Abstract
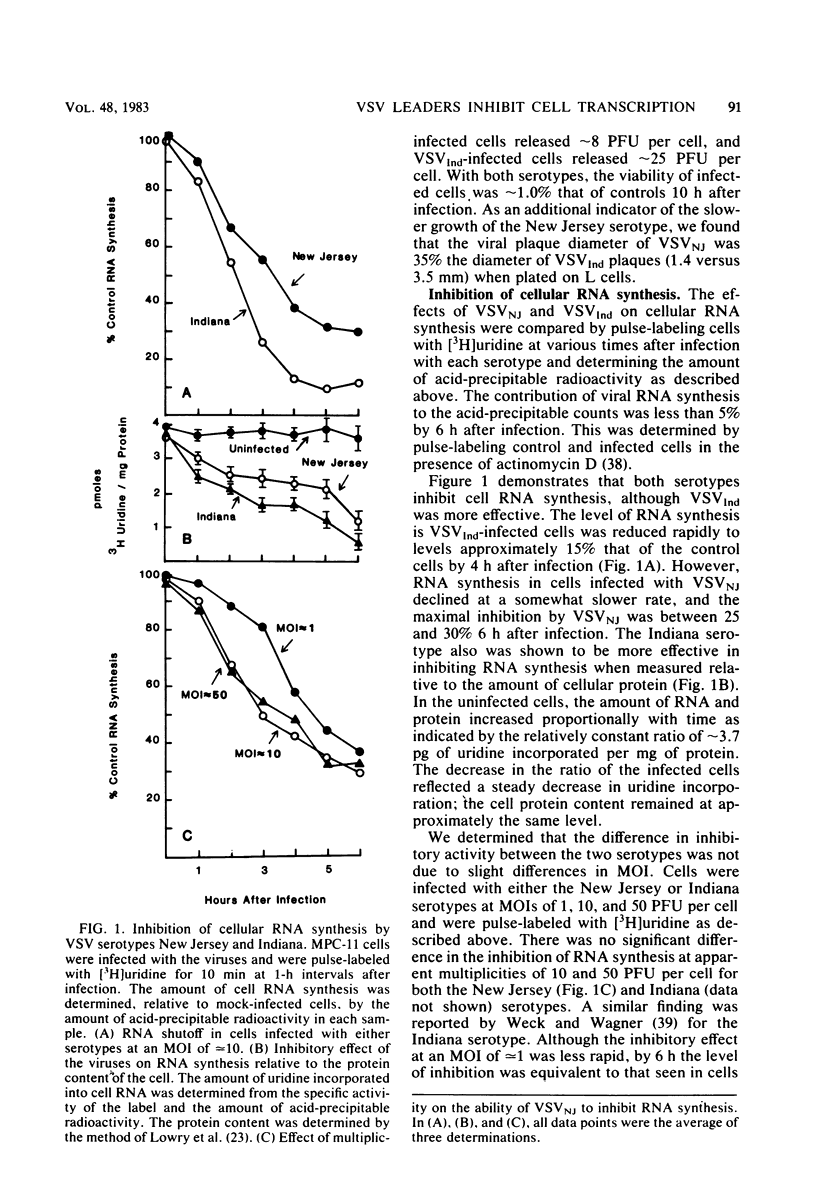
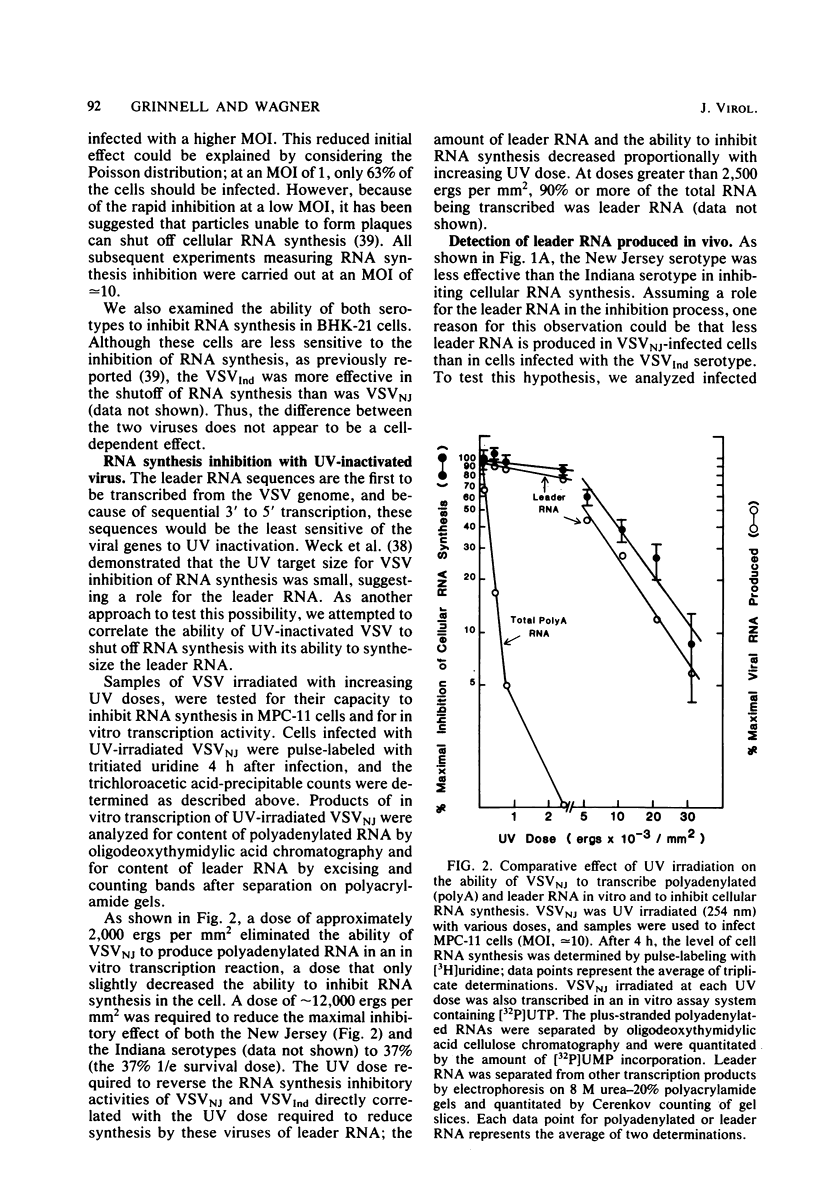
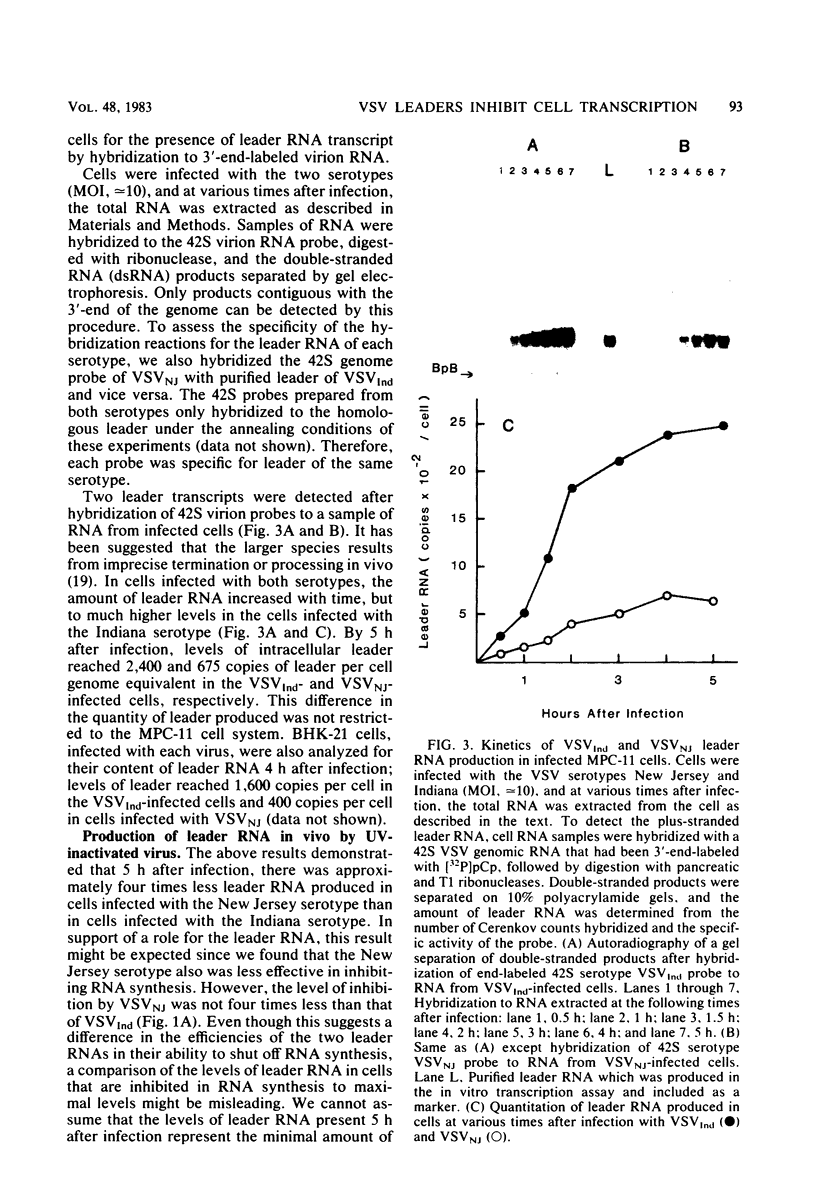
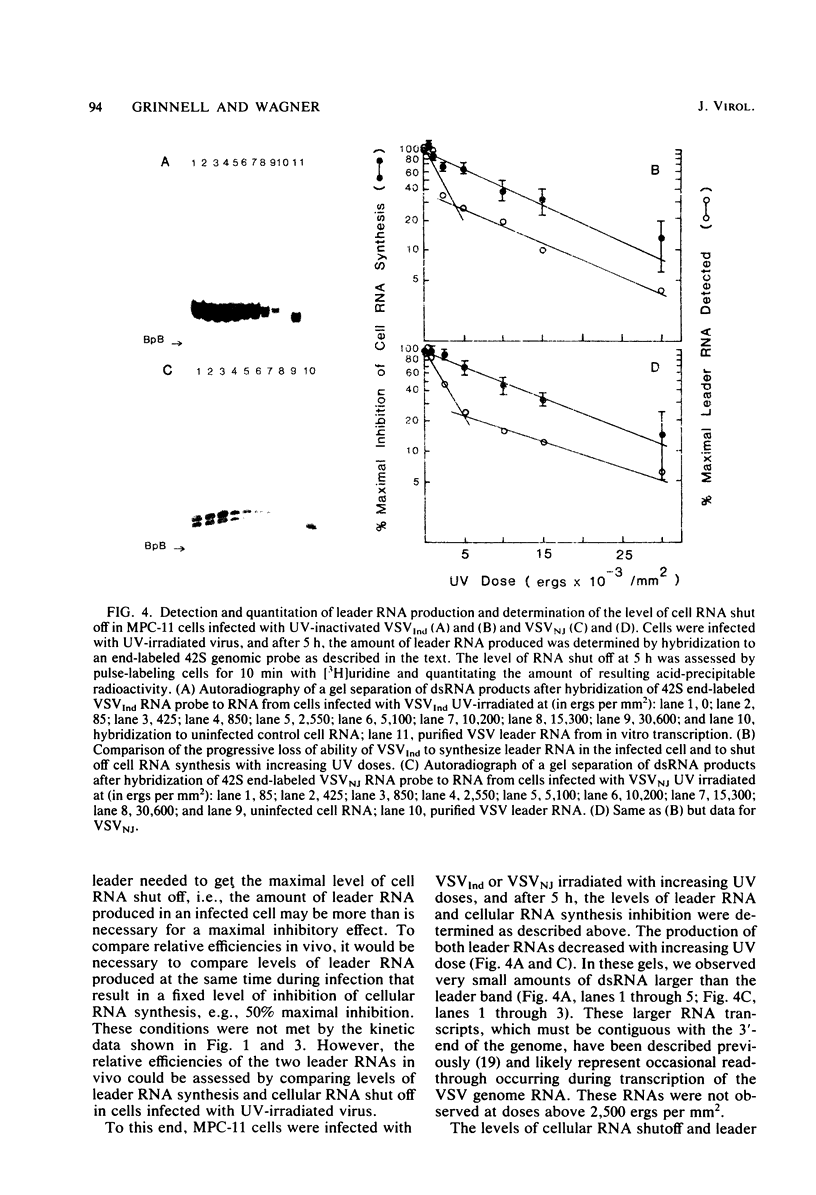
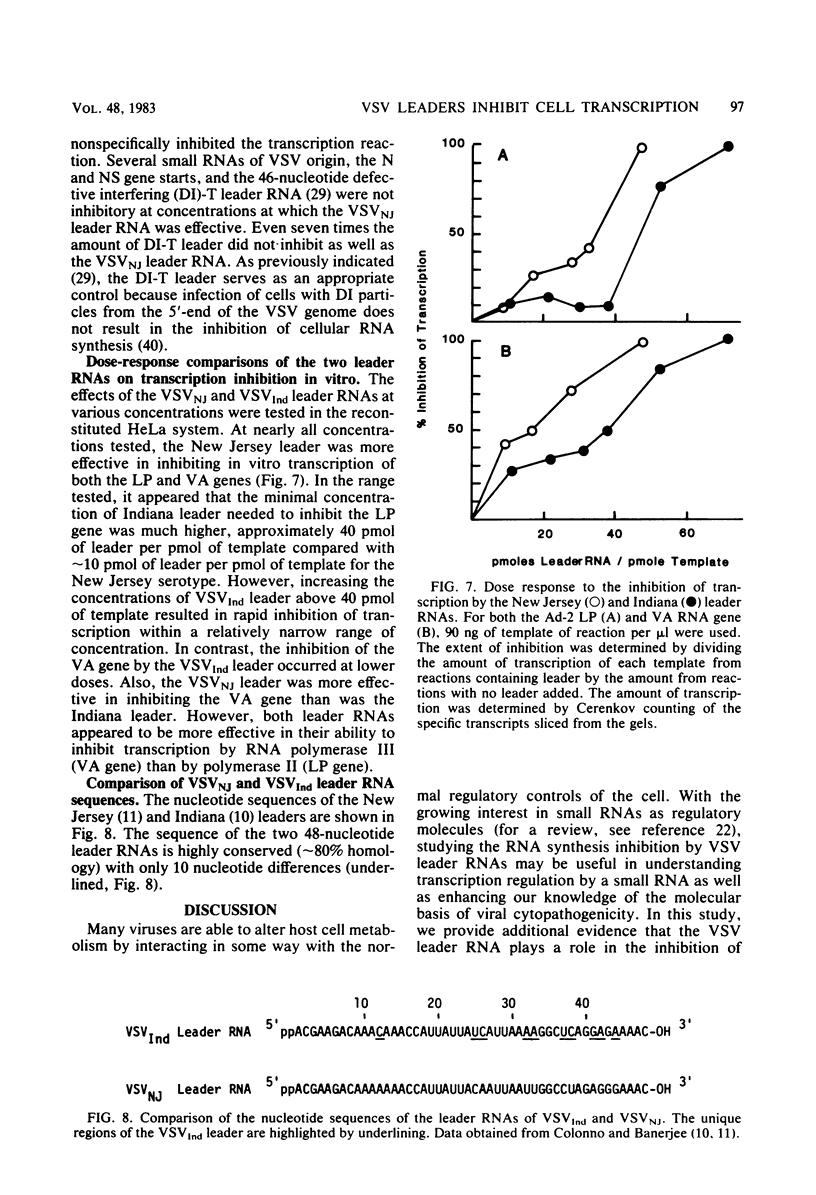
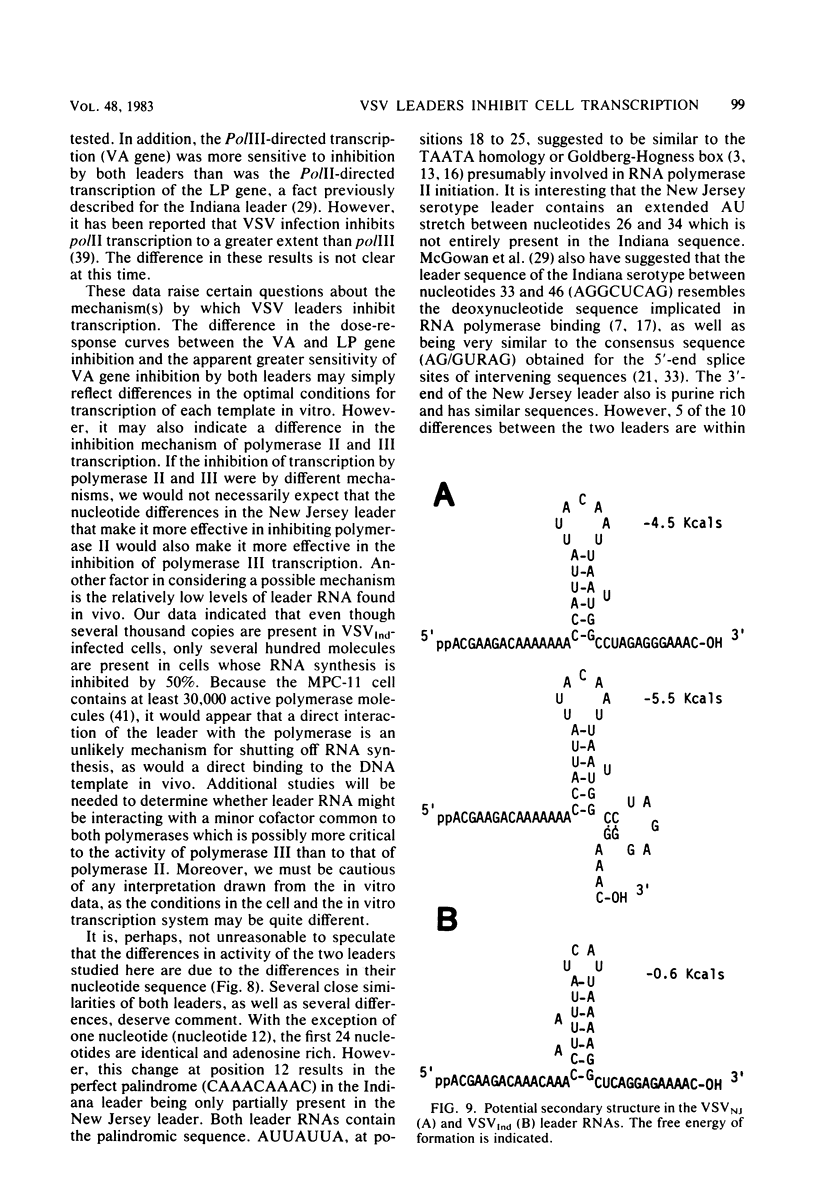
We compared the ability of the leader RNAs of the New Jersey and Indiana serotypes of vesicular stomatitis virus to inhibit transcription in infected host cells. The level of cellular RNA synthesis in cells infected with either serotype was drastically reduced by 5 h after infection. Studies with UV-inactivated virus demonstrated that shutoff of cellular RNA synthesis directly correlated with the ability of the infecting virus to transcribe its plus-stranded leader RNA. Although both serotypes inhibited cellular RNA synthesis, the Indiana serotype reduced synthesis to lower levels. In addition, an examination of the kinetics of leader RNA synthesis in vivo indicated that up to four times more leader RNA was produced in cells infected with the Indiana serotype than in those infected with the New Jersey serotype. However, in vivo studies also suggested that the leader RNA of the New Jersey serotype was a more efficient RNA inhibitor than was the Indiana serotype leader RNA. Although up to 2,900 copies of the leader RNA per cell could be detected in infected cells, only 550 copies of the Indiana and 100 copies of the New Jersey leader RNAs per cell were present in infected cells that were demonstrating 50% of the maximal inhibition of RNA synthesis. In an in vitro system, leader RNAs of both serotypes inhibited DNA-dependent transcription of the adenovirus late promoter and adenovirus-associated RNA genes, but the New Jersey serotype leader was also a better inhibitor in this reconstituted system. Data from the dose response of inhibition by each leader suggest that polymerase III transcription was more sensitive to inhibition by viral leaders than was polymerase II transcription. Polyadenylated viral mRNAs and the NS and N gene starts transcribed by both serotypes did not significantly inhibit transcription at levels at which the corresponding leader RNAs were inhibitory. Overall, our results strongly suggest a role for the plus-stranded leader RNAs of the New Jersey and Indiana serotypes of vesicular stomatitis virus in inhibiting cellular transcription in vivo. We discuss differences in the nucleotide sequences of the two leader RNAs in relation to their differences in biological activity and to potential regulatory sequences.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akusjärvi G., Mathews M. B., Andersson P., Vennström B., Pettersson U. Structure of genes for virus-associated RNAI and RNAII of adenovirus type 2. Proc Natl Acad Sci U S A. 1980 May;77(5):2424–2428. doi: 10.1073/pnas.77.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. C., Ziff E. B. Promoters and heterogeneous 5' termini of the messenger RNAs of adenovirus serotype 2. J Mol Biol. 1981 Jun 25;149(2):189–221. doi: 10.1016/0022-2836(81)90298-9. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenholz Y., Moore N. F., Wagner R. R. Enveloped viruses as model membrane systems: microviscosity of vesicular stomatitis virus and host cell membranes. Biochemistry. 1976 Aug 10;15(16):3563–3570. doi: 10.1021/bi00661a026. [DOI] [PubMed] [Google Scholar]
- Baxt B., Bablanian R. Mechansims of vesicular stomatitis virus-induced cytopathic effects. II. Inhibition of macromolecular synthesis induced by infectious and defective-interfering particles. Virology. 1976 Jul 15;72(2):383–392. doi: 10.1016/0042-6822(76)90167-7. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978 Sep;15(1):93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Mapping and initiation studies on the leader RNA of vesicular stomatitis virus. Virology. 1977 Mar;77(1):260–268. doi: 10.1016/0042-6822(77)90423-8. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Nucleotide sequence of the leader RNA of the New Jersey serotype of vesicular stomatitis virus. Nucleic Acids Res. 1978 Nov;5(11):4165–4176. doi: 10.1093/nar/5.11.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Dierks P. M., Parsons J. T. In vitro synthesis of a unique RNA species by a T particle of vesicular stomatitis virus. J Virol. 1977 Sep;23(3):708–716. doi: 10.1128/jvi.23.3.708-716.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. D., Clarkson S. G., Tocchini-Valentini G. Transcription initiation of eucaryotic transfer RNA genes. Cell. 1982 May;29(1):3–5. doi: 10.1016/0092-8674(82)90083-6. [DOI] [PubMed] [Google Scholar]
- Hay N., Skolnik-David H., Aloni Y. Attenuation in the control of SV40 gene expression. Cell. 1982 May;29(1):183–193. doi: 10.1016/0092-8674(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Manley J. L. DNA sequence required for initiation of transcription in vitro from the major late promoter of adenovirus 2. Proc Natl Acad Sci U S A. 1981 Feb;78(2):820–824. doi: 10.1073/pnas.78.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla M. G., Piwnica-Worms H., Keene J. D. Rapid and transient localization of the leader RNA of vesicular stomatitis virus in the nuclei of infected cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5240–5244. doi: 10.1073/pnas.79.17.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Snurps and scyrps. Cell. 1981 Aug;25(2):298–300. doi: 10.1016/0092-8674(81)90047-7. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. I. Comparison of cell-killing, plaque-forming, and defective-interfering particles of vesicular stomatitis virus. Virology. 1974 Feb;57(2):321–338. doi: 10.1016/0042-6822(74)90172-x. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. II. Cell killing by vesicular stomatitis virus: a requirement for virion-derived transcription. Virology. 1975 Jan;63(1):176–190. doi: 10.1016/0042-6822(75)90383-9. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J., Johnson L. D., Lazzarini R. A. Cell killing by viruses. V. Transcribing defective interfering particles of vesicular stomatitis virus function as cell-killing particles. Virology. 1977 Oct 1;82(1):242–246. doi: 10.1016/0042-6822(77)90048-4. [DOI] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Differential inhibition of host protein synthesis in L cells infected with RNA - temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1976 May;18(2):550–558. doi: 10.1128/jvi.18.2.550-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. J., Emerson S. U., Wagner R. R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982 Feb;28(2):325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- McGowan J. J., Wagner R. R. Inhibition of cellular DNA synthesis by vesicular stomatitis virus. J Virol. 1981 Apr;38(1):356–367. doi: 10.1128/jvi.38.1.356-367.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Alterations in the protein synthetic apparatus of Friend erythroleukemia cells infected with vesicular stomatitis virus or herpes simplex virus. J Virol. 1978 Jan;25(1):422–426. doi: 10.1128/jvi.25.1.422-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R., LEVEY A. H., SNYDER R. M., RATCLIFF G. A., Jr, HYATT D. F. BIOLOGIC PROPERTIES OF TWO PLAQUE VARIANTS OF VESICULAR STOMATITIS VIRUS (INDIANA SEROTYPE). J Immunol. 1963 Jul;91:112–122. [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Inhibition of RNA synthesis in mouse myeloma cells infected with vesicular stomatitis virus. J Virol. 1978 Mar;25(3):770–780. doi: 10.1128/jvi.25.3.770-780.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Transcription of vesicular stomatitis virus is required to shut off cellular RNA synthesis. J Virol. 1979 Apr;30(1):410–413. doi: 10.1128/jvi.30.1.410-413.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Vesicular stomatitis virus infection reduces the number of active DNA-dependent RNA polymerases in myeloma cells. J Biol Chem. 1979 Jun 25;254(12):5430–5434. [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Goodman T. C., Hillen W., Horn G. T., Klein R. D., Larson J. E., Müller U. R., Neuendorf S. K., Panayotatos N., Stirdivant S. M. DNA structure and gene regulation. Prog Nucleic Acid Res Mol Biol. 1980;24:167–267. doi: 10.1016/s0079-6603(08)60674-1. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J Virol. 1972 Jan;9(1):85–89. doi: 10.1128/jvi.9.1.85-89.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. S., Lucas-Lenard J. M. Inhibition of ribonucleic acid accumulation in mouse L cells infected with vesicular stomatitis virus requires viral ribonucleic acid transcription. Biochemistry. 1980 Feb 19;19(4):804–810. doi: 10.1021/bi00545a029. [DOI] [PubMed] [Google Scholar]
- Yaoi Y., Amano M. Inhibitory effect of ultraviolet-inactivated vesicular stomatitis virus on initiation o DNA synthesis in cultured chick embryo cells. J Gen Virol. 1970 Oct;9(1):69–75. doi: 10.1099/0022-1317-9-1-69. [DOI] [PubMed] [Google Scholar]
- Yaoi Y., Mitsui H., Amano M. Effect of U.v.-irradiated vesicular stomatitis virus on nucleic acid synthesis in chick embryo cells. J Gen Virol. 1970 Sep;8(3):165–172. doi: 10.1099/0022-1317-8-3-165. [DOI] [PubMed] [Google Scholar]