Abstract
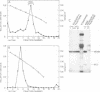
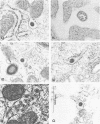
Cell-free cytoplasmic extracts of the Syrian hamster cell lines C13/SV28 and BHK-21F were immunogenic in Syrian hamsters. The resulting antisera cross-reacted completely with antisera against lymphocytic choriomeningitis virus (LCMV) in an immunoradiometric assay employing BHK-21F antigen. Several other Syrian hamster cell lines not previously known to be infected with LCMV were also strongly positive when assayed for viral antigens. Also, several mouse sera and antisera raised in Syrian hamsters against cells transformed by papovaviruses had high titers of anti-LCMV activity. No cytopathic effect was evident in any of the persistently infected cell lines. Culture media from these cells were not infectious and showed no evidence of defective interfering particles. However, cell-free extracts of all the persistently infected cells contained material capable of transmitting the persistent infection to uninfected cells of Syrian hamsters, rats, mice, green monkeys, and humans. The onset of infection is much slower than when LCMV virions are used. When 2 X 10(6) uninfected BHK cells were treated with an extract from 100 persistently infected cells, the new infection was apparent within about 12 days. When an extract from 10(6) cells was used, the new infection was apparent within about 5 days, but not sooner. The intracellular infectious material was sensitive to treatment with deoxycholate, Nonidet P-40, or ether but resistant to treatment with RNase or trypsin. It was also large (5,000S) and heterodisperse on sucrose gradients. The infectious material was probably contained in large lipid vesicles and their integrity was probably essential for infection. When a few persistently infected cells were cocultivated with many uninfected cells, a few discrete colonies positive for LCMV antigens were observed after about 5 days. Since the culture media were not infectious, the infection probably spread by cell-cell contact. Several different experiments indicated that interferon did not play a major role in mediating persistence in this case. Persistent infections by LCMV can be maintained without expression of extracellular virus particles and without appearance of large amounts of viral antigens on the cell surface. Cell-cell contact could still allow transmission of intracellular infectious material. In an animal, these properties could circumvent immune surveillance.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Smith G. H., Hoffman H. A., Rowe W. P. Use of enzyme-labeled antibody for electron microscope localization of lymphocytic choriomeningitis virus antigens in infected cell cultures. J Natl Cancer Inst. 1969 Mar;42(3):497–515. [PubMed] [Google Scholar]
- Albu E., Holmes K. V. Isolation and preliminary characterization of the RNA-containing R-type, virus-like particle of BHK-21 cells. J Virol. 1973 Nov;12(5):1164–1172. doi: 10.1128/jvi.12.5.1164-1172.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Oldstone M. B. Virus-induced immune complex disease: identification of specific viral antigens and antibodies deposited in complexes during chronic lymphocytic choriomeningitis virus infection. J Immunol. 1978 Apr;120(4):1297–1304. [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Doyle M. V., Oldstone M. B. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J Immunol. 1978 Oct;121(4):1262–1269. [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology. 1979 Dec;99(2):443–447. doi: 10.1016/0042-6822(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin J., Sikora E. Low-pathogenicity variant of lymphocytic choriomeningitis virus. Infect Immun. 1973 May;7(5):825–826. doi: 10.1128/iai.7.5.825-826.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Grube F. A carrier state of lymphocytic choriomeningitis virus in L cell cultures. Nature. 1967 Feb 25;213(5078):770–773. doi: 10.1038/213770a0. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Slenczka W., Tees R. A persistent and inapparent infection of L cells with the virus of lymphocytic choriomeningitis. J Gen Virol. 1969 Jul;5(1):63–81. doi: 10.1099/0022-1317-5-1-63. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rowe W. P., Turner H. C., Huebner R. J. Lymphocytic-choriomeningitis virus in hamster tumor: spread to hamsters and humans. Science. 1965 Oct 15;150(3694):363–364. doi: 10.1126/science.150.3694.363. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Miles L. E., Lipschitz D. A., Bieber C. P., Cook J. D. Measurement of serum ferritin by a 2-site immunoradiometric assay. Anal Biochem. 1974 Sep;61(1):209–224. doi: 10.1016/0003-2697(74)90347-9. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Reed S. I., Stark G. R. An antigen associated with messenger RNA in a transformed hamster cell line. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):295–303. doi: 10.1101/sqb.1974.039.01.039. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Buchmeier M. J. Restricted expression of viral glycoprotein in cells of persistently infected mice. Nature. 1982 Nov 25;300(5890):360–362. doi: 10.1038/300360a0. [DOI] [PubMed] [Google Scholar]
- Pedersen I. R. Structural components and replication of arenaviruses. Adv Virus Res. 1979;24:277–330. doi: 10.1016/s0065-3527(08)60396-6. [DOI] [PubMed] [Google Scholar]
- Popescu M., Lehmann-Grube F. Diversity of lymphocytic choriomeningitis virus: variation due to replication of the virus in the mouse. J Gen Virol. 1976 Jan;30(1):113–122. doi: 10.1099/0022-1317-30-1-113. [DOI] [PubMed] [Google Scholar]
- Popescu M., Schaefer H., Lehmann-Grube F. Homologous interference of lymphocytic choriomeningitis virus: detection and measurement of interference focus-forming units. J Virol. 1976 Oct;20(1):1–8. doi: 10.1128/jvi.20.1.1-8.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands K., Grabau E., Spindler K., Jones C., Semler B., Holland J. Virus protein changes and RNA termini alterations evolving during persistent infection. Cell. 1980 Apr;19(4):871–880. doi: 10.1016/0092-8674(80)90078-1. [DOI] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. II. Effect of measles antibody on persistently infected HeLa sublines and recovery of a HeLa clonal line persistently infected with incomplete virus. J Bacteriol. 1966 Dec;92(6):1805–1811. doi: 10.1128/jb.92.6.1805-1811.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck L. D., Trowbridge R. S., Welsh R. M., Wright E. A., Pfau C. J. Arenaviruses: cellular response to long-term in vitro infection with parana and lymphocytic choriomeningitis viruses. Infect Immun. 1972 Oct;6(4):444–450. doi: 10.1128/iai.6.4.444-450.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwick T. L., Kirk B. E. Analysis of baby hamster kidney cells persistently infected with lymphocytic choriomeningitis virus. J Gen Virol. 1976 Sep;32(3):361–367. doi: 10.1099/0022-1317-32-3-361. [DOI] [PubMed] [Google Scholar]
- Tilz G. P., de Vaux Saint Cyr C., Grabar P. New antigens in cells transformed by the SV40 virus. II. Evidence of their cytoplasmic localization in a cell line (TSV5 CL2) of hamster fibroblasts transformed by SV40. Int J Cancer. 1969 Sep 15;4(5):641–647. doi: 10.1002/ijc.2910040509. [DOI] [PubMed] [Google Scholar]
- Traub E. A FILTERABLE VIRUS RECOVERED FROM WHITE MICE. Science. 1935 Mar 22;81(2099):298–299. doi: 10.1126/science.81.2099.298. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Buchmeier M. J. Protein analysis of defective interfering lymphocytic choriomeningitis virus and persistently infected cells. Virology. 1979 Jul 30;96(2):503–515. doi: 10.1016/0042-6822(79)90107-7. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Oldstone M. B. Inhibition of immunologic injury of cultured cells infected with lymphocytic choriomeningitis virus: role of defective interfering virus in regulating viral antigenic expression. J Exp Med. 1977 Jun 1;145(6):1449–1468. doi: 10.1084/jem.145.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Pfau C. J. Determinants of lymphocytic choriomeningitis interference. J Gen Virol. 1972 Feb;14(2):177–187. doi: 10.1099/0022-1317-14-2-177. [DOI] [PubMed] [Google Scholar]
- Wiblin C. N., MacPherson I. A. The transformation of BHK 21 hamster cells by simian virus 40. Int J Cancer. 1972 Sep 15;10(2):296–309. doi: 10.1002/ijc.2910100210. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Snider M. D., Porter M., Lodish H. F. Mutants of vesicular stomatitis virus blocked at different stages in maturation of the viral glycoprotein. Cell. 1980 Sep;21(2):417–427. doi: 10.1016/0092-8674(80)90478-x. [DOI] [PubMed] [Google Scholar]
- van der Zeijst B. A., Bleumink N., Crawford L. V., Swyryd E. A., Stark G. R. Viral proteins and RNAs in BHK cells persistently infected by lymphocytic choriomeningitis virus. J Virol. 1983 Oct;48(1):262–270. doi: 10.1128/jvi.48.1.262-270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]