Abstract
In muscles, sarcopenia, the loss of muscle mass, is the major cause of aging-related functional decline and frailty. Several factors are correlated with sarcopenia during aging, including contraction-related cellular injury, oxidative stress, endocrine changes and reduced regenerative potential. However the involvement of these factors has not been experimentally investigated. Here, we report that contraction-related injury may significantly promote the progression of sarcopenia in the pharynx of the nematode, Caenorhabditis elegans, a model of aging in non-regenerative tissues. Both functional and structural declines in the pharynx during aging were significantly delayed in mutants with reduced muscle contraction rates. We also examined the role of bacteria in pharynx muscle decline during aging, as previous studies reported that antimicrobial treatments could extend C. elegans lifespan. Although microbial infection may have enhanced functional decline in the pharynx during aging, it was not the sole cause of decreased pumping rates in old animals. This study identifies contraction-related injury as a factor affecting the initiation and progression of sarcopenia during aging. Further, characterization of the specific types of damage induced by muscle contraction will be helpful for understanding the underlying causes of sarcopenia.
Keywords: Caenorhabditis elegans, Pharynx, Sarcopenia, Muscle, Aging, Insulin, Acetylcholine, Bacteria, Microbial stress
1. Introduction
During normal aging, most animals experience declines in muscle strength and coordination that can significantly impair motility and function. Although the gross changes to tissue during aging are readily apparent, little is known about the types of cellular changes associated with aging that impair functionality (Kamel, 2003). One approach to studying aging-related functional decline is to dissect these changes in a genetically amenable model organism. The nematode, Caenorhabditis elegans, is a well-studied organism that provides a useful model for dissecting the genetics of longevity and aging (Guarente et al., 2000). As in people, aging in C. elegans is accompanied by muscle deterioration, or sarcopenia, which correlates with reduced muscle function (Glenn et al., 2005; Herndon et al., 2002; Johnson, 1987). C. elegans muscles lack regenerative potential in the adult. Thus, C. elegans offers the opportunity to dissect the effects of a variety of stresses associated with aging in a non-regenerating tissue. Identifying the causes of sarcopenia in C. elegans muscles will be useful for learning why human muscles suffer from sarcopenia during aging.
C. elegans nematodes feed on bacteria that are injested by rhythmic contractions of the pharynx muscles, a neuromuscular organ in the head composed of 20 muscle cells and 20 neurons (Albertson et al., 1976). Aging-related declines are easily studied in the pharynx, as the overall structure and function of the pharynx muscles can be monitored under a light microscope. In young adults, the pharynx contracts, or pumps, approximately 200–300 times/min and this pumping rate declines progressively with aging (Bolanowski et al., 1981; Huang et al., 2004). Aging is also associated with structural deterioration of the pharynx muscles (Garigan et al., 2002; Herndon et al., 2002). The causes of pharynx decline during aging have not been identified. One possibility is that the muscles suffer cellular damage over a lifetime of rapid contractions. Alternatively, these muscles may be capable of withstanding contraction-related stresses, but suffer damage from contraction-independent causes, such as microbial infection or ROS byproducts of basal metabolism.
Here, we present evidence that implicates contraction-related injury as a major factor in the progression of sarcopenia in the aging pharynx. By analyzing mutant strains with reduced pumping rates, we found that pharynx function was preserved at older ages in animals whose muscles performed fewer contractions during their lifetime. In addition, structural declines were less severe in the slow-pumping mutants. We also examined the role of bacteria in pharynx decline and found that bacterial infection was not the sole cause of pharyngeal decline, although live bacteria were inhibitory to pumping at most ages. Our findings indicate that muscle contractions over a lifetime may contribute to sarcopenia either directly through mechanical damage to cells, or indirectly through increased respiration to meet high ATP demands.
2. Materials and methods
2.1. Strains and nematode growth
C. elegans strains used in this work were N2, Bristol (wildtype); BA17, fem-1 (hc 17); CB1370, daf-2(e1370); DR1572, daf-2(e1368); DA465, eat-2(ad465); DAI 110, eat-18(ad110); GR1321, tph-1(mg280). Strains were provided by the Caenorhabditis Genetics Center at the University of Minnesota.
All animals were cultivated on NGM agar plates with the Escherichia coli strain OP50 following standard protocols (Brenner, 1974). In these experiments, we used the temperature-sensitive sterile strain, fem-1(hc17), to eliminate the problem of progeny overgrowth in age-synchronized populations. Similar results were obtained with the wildtype N2 Bristol strain. For age-synchronized populations, we collected eggs from 5–10 adult hermaphrodites over several hours. Adults were transferred to 25 °C on the first day of adulthood and maintained at this temperature until death. The first day of adulthood was designated as day 0 and animals were transferred to fresh media to eliminate overcrowding by progeny as needed. fem-1(hc17) larvae were raised at 25 °C, the non-permissive temperature for adult sterility. For daf-2(e1368) and daf-2(e1370), larvae were raised at 15 °C to bypass the temperature-sensitive period for dauer arrest.
2.2. Analysis of pumping rates
Pumping assays were performed on agar plates at room temperature (approximately 22 °C) using a Nikon SMZ1500 stereomicroscope. Pumping rate was defined as the number of contractions in the terminal bulb in a 1-min period. For pumping rates in the presence of bacteria, pumping was measured in NGM agar with confluent lawns of OP50 bacteria, the standard laboratory diet for C. elegans. Pumping measurements on ampicillin-treated bacteria were performed on NGM agar supplemented with ampicillin (100 μg/mL) and spread with ampicillin-sensitive OP50 bacteria concentrated from a liquid culture grown in LB broth. For serotonin treatments, basal pumping rate on bacteria was recorded and animals were transferred onto clean agar for approximately 5 min to remove bacteria from the cuticle. Animals were then transferred to NGM agar supplemented with serotonin (creatine sulfate, 5 mg/mL, Sigma) and pumping rates were recorded after 30 min.
2.3. Structural analysis of pharynx and body muscle during aging
For pharynx muscles, 10–20 animals for each age and genotype were mounted on 2% agarose pads and paralyzed with 0.2% sodium azide in M9 buffer. Differential-interference contrast (DIC) images were collected of pharynx terminal bulb muscles using a Hamamatsu ORCA-ER CCD digital camera mounted on a Nikon E800 microscope with OpenLab software (Improvision, Inc. Lexington, MA). Each image was cropped around the terminal bulb and presented to group-blind respondents who scored the structure as 1—least, 2—somewhat, or 3—most deteriorated. Six respondents’ scores for each animal were averaged to give a single structural deterioration score. Student’s t-tests were performed to analyze group differences. Group averages and individual animal scores are presented.
For structural analysis of body muscles, phalloidin staining of actin filaments was performed as described (Glenn et al., 2005). Briefly, animals were transferred in M9 onto a lysine-spread slide, freeze-cracked, fixed in cold methanol for 4 min and stained with AlexaFluor 488 phalloidin (Molecular Probes, Eugene, OR). For each specimen, digital images were collected of the head or body muscles in the head, middle and tail regions, using an Endow GFP filter set (Chroma Technology Corp., Rockingham, VT), with hardware and software as described for pharynx images. Images were scored in a group-blind manner for signs of sarcomere damage as indicated by irregular staining or deformed (wavy) sarcomeres. An animal was designated as having muscle deterioration if at least one of the three images showed signs of sarcomere damage. The percent of animals with muscle damage was then calculated for each group.
2.4. Statistical analysis
Statistical significance (P) was determined from a two-tailed Student’s t-test by comparing data from different strains or treatments on same-aged animals, as indicated.
3. Results
3.1. Inhibition of bacterial growth did not rescue pharynx functional decline during aging
In order to investigate factors leading to sarcopenia, we studied aging of the pharynx of the nematode, C. elegans. The C. elegans pharynx is a neuromuscular organ composed of 20 muscle cells and 20 neurons and enables the animal to ingest and crush bacterial food (Albertson et al., 1976). In young animals, the pharynx pumps approximately 250 times per min (ppm), and pumping declines during aging (Fig. 2A) (Bolanowski et al., 1981; Huang et al., 2004). The pharyngeal nervous system is dispensable for pharynx contraction, as a muscle-intrinsic pathway promotes slower pumping in the absence of the nervous system (Avery et al., 1989). Instead, the pharyngeal nervous system coordinates efficient contractions and stimulates fast pumping in response to food. Acetylcholine and serotonin both stimulate fast pumping and glutamate acts as an inhibitory neurotransmitter in the pharynx (Dent et al., 1997; Horvitz et al., 1982; McKay et al., 2004).
Fig. 2.
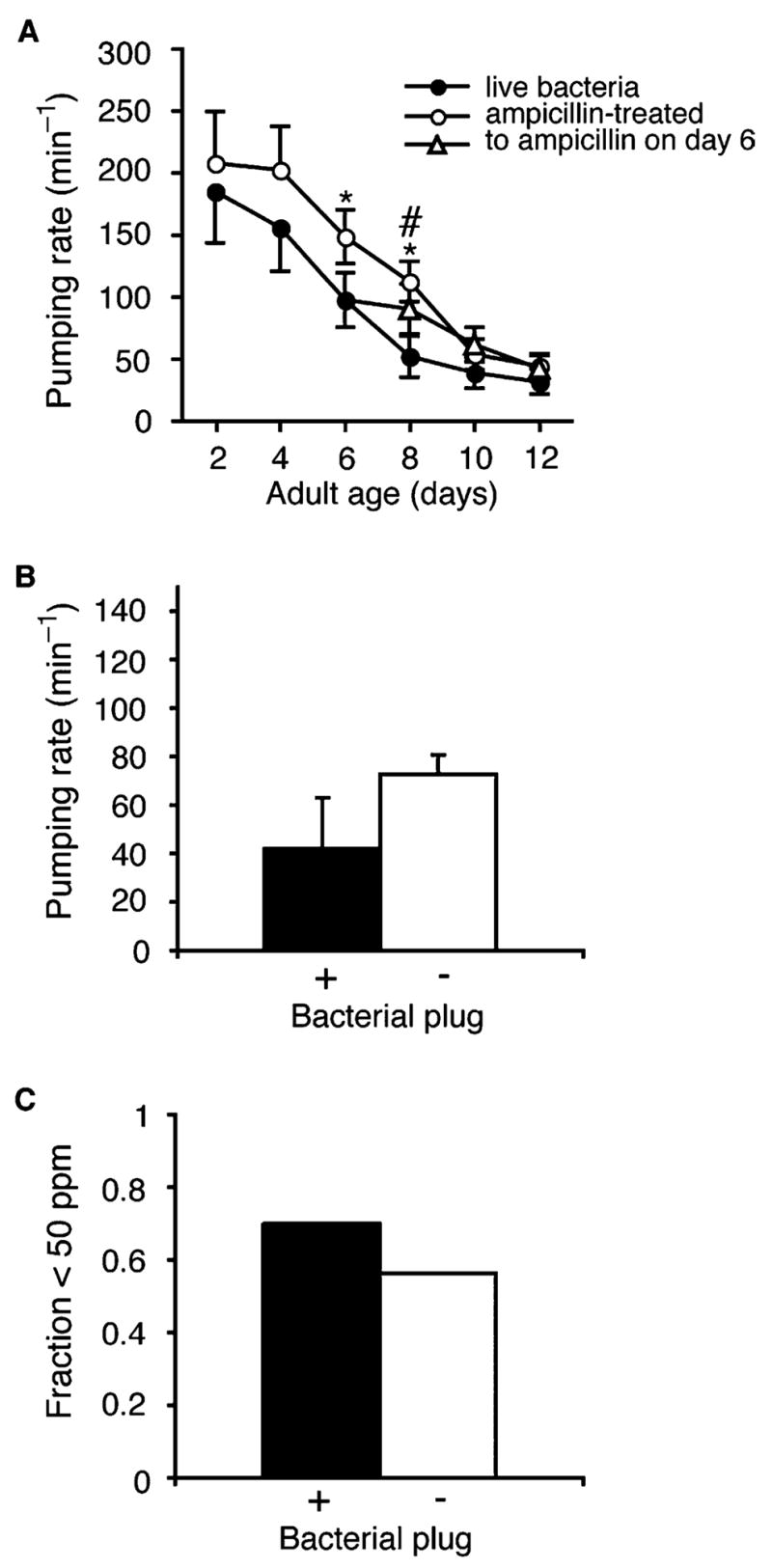
Bacteria were a minor contributor to pharynx functional decline during aging in fem-1(hc17) animals. (A) Functional decline in pharynx of animals cultured on growing bacteria (
 ), or ampicillin-treated non-growing bacteria (
), or ampicillin-treated non-growing bacteria (
 ), or transferred to ampicillin-treated bacteria on adult day 6 (
), or transferred to ampicillin-treated bacteria on adult day 6 (
 ). *P=0.006, live versus ampicillin-treated bacteria; #P=0.019, live bacteria versus transferred on day 6; (n), 20 animals, except n=10 animals for days 8, 10 on live bacteria. (B) Average pumping rate in aged (days 7–10) adults with or without bacterial plugging. Error bars, SEM; plugged, n=10 animals; unplugged, n=49 animals; P=0.2, t-test. (C) Fraction of day 7–10 adult animals that pumped fewer than 50 times/min; plugged, n=10 animals; unplugged pharynx, n=49 animals.
). *P=0.006, live versus ampicillin-treated bacteria; #P=0.019, live bacteria versus transferred on day 6; (n), 20 animals, except n=10 animals for days 8, 10 on live bacteria. (B) Average pumping rate in aged (days 7–10) adults with or without bacterial plugging. Error bars, SEM; plugged, n=10 animals; unplugged, n=49 animals; P=0.2, t-test. (C) Fraction of day 7–10 adult animals that pumped fewer than 50 times/min; plugged, n=10 animals; unplugged pharynx, n=49 animals.
For aging experiments, we used the adult sterile strain, fem-1(hc17), as the wildtype control to avoid progeny overgrowth in age-synchronized populations. During aging, the pharynx suffered structural and functional declines (Figs. 1A–E and 2A, filled circles) (Bolanowski et al., 1981; Croll et al., 1977; Garigan et al., 2002; Herndon et al., 2002; Huang et al., 2004). The pharynx is composed of three anatomical regions, the corpus, the isthmus and the terminal bulb where injested bacteria are crushed (Fig. 1A). Each anatomical region was observed to deteriorate during aging, although damage was most evident in the large muscles cells of the terminal bulb. In some older animals, the pharynx isthmus appeared plugged with bacteria (Fig. 1D, arrowheads) (Garigan et al., 2002). In addition, the isthmus appeared bent in older animals, possibly a sign of frailty or weakness (Fig. 1E).
Fig. 1.
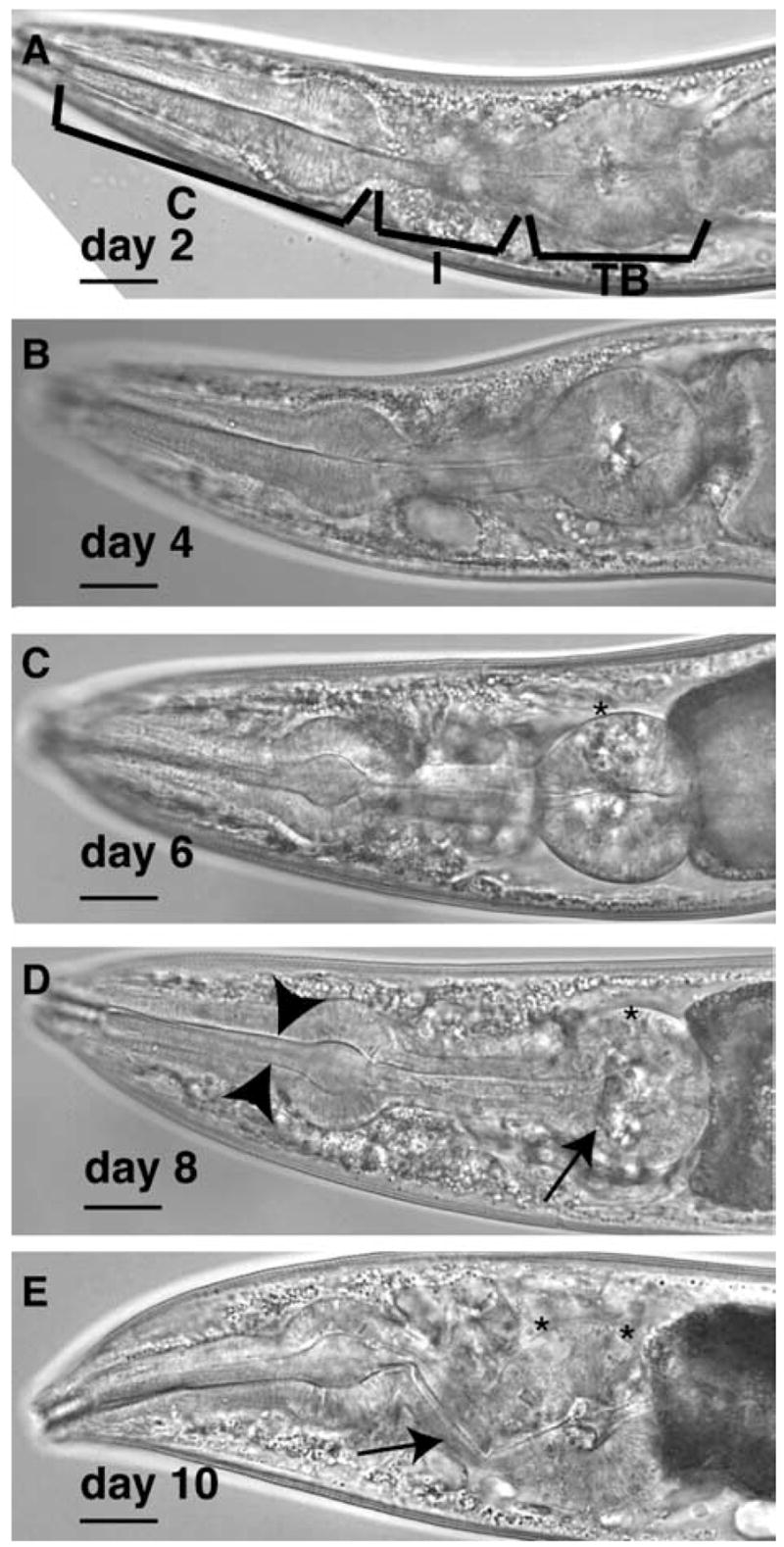
Aging is associated with structural and functional declines in the C. elegans pharynx. (A–E) Structural decline in the C. elegans pharynx over lifespan, between adult days 2 (A) and day 10 (E) in fem-1(hc17) adults. In these populations, 50% survival was approximately 15 days. (A) The anatomical regions of the pharynx are the corpus (C), isthmus (I), and terminal bulb (TB). After adult day 6, structural declines were evident in the terminal bulb (D, arrow), or isthmus (E, arrow). Vacuole-like pits appeared in the terminal bulb region (*). The isthmus also became plugged with bacteria (D, arrowheads). Scale bars, 20 μm.
Bacterial plugging might interfere with pumping and, ultimately, survival (Steger et al., 2004). Consistent with this idea, pumping rates were higher in animals living on growth-inhibited bacteria than on growing bacteria (Fig. 2A). However, further investigation revealed that bacterial plugging was not the major cause of slow pumping in old animals. First, pumping rates still declined with aging even when animals were fed growth-inhibited bacteria (Fig. 2A, open circles). Second, transfer onto growth-inhibited bacteria at adult day 6 did not arrest pumping decline at older ages (Fig. 2A, triangles). Finally, slow pumping in old animals was not strictly correlated with bacterial plugging of the pharynx. Although pumping rates were generally lower in day 7–10 adults with bacterial plugs, many same-aged non-plugged animals also pumped slowly (<50 ppm) (Fig. 2B and C). Together, these results showed that bacterial plugging of the pharynx was not the sole cause of slow pumping in old animals.
3.2. Serotonin stimulates pumping in old adults
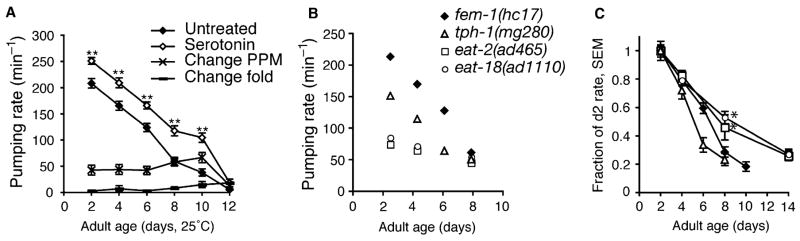
We examined whether pharynx pumping in old animals could be stimulated by treatment with serotonin, which stimulates pumping in response to the presence of food (Horvitz et al., 1982). Throughout adult life, serotonin stimulated pumping by about 50 ppm, until day 12, when serotonin only increased pumping by 12 ppm (Fig. 3A). Interestingly, since pumping rates declined overall between adult days 2 and 12, the fold-effect of serotonin on pumping increased over this interval. These observations indicate that intrinsic pumping rate in old animals is not fully reflective of functional capacity, as pumping could be further stimulated pharmacologically. However, the steady decline in pumping rate, even with the addition of serotonin, indicates that structural declines over this period (as in Fig. 1) are correlated with functional declines.
Fig. 3.
Pharynx functional decline during aging in the presence of serotonin and in pumping-impaired mutants. (A) Serotonin stimulated pumping at all ages (
 untreated;
untreated;
 5 mg/mL serotonin creatine sulfate) and the change between untreated and serotonin-treatment was not different at different ages (
5 mg/mL serotonin creatine sulfate) and the change between untreated and serotonin-treatment was not different at different ages (
 change in pump rate;
change in pump rate;
 fold change by serotonin); **P<0.001, t-test versus day 2, untreated; n=32–44, except n=12 for day 12. (B) Pumping rate during aging in fem-1(hc17) (◆), tph-1(mg280) (△), eat-2(ad465) (□) and eat-18(ad1110) (○) animals. (C) Relative decline of pumping rate, expressed as a fraction of pumping rate on adult day 2, in strains as presented in part (B); n> 14 animals; *P<0.05, t-test versus same-age wildtype.
fold change by serotonin); **P<0.001, t-test versus day 2, untreated; n=32–44, except n=12 for day 12. (B) Pumping rate during aging in fem-1(hc17) (◆), tph-1(mg280) (△), eat-2(ad465) (□) and eat-18(ad1110) (○) animals. (C) Relative decline of pumping rate, expressed as a fraction of pumping rate on adult day 2, in strains as presented in part (B); n> 14 animals; *P<0.05, t-test versus same-age wildtype.
3.3. Sarcopenia was delayed in slow-pumping strains
One possible cause for functional decline in the pharynx may be contraction-related damage to muscles from fast pumping. To investigate this possibility, we examined pharynx aging in slow-pumping mutants. Mutations in the genes encoding the EAT-2 nicotinic acetylcholine receptor subunit or EAT-18, a novel protein, impair excitatory nicotinic neurotransmission in the pharynx and reduce pumping rate by approximately 70%, compared with fem-1(hc17) animals (Fig. 3B) (McKay et al., 2004). While pumping declined during aging of eat-2(ad465) and eat-18(ad1110) adults, the rate of decline was slower than in control fem-1(hc17) animals (Fig. 3C).
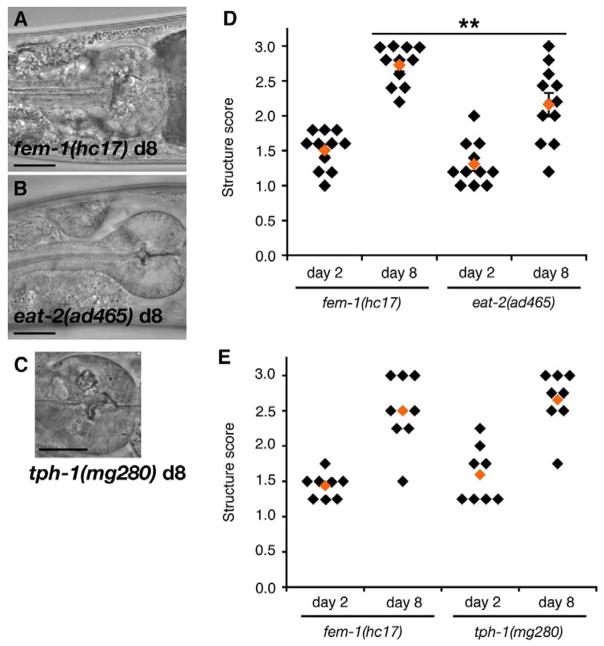
We next examined whether pharynx muscle structure was better preserved in slow-pumping eat-2(ad465) animals as compared with fem-1(hc17). For scoring structural decline, we asked several human respondants to score the state of the pharynx terminal bulb muscles in animals of each genotype at adult days 2 and 8. The respondants were blinded to the genotype and age of each animal imaged and had to rank the state of the muscle on a score from 1 to 3, with 1 being the ‘best’and 3 being the ‘worst’ structure. In general, older muscles were given higher scores, reflecting structural greater deterioration (Fig. 4D). It should be noted that this scoring strategy was subjective and usually based on the distribution of structures in the image set. Therefore, there is not a linear scale of structures between ‘1’ and ‘3’. Microscopic examination showed that structural deterioration in the pharynx was less severe in day 8 eat-2(ad465) adults than in day 8 fem-1(hc17) controls (Fig. 4A,B,D). The preservation of pharynx structure and function during aging in slow-pumping eat-2(ad465) animals suggested that pharynx aging was delayed in these animals.
Fig. 4.
Slow pumping delayed structural aging of the pharynx in eat-2(ad465) animals. (A–C) Pharynx structure in representative adult day 8 fem-1(hc17) (A), eat-2(ad465) (B) or tph-1(mg280) (C) animals. Scale bars, 20 μrn. (D, E) Classification of pharynx structure in fem-1(hc17) versus eat-2(ad465) (D) or fem-1(hc17) versus tph-1(mg280) (E) animals at adult days 2 and 8. Each diamond designates the average score for structural integrity in the terminal bulb of each pharynx examined. Smaller scores indicate better-organized structure, i.e. more like younger animals. Mean score for all images is shown as an orange diamond. Significance, West for average of all images, (D) day 8 eat-2(ad465) versus fem-1(hc17),P =0.007 (**), day 2 P = 0.127; day 2 versus 8 fem-1(hc17), P <0.0001; day 2 versus 8 eat-2(ad465), P=0.0002; (E) day 8 tph-1(mg280) versus fem-1(hc17), P=0.51, day 2 P=0.34; day 2 versus 8 fem-1(hc17), P=0.0004; day 2 versus 8 tph-1(mg280), P=0.0001.
We next examined pharynx aging in a mutant with modestly reduced pumping rate. For this study, we examined pharynx aging in tph-1(mg280) animals, which do not synthesize serotonin due to a deletion in tph-1, encoding tryptophan hydroxylase, the rate-limiting biosynthetic enzyme for serotonin (Sze, et al., 2000). Due to the absence of serotonin, tph-1(mg280) animals pump approximately 30% slower than wildtype animals (Fig. 3B). Pumping rate also declined with aging in tph-1(mg280), and the decline in tph-1(mg280) animals was similar to that in fem-1(hc17) animals (Fig. 3C). Structural analysis showed that pharynx structure had deteriorated similarly in tph-1(mg280) and fem-1(hc17) animals by adult day 8 (Fig. 4C,E). Together, these results indicate structural decline was not significantly delayed during aging of tph-1(mg280), perhaps indicating a threshold effect in the progression of sarcopenia.
3.4. Sarcopenia in body muscles was not delayed in eat-2(ad465) compared to wildtype animals
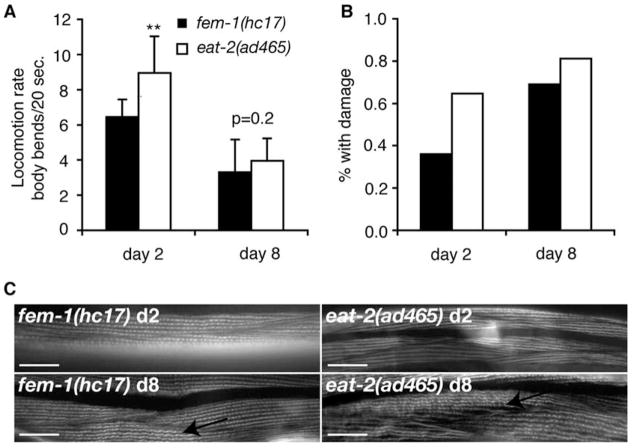
An alternative explanation for the protective effect of the eat-2(ad465) mutation on pharynx sarcopenia may result from calorie restriction, and not slow pumping per se, as slow pumping in eat-2(ad465) animals has been shown to cause lifespan extension via caloric restriction (CR) (Lakowski et al., 1998). Since it was possible that CR, instead of slow pumping itself, delayed pharynx aging in eat-2(ad465) adults, we examined whether functional aging of body muscles was also delayed in eat-2(ad465) animals. Reductions in locomotion during C. elegans aging have been correlated with muscle cell damage (Glenn et al., 2005; Herndon et al., 2002). Locomotion rate declined during aging of both eat-2(ad465) and fem-1(hc17) adults, suggesting that the body muscles aged similarly in both strains (Fig. 5A). Body muscle deterioration was detected using phalloidin to visualize sarcomeric actin, as previously described (Glenn et al., 2005). Both fem-1(hc17) and eat-2(ad465) adults exhibited body muscle deterioration by adult day 8 in this assay (Fig. 5B,C). We noted that muscles in eat-2(ad465) young adults were slightly more damaged than controls, possibly an indication of undernourishment during development. Nevertheless, these findings show that functional and structural aging in the locomotory body muscles was not delayed in eat-2(ad465) animals. This supports the hypothesis that slow pumping in eat-2(ad465) animals specifically delayed pharynx decline during aging.
Fig. 5.
Body muscle aging was not delayed in eat-2(ad465). (A) Body movement in fem-1(hc17) (■) and eat-2(ad465) (□) adults declined between days 2 and 8 of adulthood (25 °C); **P=0.00005 for day 2 fem-1(hc17) versus day 2 eat-2(ad465), t-test. (B, C) Structural damage occurred similarly in both fem-1(hc17) and eat-2(ad465) animals between days 2 and 8. (B) The fraction of animals with damage (wrinkled or faded) to sarcomeric actin, visualized by phalloidin;fem-1(hc17), day 2, n = 27 animals, day 8, n = 29; eat-2(ad465), day 2, n = 17; day 8, n= 16. (C) Representative images of phalloidin-stained sarcomeric actin in body wall muscles; scale bars, 20 μm.
3.5. Slow pumping in daf-2(e1370) animals protected against functional decline and sarcopenia during aging
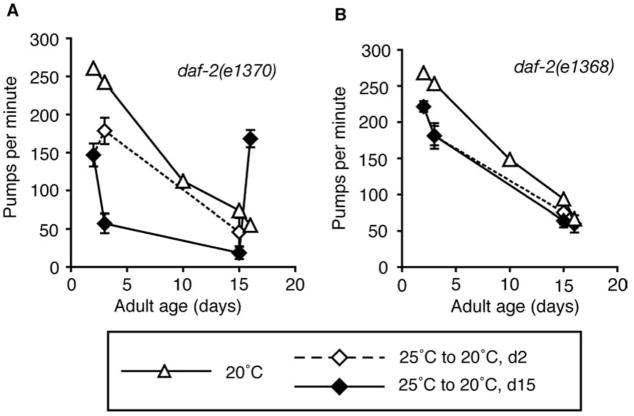
We further investigated the role of fast pumping in pharynx decline in daf-2 mutants that reduce pumping rate in a temperature-dependent manner, daf-2 encodes an insulin/IGF-I receptor regulating reproductive development, stress resistance and adult lifespan in C. elegans (Kenyon, 2001; Kimura et al., 1997). Defects in signaling through the DAF-2 pathway extend adult lifespan and delay behavioral declines related to muscle aging and slow sarcopenia (Garigan et al., 2002; Glenn et al., 2005; Herndon et al., 2002; Kenyon et al., 1993). The known daf-2 mutants fall into two classes which both increase adult lifespan and stress tolerance, but differentially affect pharynx function (Gems et al., 1998). Class 1 mutants pump normally, while class 2 mutants reduce pumping at restrictive temperature (25 °C). To corroborate the results with eat-2(ad465), we examined the effect of pumping arrest on pharynx aging in class 2 daf-2(e1370) animals. On the first day of adulthood, daf-2(e1370) animals were transferred to non-permissive temperature (25 °C) to arrest pumping. After 2 or 15 days, animals were transferred back to permissive temperature (20 °C) to resume pumping, and pumping rates were measured. Pumping rates were significantly higher in daf-2(e1370) animals which had arrested pumping for 15 days than in animals that had remained at 20 °C throughout adulthood (Fig. 6A). A short period (2 days) of slow pumping did not effectively delay pharynx decline (Fig. 6A). As a control, we examined class 1 daf-2(e1368) animals that pump normally at all temperatures. In these animals, pharynx function was not preserved when animals were maintained at 25 °C for the first 2 or 15 days of adulthood (Fig. 6B).
Fig. 6.
Slow pumping only in adulthood could protect against functional decline during aging in daf-2(e1370) animals. Functional decline of the pharynx in (A) daf-2(e1370) animals that reduce pumping at 25 °C and (B) daf-2(e1368) animals that do not. Pumping rates across lifespan are shown for animals maintained throughout adulthood at 20 °C (
 ) or transferred to 20 °C after 2 days (
) or transferred to 20 °C after 2 days (
 ) or 15 days (
) or 15 days (
 ) at 25 °C. Significance between e1370 and e1368 pumping rates after d15 transfer, P = 3 × 10−9, t-test, measured on d1 6; n ≥ 20 animals for populations maintained at 20 °C throughout adulthood; for transfers, n=30 (e1370, d2 transfer), 34 (e1370, d15 transfer) and n=29 (e1368 d2 transfer), 33 (d15 transfer).
) at 25 °C. Significance between e1370 and e1368 pumping rates after d15 transfer, P = 3 × 10−9, t-test, measured on d1 6; n ≥ 20 animals for populations maintained at 20 °C throughout adulthood; for transfers, n=30 (e1370, d2 transfer), 34 (e1370, d15 transfer) and n=29 (e1368 d2 transfer), 33 (d15 transfer).
Structural deterioration of the pharynx terminal bulb was also delayed in daf-2(e1370) animals maintained at 25 °C for the first 15 or 20 days of adulthood, compared with daf-2(e1368) animals treated the same way (Fig. 7A). However, the isthmus region in old daf-2(e1370) animals appeared thinned and was often bent, and these changes were not observed in daf-2(e1368) animals (Fig. 7A, arrows). Isthmus thinning may result from autophagy, which could be elevated in non-pumping daf-2(e1370) animals (Melendez, et al., 2003). Stress resistance is increased in both class 1 and 2 daf-2 mutants (Gems, et al., 1998). Since pharynx deterioration occurred more rapidly in daf-2(e1368) animals, this data suggests that the differences in pharynx pumping over lifespan more reasonably account for aging differences between these strains, rather than differences in oxidative stress resistance.
Fig. 7.
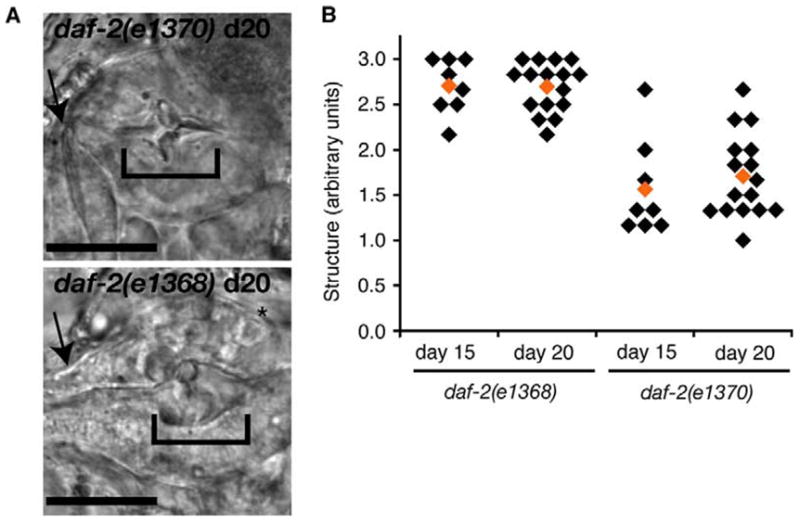
Structural aging was delayed in daf-2(e1370) animals that reduce pumping rate. (A) Structural decline correlated with functional decline. Pharynxs of day 20 daf-2(e1370) (upper) and daf-2(e1368) (lower) animals showed different extents of structural deterioration. Terminal bulb deterioration was reduced in daf-2(e1370) animals maintained at 25 °C as adults, than in daf-2(e1368) animals (brackets), although the isthmus of daf-2(e1370) animals appeared thinner and weaker (arrows). Scale bars, 20 μm. (B) Classification of terminal bulb structure from daf-2(e1368) and daf-2(e1370) after 15 or 20 adult days at 25 °C, evaluated by human scorers as Fig. 4, statistical analysis: d15, P=0.0002; d20, P=3 × 10−8, e1370 versus e1368, average of all images, t-test.
4. Discussion
In this report, we have examined several factors that could contribute to sarcopenia in the C. elegans pharynx during aging. Bacteria appeared to be one negative factor in maintaining pharynx function during aging, as worms grown on ampicillin-treated bacteria maintained higher pumping rates during adult lifespan than animals grown on untreated bacterial lawns. However, functional declines were still observed during adult lifespan in animals grown on ampicillin-treated bacteria. In addition, bacterial plugging of the pharynx lumen appeared more likely to be a consequence, rather than a cause, of slowed pumping in older animals, as many older animals pumped slowly, but did not exhibit plugged lumens. Bacterial plugging of the lumen has been observed previously in animals lacking several interneurons due to laser ablation (Avery, 1993). We interpreted this to show that pharynx decline was not solely due to negative effects of bacteria.
Since microbial effects only accounted for a fraction of the pharynx functional decline during aging, we examined whether the rate of muscle contraction was correlated with functional and structural aspects of sarcopenia in the pharynx. Indeed, these studies showed that declines during aging were delayed in two different strains, eat-2(ad465) and daf-2(e1370), which had reduced rates of muscle contractions over lifespan. These findings are consistent with the hypothesis that muscle contraction is one factor that contributes to sarcopenia during aging in the pharynx. However, care must be used in this interpretation due to confounding pleitropic phenotypes in these mutants. First, slow pumping in eat-2(ad465) animals leads to lifespan extension by calorie restriction, which could have effects on sarcopenia independent of contraction (Lakowski et al., 1998). We showed that sarcopenia in eat-2(ad465) body muscles was not delayed compared to fem-1(hc17) controls. Thus, caloric restriction did not affect sarcopenia in the body muscles of eat-2(ad465), supporting the conclusion that pharynx muscles were protected from sarcopenia as a result of slow pumping. However, we cannot rule out the possibility that beneficial effects of calorie restriction may act preferentially on pharynx muscles, and not locomotory muscles.
To validate the findings with eat-2(ad465), we investigated pharynx sarcopenia in slow-pumping daf-2(e1370) animals, as compared with fast-pumping daf-2(e1368) animals. Both of these daf-2 mutations can extend adult lifespan and delay the progression of aging-related functional and structural declines (Garigan et al., 2002; Glenn et al., 2005). Therefore, it is reasonable to conclude that the differences in pharynx sarcopenia in daf-2(e1368) and daf-2(e1370) adults reflect different rates of pharynx contraction during adulthood. An alternative explanation is that the e1370 allele causes a more severe decrement in daf-2 activity than the e1368 allele, and these differences in daf-2 pathway activity account for differences in pharynx sarcopenia. Further study of the behavioral pleitropies of these mutations should illuminate the possible effects on sarcopenia.
Interestingly, pharynx sarcopenia was not altered in tph-1(mg280) animals with moderate (30%) reductions in pumping rate compared with fem-1(hc17) controls. This could reflect a threshold effect in the relationship between sarcopenia and pumping rate. Alternatively, the absence of serotonin could have secondary effects on muscle aging that were independent of pumping rate. It should be noted that tph-1(mg280) animals are reported to live 25% longer than wildtype, indicating that these animals are not generally less healthy than wildtype (Finch et al., 2001). One possibility is that the design of the current experiments could not distinguish slight reductions in sarcopenia. Therefore, further investigation of the effects of moderate reductions in muscle contraction may reveal more subtle changes in sarcopenia development.
The structural analysis of terminal bulbs that was used to assess the progression of sarcopenia indicated a relationship between structure and function in older animals. Throughout these experiments, we observed that strains with higher pumping rates such as fem-1(hc17), tph-1(mg280) and daf-2(e1368) showed relatively greater changes in terminal bulb structure than slower-pumping strains, such as eat-2(ad465) and daf-2(e1370). In particular, the distribution of terminal bulb structure scores shifted from 1 to 3 between days 2 and 8 in the faster pumping strains, but remained closer to 2 at day 8 in the slower pumping strains. This difference suggests a correlation in change in structure between days 2 and 8 and overall pumping rate during this time.
There are multiple scenarios for development of sarcopenia as a result of contraction-related injury to pharynx muscles. First, the high ATP demands of rapid muscle contraction may cause increased respiration, thereby increasing ROS production and cellular oxidative stress. In this case, damage may be be localized to mitochondria. In support of this theory, sarcopenia and aging have been correlated with decreased mitochondrial function in rodents, primates and humans (Bua et al., 2004; McArdle et al., 2002; Short et al., 2005; Wanagat et al., 2001). Alternatively, muscle contractions could cause mechanical damage to cellular components. Initially, mechanical damage should be localized to the contractile apparatus and may be removed by cellular clearance pathways. In time, however, accumulation of damaged myofilaments could overwhelm clearance pathways, impairing clearance of damage throughout the cell. In mammals, pathways for repairing damage are active in aged muscle, but appear to be impaired, consistent with an accumulation of damage that impairs clearance pathways (Close et al., 2005; Edstrom et al., 2005). A third possibility is that muscle tissue may weaken during aging due to contraction-independent causes, and muscle contraction in the weakened tissue causes the weakened tissue to deteriorate.
Previous analyses of cellular aging in C. elegans found that structural decline occurred stochastically in individual body muscle cells and was variable between individual animals (Herndon et al., 2002; Rea et al., 2005). We also observed variability in pharynx decline between individual animals (i.e. Figs. 4 and 7). The stochastic nature of tissue aging may reflect combinatorial effects from independent factors. Our findings implicate contraction-related factors as one contributor to sarcopenia in the C. elegans pharynx. The impaired cellular environment may be susceptible to secondary stresses, such as from microbial attack, which progressively impair pharynx function. Finally, we envision that contraction-related injury is likely to be a factor only during youth and middle age, when the muscles actively contract. Once muscle function is significantly impaired, further accumulation of contraction-related damage likely ceases and other factors may rise in relative importance in the progression of sarcopenia.
Acknowledgments
We thank Frank Rothman for sharing unpublished data and NIA colleagues for helpful discussions. We are grateful to Wolkow and Mattson lab members for performing pharynx structure classifications. This work was supported by the NIA Intramural Research Program.
References
- Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond. 1976;275:299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- Avery L. Motor neuron m3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J Exp Biol. 1993;175:283–297. doi: 10.1242/jeb.175.1.283. [DOI] [PubMed] [Google Scholar]
- Avery L, Horvitz H. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3:473–485. doi: 10.1016/0896-6273(89)90206-7. [DOI] [PubMed] [Google Scholar]
- Bolanowski M, Russell R, Jacobson L. Quantitative measures of aging in the nematode Caenorhabditis elegans. I. Population and longitudinal studies of two behavioral parameters. Mech Ageing Dev. 1981;15:279–295. doi: 10.1016/0047-6374(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bua E, McKiernan S, Aiken J. Calorie restriction limits the generation but not the progression of mitochondrial abnormalities in aging skeletal muscle. FASEB J. 2004;18:582–584. doi: 10.1096/fj.03-0668fje. [DOI] [PubMed] [Google Scholar]
- Close G, Kayani A, Vasilaki A, McArdle A. Skeletal muscle damage with exercise and aging. Sports Med. 2005;35:413–427. doi: 10.2165/00007256-200535050-00004. [DOI] [PubMed] [Google Scholar]
- Croll N, Smith J, Zuckerman B. The aging process of the nematode Caenorhabditis elegans in bacterial and axenic culture. Exp Aging Res. 1977;3:175–199. doi: 10.1080/03610737708257101. [DOI] [PubMed] [Google Scholar]
- Dent J, Davis M, Avery L. avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 1997;16:5867–5879. doi: 10.1093/emboj/16.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstrom E, Ulfhake B. Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell. 2005;4:65–77. doi: 10.1111/j.1474-9728.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- Finch CE, Ruvkun G. The genetics of aging. Annu Rev Genomics Hum Genet. 2001;2:435–462. doi: 10.1146/annurev.genom.2.1.435. [DOI] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Graser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutations affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn C, Chow D, David L, Cooke C, Gami M, Iser W, Hanselman K, Goldberg I, Wolkow C. Behavioral deficits during early stages of aging in Caenorhabditis elegans result from locomotory deficits possibly linked to muscle frailty. J Gerontol A Biol Sci Med Sci. 2005;59A:1251–1260. doi: 10.1093/gerona/59.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Horvitz H, Chalfie M, Trent C, Sulston J, Evans P. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE. Aging can be genetically dissected into component processes using long-lived lines of Caenorhabditis elegans. Proc Natl Acad Sci. 1987;84:3777–3781. doi: 10.1073/pnas.84.11.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel H. Sarcopenia and aging. Nutr Rev. 2003;61:157–167. doi: 10.1301/nr.2003.may.157-167. [DOI] [PubMed] [Google Scholar]
- Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. PNAS. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Res Rev. 2002;1:79–93. doi: 10.1016/s0047-6374(01)00368-2. [DOI] [PubMed] [Google Scholar]
- McKay J, Raizen D, Gottschalk A, Schafer W, Avery L. eat-2 and eat-18 are required for nicotinic neurotransmission in the Caenorhabditis elegans pharynx. Genetics. 2004;166:161–169. doi: 10.1534/genetics.166.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen E, Hall D, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short K, Bigelow M, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair K. Decline in skeletal muscle mitochondrial function with aging in humans. PNAS. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger K, Avery L. The gar-3 muscarinic receptor cooperates with calcium signals to regulate muscle contraction in the Caenorhabditis elegans pharynx. Genetics. 2004:167. doi: 10.1534/genetics.103.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze J, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Wanagat J, Cao Z, Pathare P, Aiken J. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15:322–332. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]