Abstract
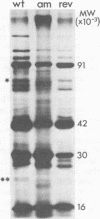
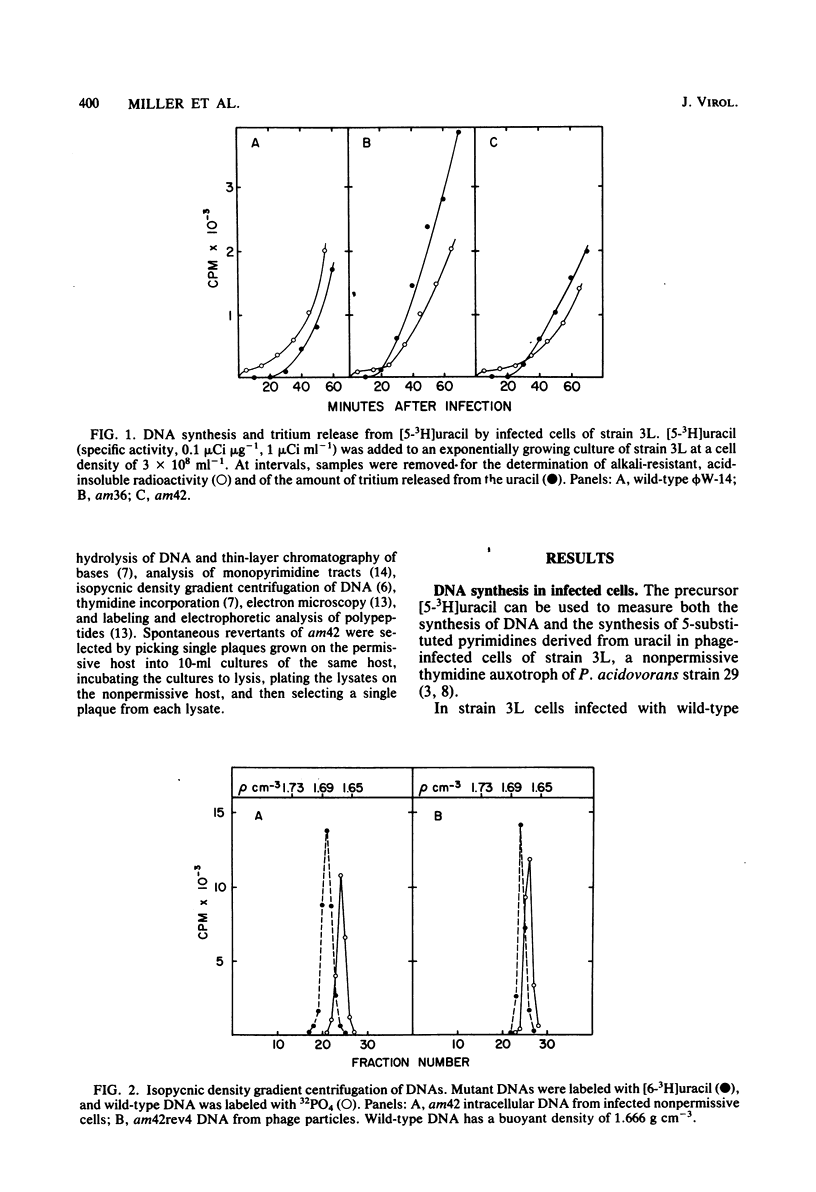
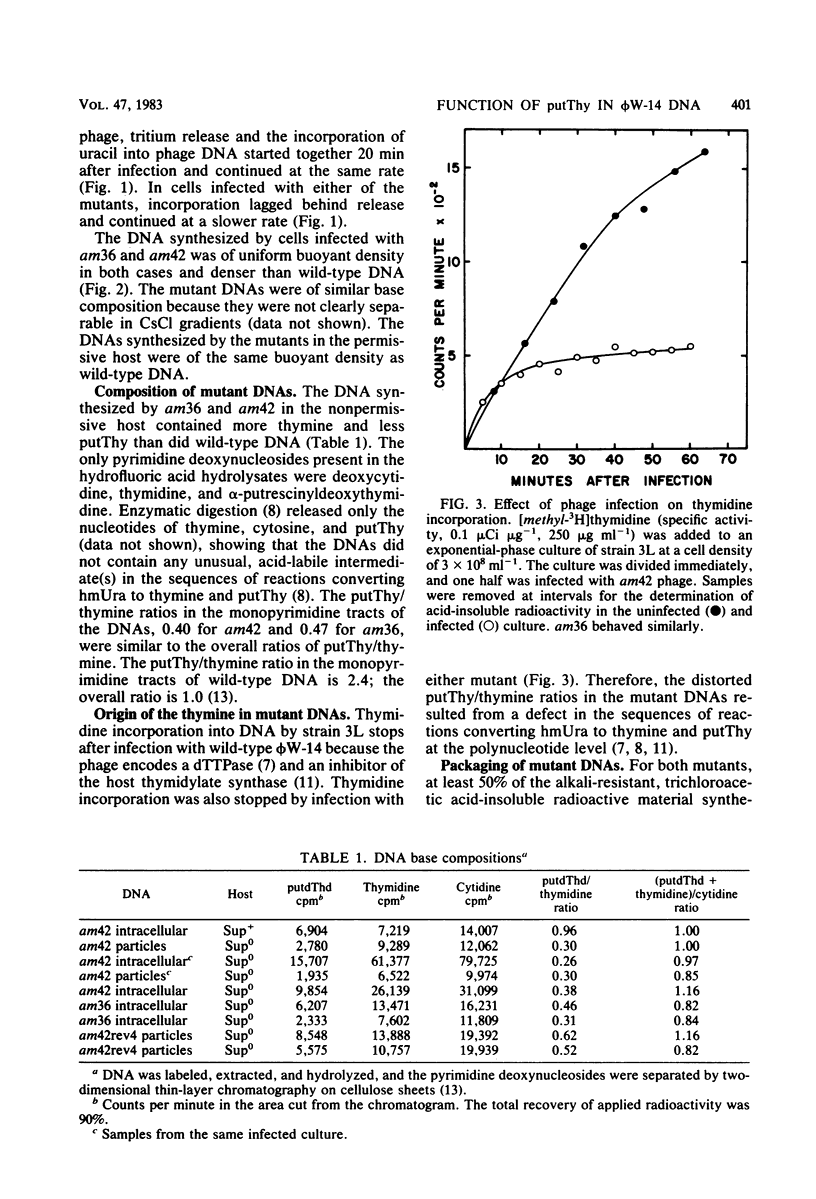
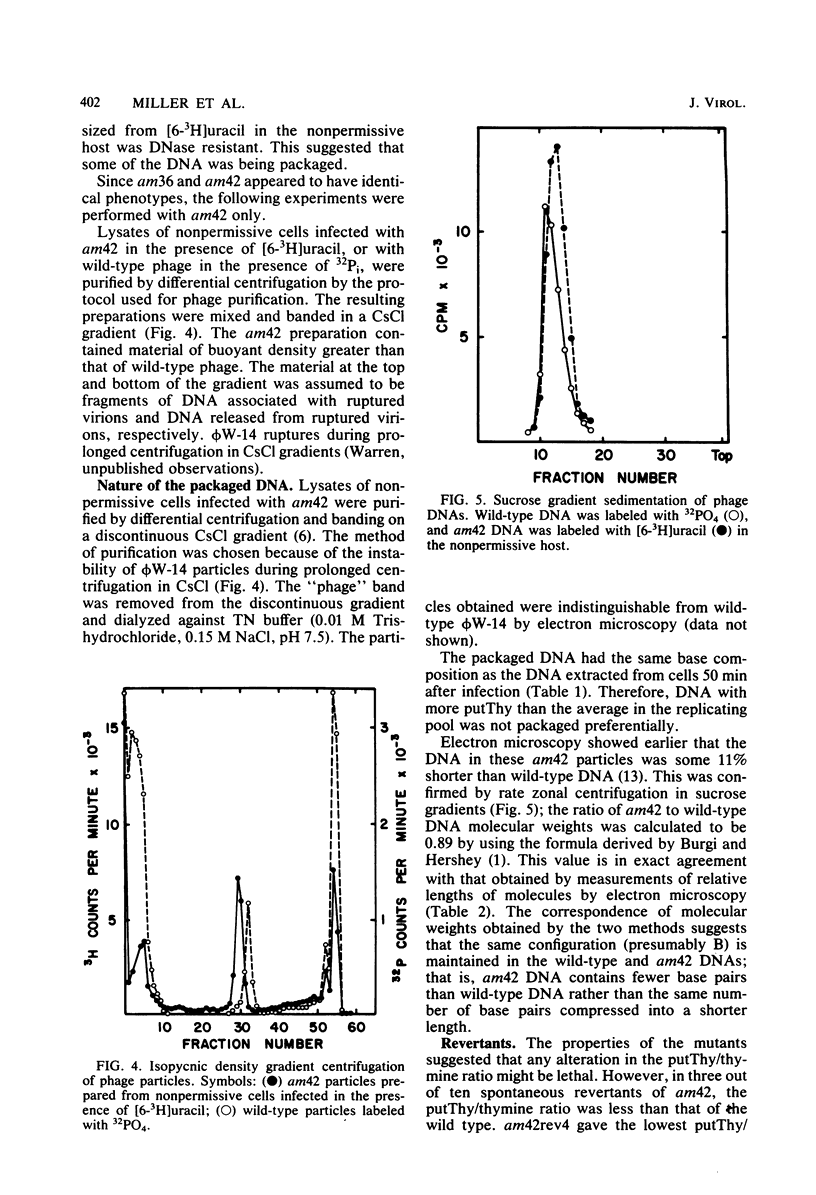
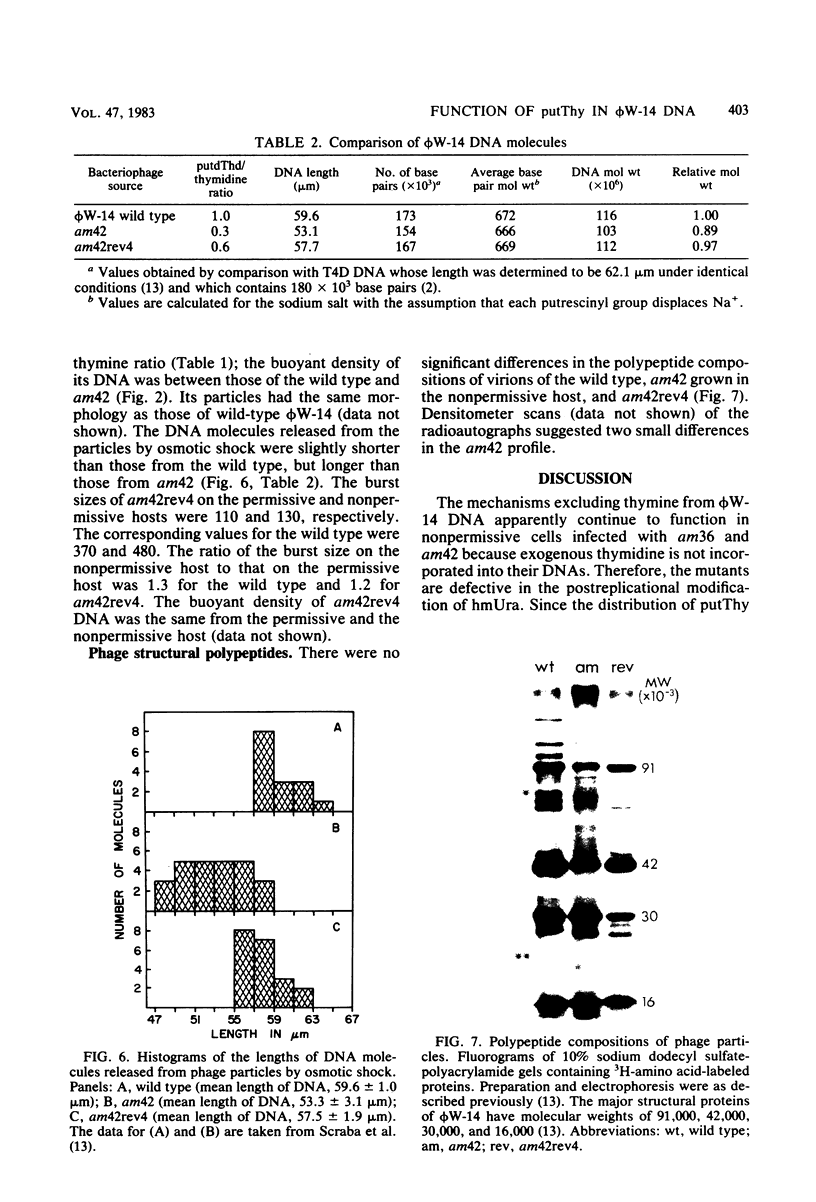
The DNA synthesized in the nonpermissive host by the noncomplementing mutants am36 and am42 of bacteriophage phi W-14 contains about half the wild-type level of alpha-putrescinylthymine (putThy) and a correspondingly greater level of thymine. The mechanisms whereby thymine nucleotides are excluded from replicating DNA are functional in both mutants because neither of them incorporates exogenous thymidine into DNA. It is proposed that (i) in wild-type phi W-14, the conversion of hydroxymethyluracil to putThy at the polynucleotide level is sequence specific, but that to thymine is nonspecific; and (ii) in the mutants, the sequence-specific recognition is impaired so that more thymine and less putThy are formed. The thymine-rich DNA can be packaged into phage particles. In the case of am42, the phage particles are morphologically indistinguishable from and have essentially the same polypeptide composition as wild-type particles. However, the DNA molecules they contain are about 11% shorter than those in wild-type phage, am42rev4, a revertant of am42, contains DNA with about 70% of the normal level of putThy; these molecules are about 3% shorter than wild-type DNA. The properties of am42 and am42rev4 are consistent with the suggestion that putThy facilitates the very tight packing of phi W-14 DNA (Scraba et al., Virology 124:152-160, 1983). It also appears that the putThy content of phi W-14 DNA can be reduced by no more than 30% without adversely affecting the production of viable progeny; for example, the burst size of am42rev4 is about 25% of that of the wild type.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. W., Wever G. H., Wiberg J. S. High-molecular-weight DNA and the sedimentation coefficient: a new perspective based on DNA from T7 bacteriophage and two novel forms of T4 bacteriophage. J Virol. 1980 Jan;33(1):438–448. doi: 10.1128/jvi.33.1.438-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelln R. A., Warren R. A. Obligate thymidine auxotrophs of Pseudomonas acidovorans. J Bacteriol. 1973 Jan;113(1):510–511. doi: 10.1128/jb.113.1.510-511.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Bose R. J., Warren R. A. 5-(4-Aminobutylaminomethyl)uracil, an unusual pyrimidine from the deoxyribonucleic acid of bacteriophage phiW-14. Biochemistry. 1973 Jan 2;12(1):151–157. doi: 10.1021/bi00725a025. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Warren R. A. Isolation and properties of a Pseudomonas acidovorans bacteriophage. J Gen Virol. 1970 Jan;6(1):85–93. doi: 10.1099/0022-1317-6-1-85. [DOI] [PubMed] [Google Scholar]
- Lewis H. A., Miller R. C., Jr, Stone J. C., Warren R. A. Alkali lability of bacteriophage phi W-14 DNA. J Virol. 1975 Dec;16(6):1375–1379. doi: 10.1128/jvi.16.6.1375-1379.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltman K. L., Neuhard J., Lewis H. A., Warren R. A. Synthesis of thymine and alpha-putrescinylthymine in bacteriophage phi W-14-infected Pseudomonas acidovorans. J Virol. 1980 May;34(2):354–359. doi: 10.1128/jvi.34.2.354-359.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltman K. L., Neuhard J., Warren R. A. 5-[(Hydroxymethyl)-O-pyrophosphoryl]uracil, an intermediate in the biosynthesis of alpha-putrescinylthymine in deoxyribonucleic acid of bacteriophage phi W-14. Biochemistry. 1981 Jun 9;20(12):3586–3591. doi: 10.1021/bi00515a043. [DOI] [PubMed] [Google Scholar]
- Marcus M., Newlon M. C. Control of DNA synthesis in Bacillus subtilis by phage phi e. Virology. 1971 Apr;44(1):83–93. doi: 10.1016/0042-6822(71)90155-3. [DOI] [PubMed] [Google Scholar]
- Miller P. B., Maltman K. L., Warren R. A. Isolation and preliminary characterization of amber mutants of bacteriophage phi W-14 defective in DNA synthesis. J Virol. 1982 Jul;43(1):67–72. doi: 10.1128/jvi.43.1.67-72.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Maltman K. L., Warren R. A. Bacteriophage phi W-14-infected Pseudomonas acidovorans synthesizes hydroxymethyldeoxyuridine triphosphate. J Virol. 1980 May;34(2):347–353. doi: 10.1128/jvi.34.2.347-353.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe D. H. Thymidine triphosphate nucleotidohydrolase: a phage-induced enzyme in Bacillus subtilis. Virology. 1969 Aug;38(4):520–526. doi: 10.1016/0042-6822(69)90172-x. [DOI] [PubMed] [Google Scholar]
- Scraba D. G., Bradley R. D., Leyritz-Wills M., Warren R. A. Bacteriophage phi W-14: the contribution of covalently bound putrescine to DNA packing in the phage head. Virology. 1983 Jan 15;124(1):152–160. doi: 10.1016/0042-6822(83)90298-2. [DOI] [PubMed] [Google Scholar]