Abstract
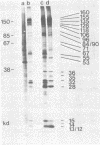
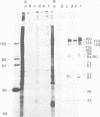
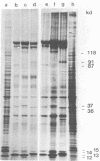
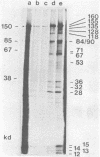
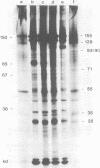
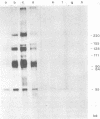
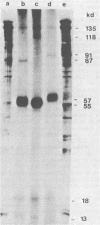
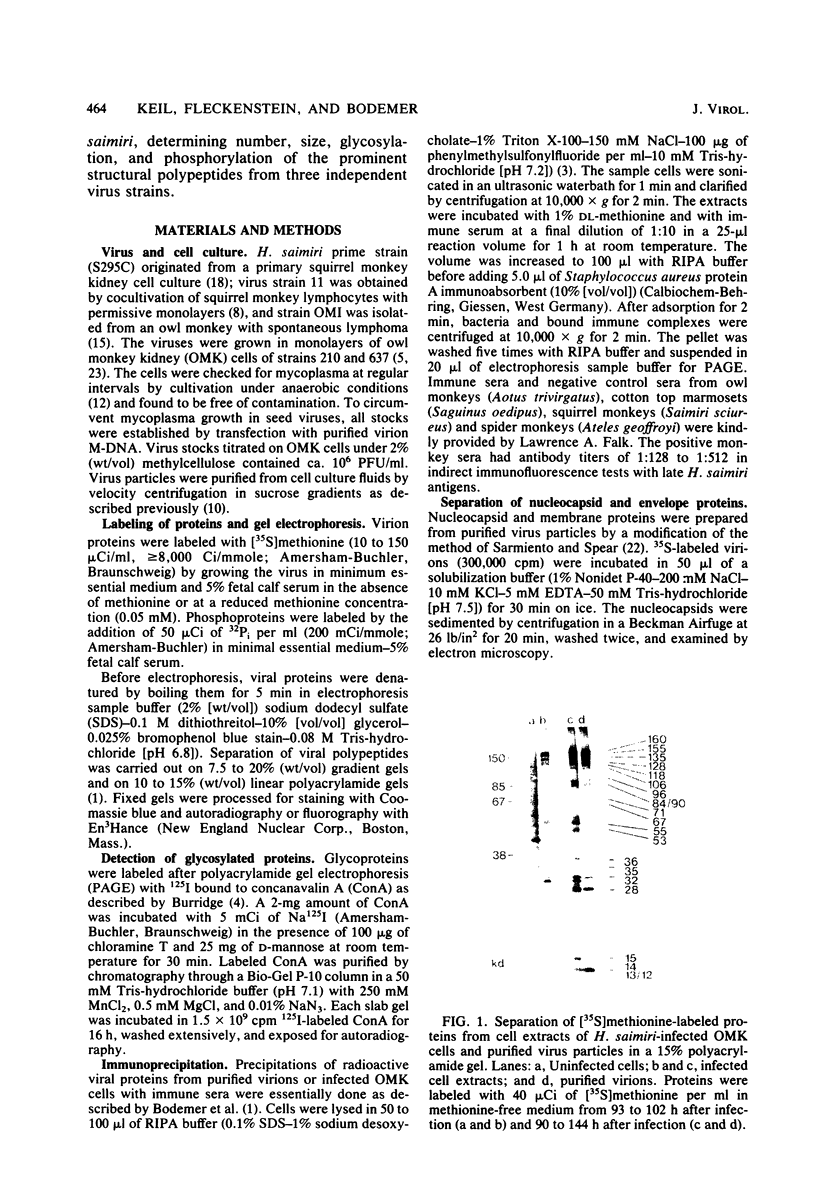
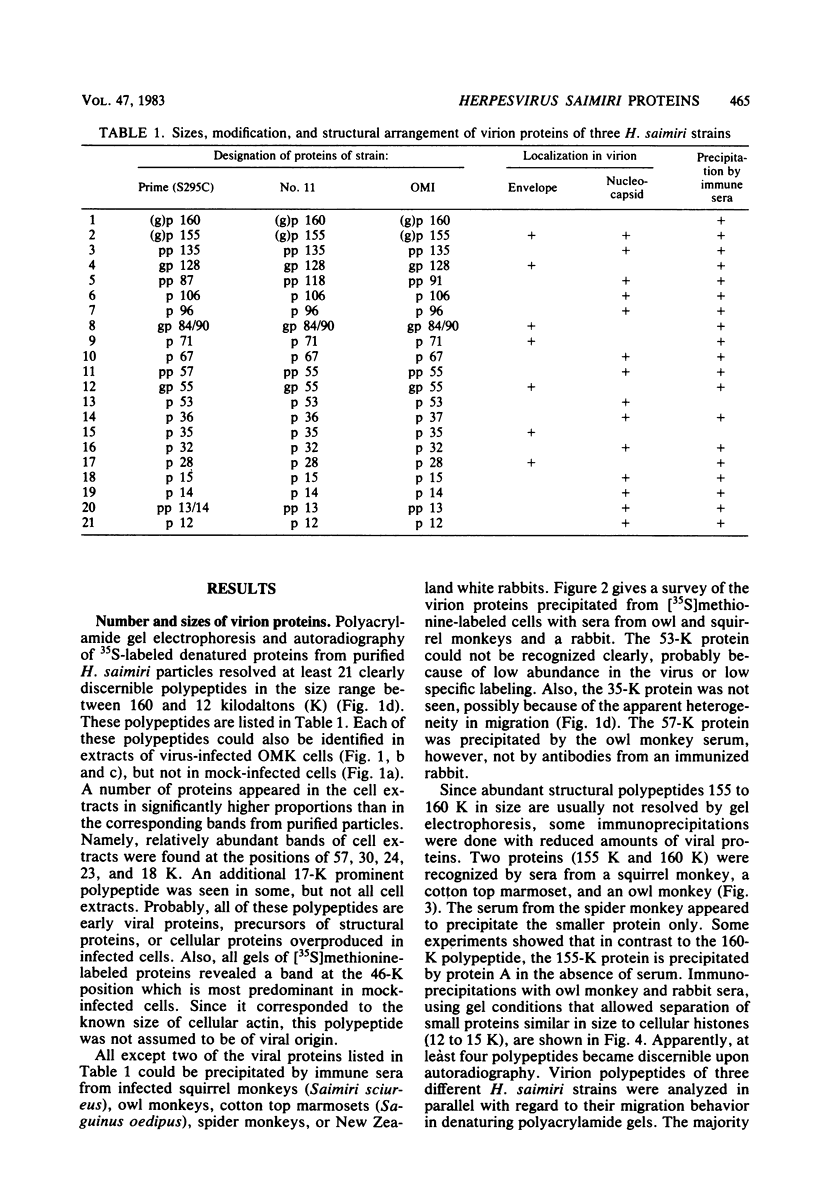
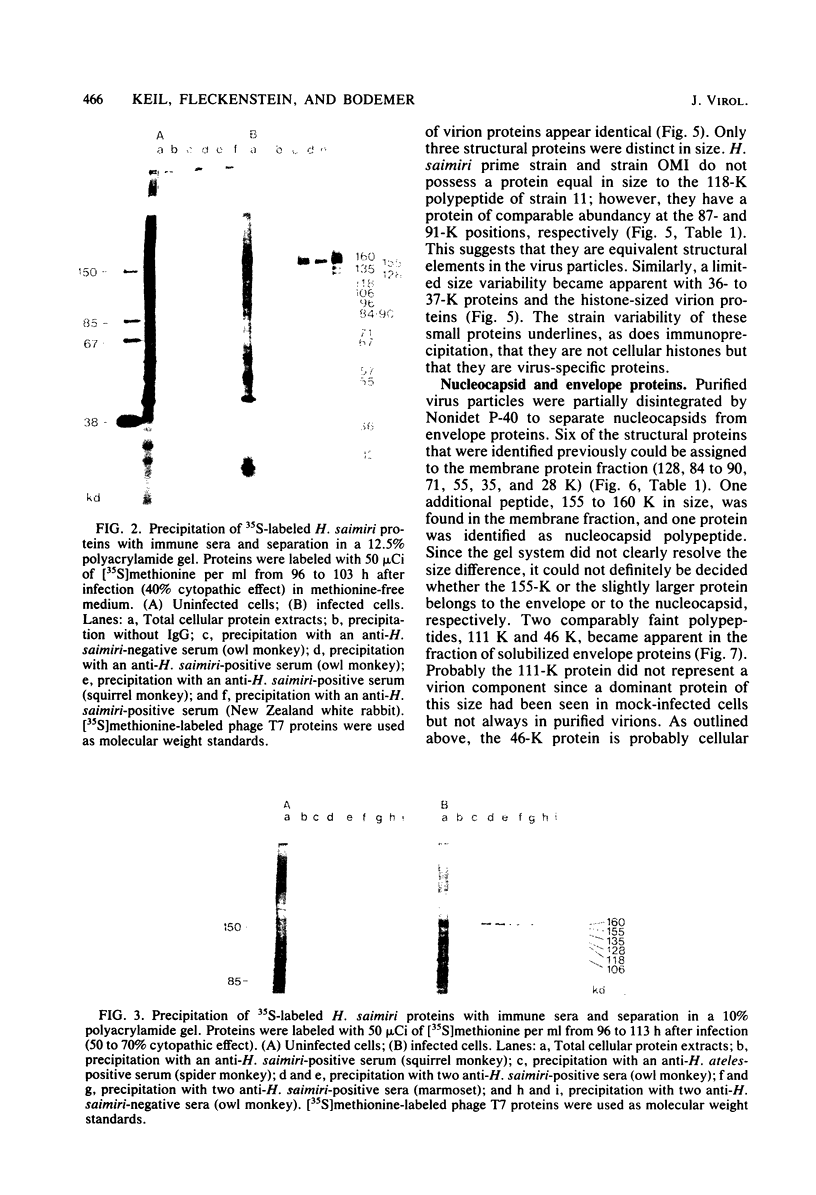
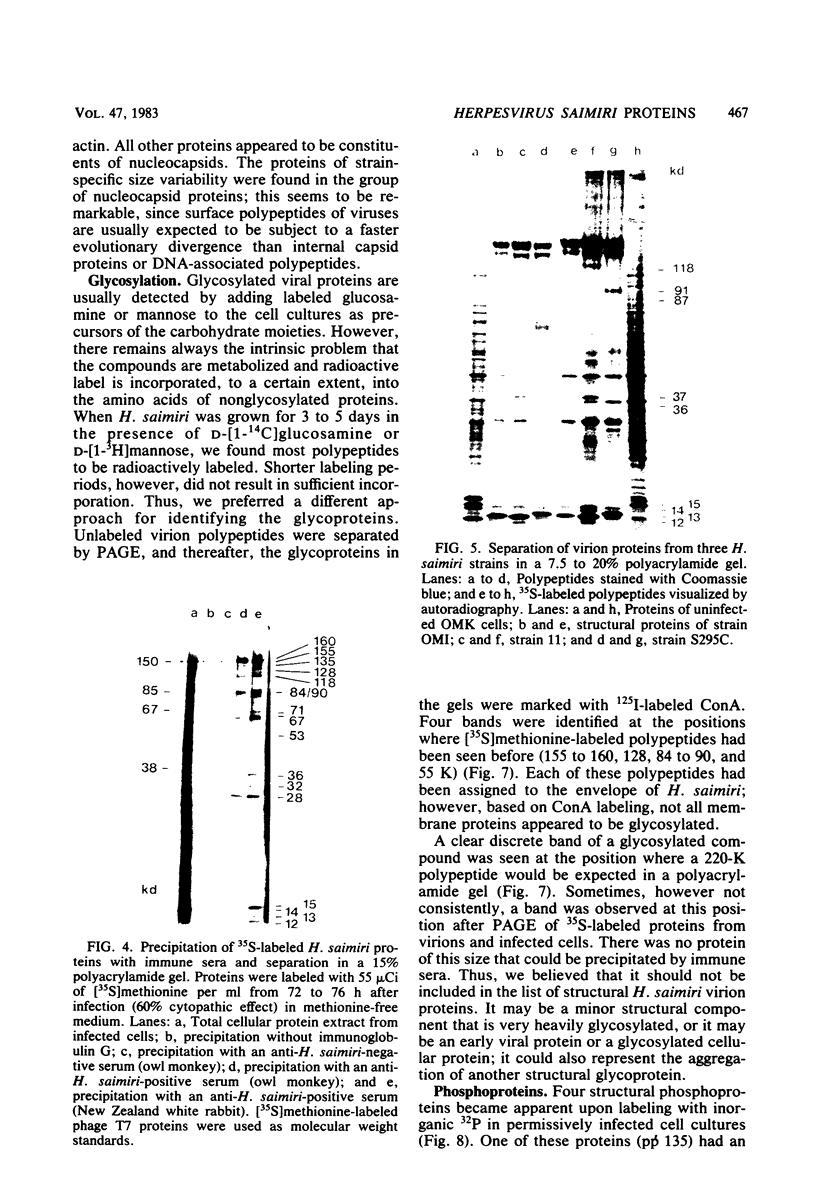
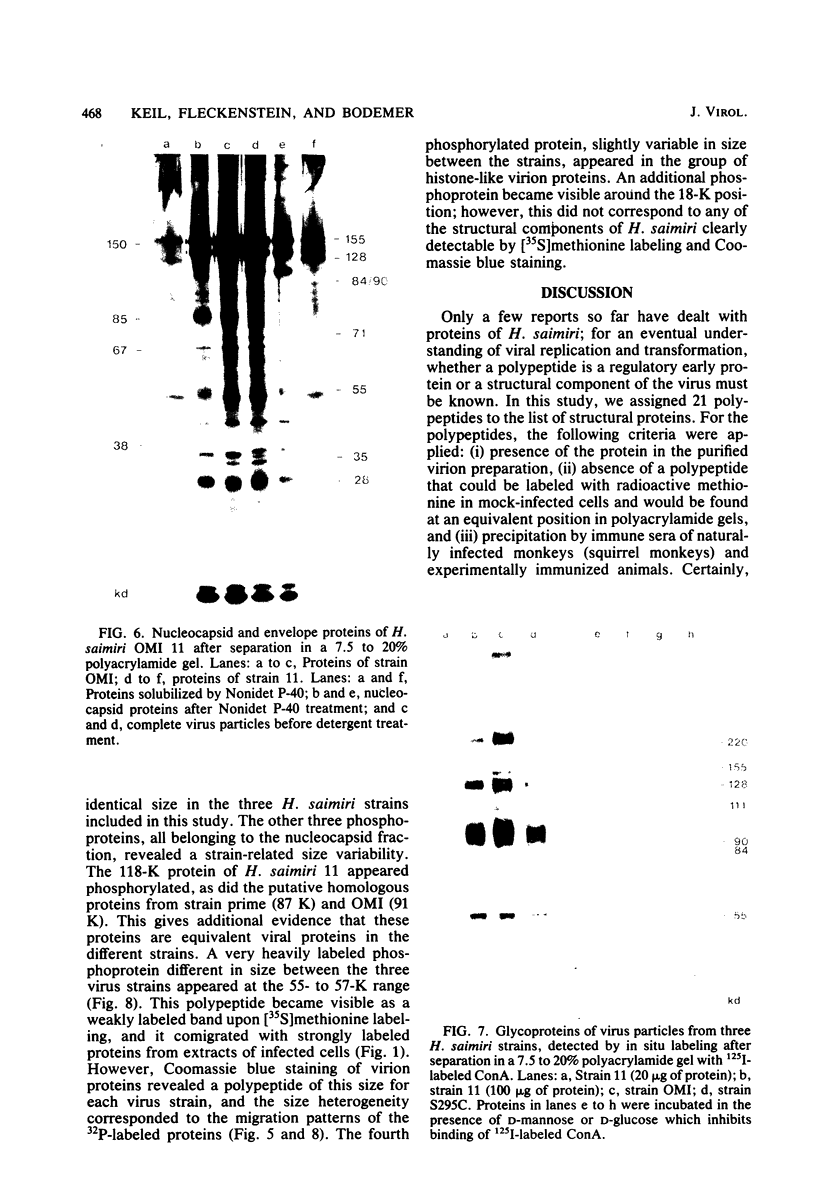
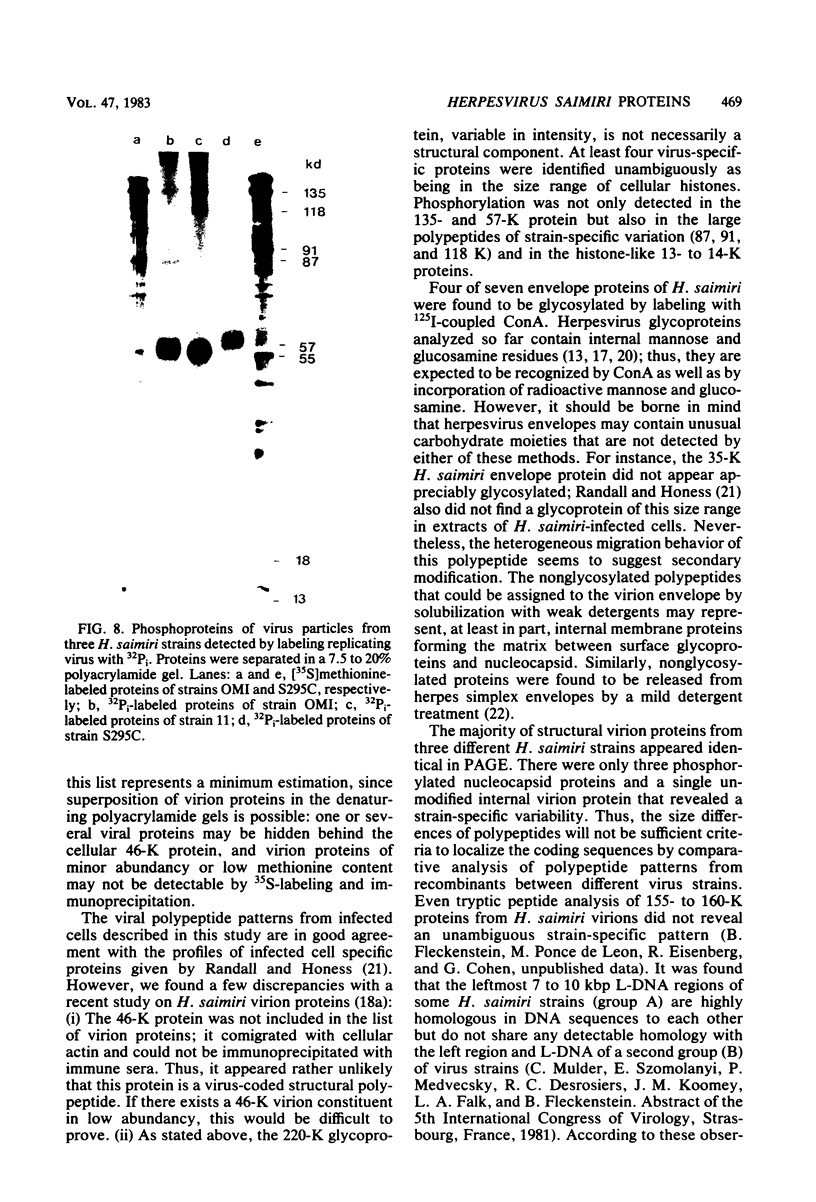
Herpesvirus saimiri particles were purified from productively infected owl monkey kidney cell cultures, and the virion polypeptides were analyzed by polyacrylamide gel electrophoresis. A total of 21 predominant proteins were found in lysates of H. saimiri 11 particles by Coomassie blue staining or by [35S]methionine labeling and autoradiography; all proteins were between 160,000 and 12,000 daltons in size. They are most probably virion constituents, as most of them were precipitated by immune sera, and no dominant proteins of equivalent sizes were found in mock-infected cultures. Four glycoproteins (gp 155/160, gp 128, gp 84/90, gp 55) and three polypeptides that appeared not to be glycosylated (p71, p35, p28) were assigned to the envelope or matrix of virions, whereas at least four phosphoproteins (pp132, pp118, pp55, pp13) and ten polypeptides without apparent secondary modification (p155/160, p106, p96, p67, p53, p36, p32, p15, p14, p12) were found in the nucleocapsid fraction. Analysis of virion proteins from different H. saimiri strains did not reveal appreciable differences in the migration behavior of most polypeptides, including all glycoproteins; however, determination of a strain-specific size pattern was possible for three of four phosphoproteins. The overall similarity in protein architecture of H. saimiri strains obviously does not reflect the variability in biology, such as oncogenic properties. In comparison, DNA sequence divergences appear to remain a better taxonomic criterion for strain distinction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodemer W. W., Summers W. C., Niederman J. C. Detection of virus-specific antigens in EB-(P3HR1) virus-superinfected Raji cells by immunoprecipitation. Virology. 1980 Jun;103(2):340–349. doi: 10.1016/0042-6822(80)90192-0. [DOI] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Da Silva M. P. Cuidados de enfermagem nos pacientes com "shunt" e fistula artério-venosa. Rev Enferm Nov Dimens. 1976 Nov;2(5):290–294. [PubMed] [Google Scholar]
- Deinhardt F. W., Falk L. A., Wolfe L. G. Simian herpesviruses and neoplasia. Adv Cancer Res. 1974;19(0):167–205. doi: 10.1016/s0065-230x(08)60054-8. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Falk L. A. Herpesvirus saimiri strain variability. J Virol. 1982 Jul;43(1):352–356. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Deinhardt F. Isolation of Herpesvirus saimiri from blood of squirrel monkeys (Saimiri sciureus). J Natl Cancer Inst. 1972 May;48(5):1499–1505. [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Ludwig H. Repetitive sequences in complete and defective genomes of Herpesvirus saimiri. J Virol. 1975 Feb;15(2):398–406. doi: 10.1128/jvi.15.2.398-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Werner J. The presence of Herpesvirus Saimiri genomes in virus-transformed cells. Int J Cancer. 1977 Apr 15;19(4):546–554. doi: 10.1002/ijc.2910190416. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B. Oncogenic herpesviruses of non-human primates. Biochim Biophys Acta. 1979 Nov 30;560(3):301–342. doi: 10.1016/0304-419x(79)90007-6. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Unity and diversity in the herpesviruses. J Gen Virol. 1977 Oct;37(1):15–37. doi: 10.1099/0022-1317-37-1-15. [DOI] [PubMed] [Google Scholar]
- Hunt R. D., Garcia F. G., Barahona H. H., King N. W., Fraser C. E., Meléndez L. V. Spontaneous Herpesvirus saimiri lymphoma in an owl monkey. J Infect Dis. 1973 Jun;127(6):723–725. doi: 10.1093/infdis/127.6.723. [DOI] [PubMed] [Google Scholar]
- Melendez L. V., Daniel M. D., Hunt R. D., Garcia F. G. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri sciureus). Lab Anim Care. 1968 Jun;18(3):374–381. [PubMed] [Google Scholar]
- Modrow S., Wolf H. Characterization of Herpesvirus saimiri and Herpesvirus ateles structural proteins. Virology. 1983 Feb;125(1):251–255. doi: 10.1016/0042-6822(83)90080-6. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S., Jeansson S., Lycke E. Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J Virol. 1981 May;38(2):564–570. doi: 10.1128/jvi.38.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R. E., Honess R. W. Proteins specified by Herpesvirus saimiri: purification and properties of a single polypeptide which elicits virus-neutralizing antibody. J Gen Virol. 1982 Jan;58(Pt 1):149–161. doi: 10.1099/0022-1317-58-1-149. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Spear P. G. Membrane proteins specified by herpes simplex viruses. IV. Conformation of the virion glycoprotein designated VP7(B2). J Virol. 1979 Mar;29(3):1159–1167. doi: 10.1128/jvi.29.3.1159-1167.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Sen A., King N., Daniel M. D., Fleckenstein B. Endogenous New World primate type C viruses isolated from owl monkey (Aotus trivirgatus) kidney cell line. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1004–1008. doi: 10.1073/pnas.75.2.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]