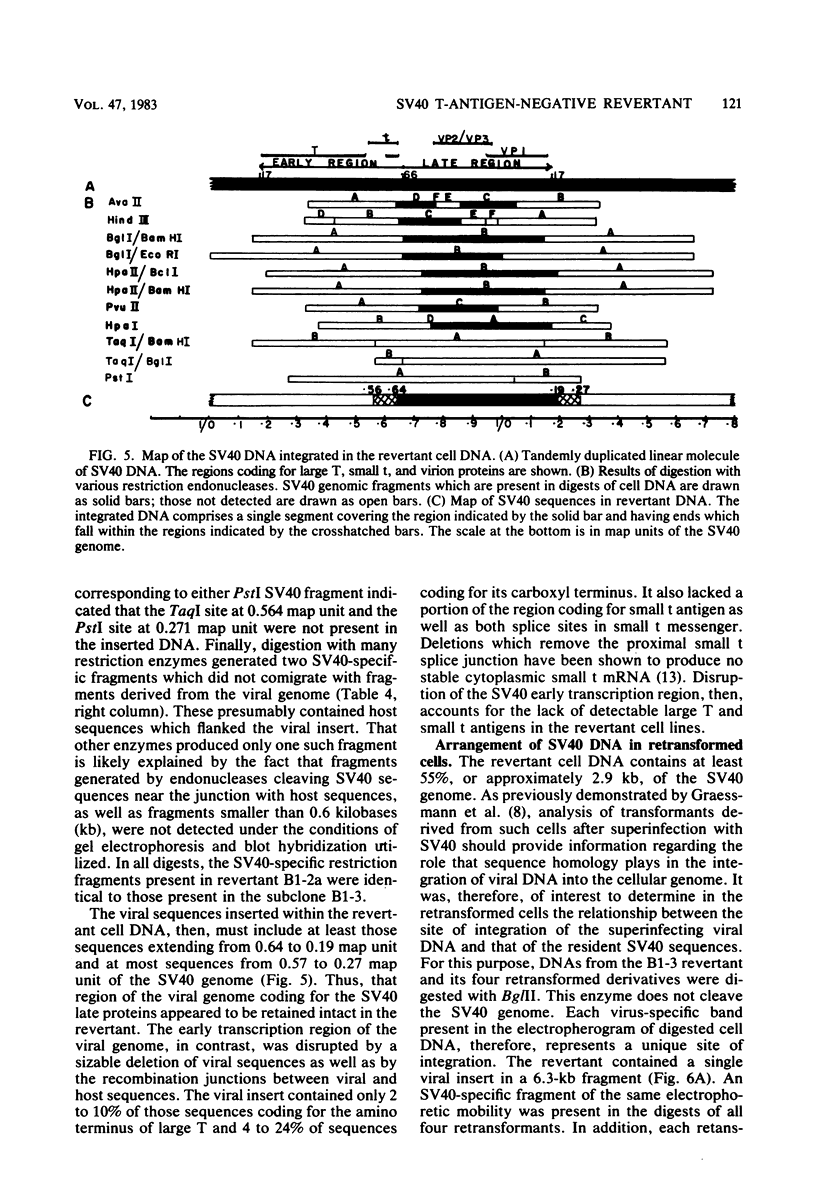
A transformation revertant has been isolated from an unusual line of simian virus 40 (SV40)-transformed BALB/c-3T3 cells in which rearrangements of integrated viral sequences are common. The revertant produces no SV40 T antigens, yields no virus on fusion with permissive cells, and can be retransformed by SV40 virions. SV40 DNA sequences are present within the cellular DNA, but interruption of the viral early transcription region by deletion and recombination with cellular sequences precludes the synthesis of T antigens. Analysis of this revertant lends further support to the notion that large T antigen plays an essential role in the maintenance of transformation in SV40-transformed BALB/c-3T3 cells. Examination of integration of SV40 DNA in this revertant, as well as in a temperature-sensitive A transformant, after retransformation by SV40 confirms that sequence homology plays little role in the insertion of SV40 DNA into cellular chromosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Zouzias D. Regulation of viral transciption and tumor antigen expression in cells transformed by simian virus 40. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1931–1935. doi: 10.1073/pnas.73.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Brockman W. W. Rearrangement of integrated viral DNA sequences in mouse cells transformed by simian virus 40. J Virol. 1981 Jun;38(3):872–879. doi: 10.1128/jvi.38.3.872-879.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. B., Brockman W. W. Effects of large and small T antigens on DNA synthesis and cell division in simian virus 40-transformed BALB/c 3T3 cells. J Virol. 1982 Nov;44(2):574–585. doi: 10.1128/jvi.44.2.574-585.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from simian virus 40-transformed cells. 3. Concanavalin A-selected revertant cells. J Virol. 1972 Apr;9(4):611–620. doi: 10.1128/jvi.9.4.611-620.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend K. K., Chen S., Ruddle F. H. Differential staining of interspecific chromosomes in somatic cell hybrids by alkaline Giemsa stain. Somatic Cell Genet. 1976 Mar;2(2):183–188. doi: 10.1007/BF01542631. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Topp W. C., Botchan M. Retransformation of a simian virus 40 revertant cell line, which is resistant to viral and DNA infections, by microinjection of viral DNA. J Virol. 1979 Dec;32(3):989–994. doi: 10.1128/jvi.32.3.989-994.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Gurney T., Jr Density dependent inhibition of both growth and T-antigen expression in revertants isolated from simian virus 40-transformed mouse SVT2 cells. J Virol. 1979 Nov;32(2):667–671. doi: 10.1128/jvi.32.2.667-671.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley S., Bender M. A., Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: effect of transformation technique on the transformed phenotype. J Virol. 1980 Jan;33(1):550–552. doi: 10.1128/jvi.33.1.550-552.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Structure of integrated simian virus 40 DNA in transformed mouse cells. J Mol Biol. 1980 Dec 5;144(2):163–182. doi: 10.1016/0022-2836(80)90031-5. [DOI] [PubMed] [Google Scholar]
- Khoury G., Gruss P., Dhar R., Lai C. J. Processing and expression of early SV40 mRNA: a role for RNA conformation in splicing. Cell. 1979 Sep;18(1):85–92. doi: 10.1016/0092-8674(79)90356-8. [DOI] [PubMed] [Google Scholar]
- Lange M., May E., May P. Ability of nonpermissive mouse cells to express a simian virus 40 late function(s). J Virol. 1981 Jun;38(3):940–951. doi: 10.1128/jvi.38.3.940-951.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Maltzman W., Levine A. J. The SV40 A gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology. 1979 Oct 30;98(2):308–318. doi: 10.1016/0042-6822(79)90554-3. [DOI] [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Hiwasa T., Oda K. I. Characterization of flat revertant cells isolated from simian virus 40-transformed mouse and rat cells which contain multiple copies of viral genomes. J Virol. 1981 Mar;37(3):1028–1043. doi: 10.1128/jvi.37.3.1028-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B. Variants of simian virus 40-transformed 3T3 cells that are resistant to concanavalin A. J Virol. 1973 Jul;12(1):79–89. doi: 10.1128/jvi.12.1.79-89.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Wolman S., Vogel A. Reversion of virus-transformed cell lines: hyperploidy accompanies retention of viral genes. Nature. 1970 Dec 5;228(5275):938–passim. doi: 10.1038/228938a0. [DOI] [PubMed] [Google Scholar]
- Steinberg B., Pollack R., Topp W., Botchan M. Isolation and characterization of T antigen-negative revertants from a line of transformed rat cells containing one copy of the SV40 genome. Cell. 1978 Jan;13(1):19–32. doi: 10.1016/0092-8674(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. DNA sequence homology and chromosomal deletion at a site of SV40 DNA integration. Nature. 1982 Mar 25;296(5855):363–366. doi: 10.1038/296363a0. [DOI] [PubMed] [Google Scholar]
- Vogel A., Pollack R. Isolation and characterization of revertant cell lines. IV. Direct selection of serum-revertant sublines of SV40-transformed 3T3 mouse cells. J Cell Physiol. 1973 Oct;82(2):189–198. doi: 10.1002/jcp.1040820207. [DOI] [PubMed] [Google Scholar]
- Vogel A., Risser R., Pollack R. Isolation and characterization of revertant cell lines. 3. Isolation of density-revertants of SV40-transformed 3T3 cells using colchicine. J Cell Physiol. 1973 Oct;82(2):181–188. doi: 10.1002/jcp.1040820206. [DOI] [PubMed] [Google Scholar]