Abstract
In the past few years, Drosophila melanogaster cells have been employed for recombinant protein production purposes, and a comprehensive knowledge of their metabolism is essential for process optimization. In this work, the kinetic response of a Schneider S2 cell line, grown in shake flasks, in two different culture media, the serum-free SF900-II® and the serum-supplemented TC-100, was evaluated. Cell growth, amino acids and glucose uptake, and lactate synthesis were measured allowing the calculation of kinetic parameters. The results show that S2 cells metabolism was able to adjust to different environmental situations, as determined by medium formulation, as well as by the particular situation resulting from the culture conditions. Cells attained a 163% higher final cell concentration (1.4 × 107 cells mL−1) in SF900 II® medium, when compared to serum-supplemented TC-100 medium. Also, a maximum specific cell growth rate 52% higher in SF900 II® medium, when compared to serum-supplemented TC-100 one, was observed. Glutamine was the growth limiting factor in SF900 II® medium, while glucose, sometimes associated with glutamine, controlled growth in serum-supplemented TC-100 medium based formulation. The different pattern of lactate production is an example of the versatility of the metabolism of these cells. This by-product was produced only in glutamine limitation, but the amount synthesized depended not only on the excess glucose, but on other medium components. Therefore, in serum-supplemented TC-100 medium a much smaller lactate amount was generated. Besides, glucose was identified not only as a growth limiting factor, but also as a viability limiting factor, since its depletion accelerated cell death.
Keywords: S2 cells, Metabolism, Glucose, Glutamine, Lactate, Alanine, Kinetic parameters, SF900 II®, TC-100
Introduction
Insect cells has been successfully employed for the expression of heterologous proteins of industrial interest showing advantages such as ease of culture, higher tolerance to osmolality, product concentration, and expression levels, when compared to mammalian systems (Ikonomu et al. 2003). The baculovirus expression vector system (BEVS) is already widely used for large-scales applications. Specialized media, transfection reagents, and vectors have been developed in response to recent advances in insect cell culture and molecular biology methods. Nevertheless, in BEVS the infection step necessary for protein expression does not allow a continuous bioprocess system; also, proteolysis is a problem due to the BEVS lytic nature and can affect the quality and quantity of product; and finally, recombinant baculovirus may loose infectivity with increasing number of passages (Santos et al. 2007; McCarroll and King 1997).
Attempts to overcome some of these problems have led to the development of a stable, plasmid-based expression system capable of continuous protein production in Spodoptera frugiperda and Drosophila melanogaster cells (McCarroll and King 1997). Among them, the Drosophila expression system has been the most successful in achieving high level production of a number of recombinant proteins: antibodies (Kirkpatrick et al. 1995), receptors (Perret et al. 2003; Torfs et al. 2000; Millar et al. 1995; Tota et al. 1995; Vanden Broeck et al. 1995), enzymes (Banks et al. 2003; Gibson et al. 1993), inhibitors (Jeon et al. 2003; Valle et al. 2001), growth factor (Lee et al. 2000) and viral antigen (Yokomizo et al. 2007; Bachmann et al. 2004; Deml et al. 1999). This is largely due to the use of efficient promoters coupled to the ability to generate high copy numbers of the introduced plasmids.
Nevertheless, little information is available on propagation or production processes using Drosophila Schneider 2 (S2) cells (Galesi et al. 2007; Swiech et al. 2007; Valle et al. 2001; Park et al. 1999; Sondergaard 1996). The metabolism of S2 is scarcely discussed in those works and in most cases comparison is made with other insect cell lines, especially S. frugiperda 9 (Sf9) cells.
Nutrient utilization and by-product formation in cultured insect cells with potential industrial application have been investigated by several groups (Drews et al. 1995, 2000; Ferrance et al. 1993; Öhman et al. 1995; Rhiel et al. 1997). Some of these cell lines presented metabolic patterns very similar to those found in mammalian cell culture, synthesizing lactate, alanine and ammonia in response to excess of substrate (Rhiel et al. 1997). Sf9 cells are, on the other hand, distinguished by a lack of lactate and ammonia production in conditions of glucose and glutamine excess. Nonetheless, Sf9 cell lines produce significant amount of ammonia under glucose limitation (Öhman et al. 1995), confirming that by-product formation pattern, apart from being cell-line specific, can also be influenced by the cultivation conditions. Alanine is a common by-product when there is an excess of glucose in the system (Öhman et al. 1995; Drews et al. 2000). The pathways that determine the transfer of amide-nitrogen and amine-nitrogen from glutamine to alanine was investigated by Drews et al. (2000). These authors also showed the formation of glycerol and ethanol by insect cells, an alternative sink to NADH not transported into the mitochondria by the NADH shuttle model.
The focus of our investigation was primarily to characterize the growth kinetic of a wild D. melanogaster S2 cells commonly employed for gene transfection purposes. These cells were cultured in two of the most common media formulation—the serum-free SF900 II® and the serum-supplemented TC-100. Besides the kinetic evaluation, the metabolic pattern was analysed to trace the influence of environmental conditions on cell growth and metabolism.
Materials and methods
Cell line and culture media
Drosophila melanogaster Schneider (S2) cell line (Invitrogen) was stored in liquid nitrogen and cultivated in a serum-supplemented TC-100 medium (Galesi et al. 2007), formulated with TC-100 medium (Gibco) and 10% (v/v) fetal bovine serum (FBS; Gibco), and a serum-free SF900 II® (Invitrogen) medium.
Inoculum preparation
Cells were adapted to both media in T-flasks (25 cm2) for 5–7 sub-cultures. Inoculum preparation, as well as the experiments performed, were carried out in 100 mL shake flasks (“Schott” bottles with working volume of 20 mL), incubated on a orbital shaker (NBS, USA), at 100 rpm, and 28 °C. Initial cell concentration was adjusted to 5 × 105 cells mL−1.
Conditions of runs
Table 1 summarizes the conditions tested in this work. Cells were cultivated in closed 100 mL shake flasks (working volume of 20 mL), incubated on a orbital shaker, at 100 rpm, and 28 °C. Initial cells concentration was adjusted to 5 – 10 × 105 cells mL−1. Samples were taken from flasks every 12–24 h; an aliquot was used directly to determine cell concentration and viability and the remaining part was centrifuged at 200 × g for 2 min to separate cells, allowing storage of the supernatants for later analysis.
Table 1.
Conditions employed in growth experiments with S2 cells carried out in SF900 II® and serum-supplemented TC-100 medium
| Run | Meio | X0 (cells mL−1) | GLCa0 (g L−1) | GLNa0 (mg L−1) | Operation mode |
|---|---|---|---|---|---|
| SF-1 | SF900 II® | 10 × 105 | 10 | 1,900 | Batch |
| SF-2 | 10 × 105 | ||||
| SF-3 | 5 × 105 | ||||
| SF-4 | 5 × 105 | ||||
| TC-1 | Supplemented TC-100 | 5 × 105 | 1 | 600 | |
| TC-2 | 5 × 105 | ||||
| TC-3 | 10 × 105 | ||||
| TC-4 | 5 × 105 | ||||
| TC-5 | 5 × 105 | ||||
| TC-6 | 5 × 105 | 4 | |||
| TC-7 | 5 × 105 | 9 | |||
| FB-1 | 5 × 105 | 1 | Fed batch | ||
| FB-2 | 5 × 105 | 1 |
aConcentrations for SF900 II® medium correspond to the values determined in this work. Values for serum-supplemented TC-100 medium correspond to the concentration stated by producer
During the fed batch runs (FB-1 and FB-2, Table 1), 0.7 g L−1 of a glucose solution (Sigma Chemical Co.) was added to the flask when glucose concentration reached 0.5 g L−1.
Analytical methods
Cell concentration (X) and viability were determined through a Neubauer hemocytometer and viability by the trypan blue dye exclusion method in an optical microscope (Freshney 2005).
Glucose (GLC), glutamine (GLN), and lactate (LAC) concentrations were determined enzymatically with an YSI Biochemical Analyser (model 2700) in culture supernatants after sample centrifugation at 200 × g for 5 min.
Other amino acids were determined in culture supernatants, after sample centrifugation at 200 × g for 5 min, by the Pico-tag system, using a reverse-phase HPLC column (Waters).
Determination of kinetic variables and parameters
Maximum specific cell growth rate (μX,MAX) was determined through the identification of a linear region in the curve ln (XV) = f(t) followed by the linear regression of these data. XV is the viable cell concentration, and t is time.
Glucose-to-cell yield coefficient (YGLC/X) was calculated from the linear regression of the curve Xv = f(GLC), at the time interval characterized as the exponential growth phase. GLC indicates the glucose concentration. A similar methodology was applied for glutamine-to-cell yield facto (YGLN/X).
Results
Through the analysis of the following experiments, it was possible to establish the kinetic response of a Drosophila S2 cell line grown on two insect cells medium formulations: the serum-free SF900 II® medium and the serum-supplemented TC-100 medium. Distinct metabolic pattern and kinetic parameter values were observed as a function of the initial composition of those media, as well as a result of growth evolution. The study was based on data on growth, substrate consumption (glucose and amino acids), and by-product formation (lactate).
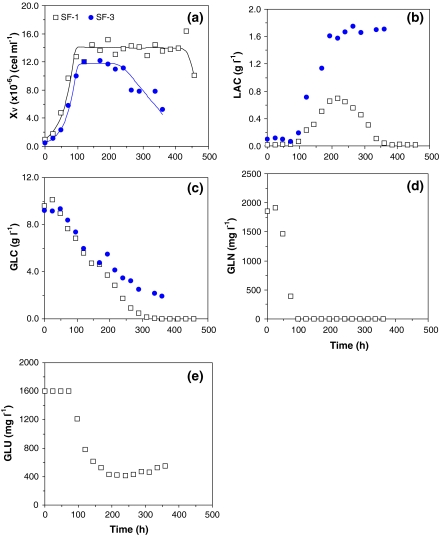
Figure 1 presents examples of typical runs carried out on SF900 II® medium, while Table 2 shows, for all experiments carried out on SF900 II® medium, the variables and parameters calculated that will be taken in account to identify possible factors that can promote cell growth, or determine cell death. Thus, the kinetic behaviour of S2 cells was evaluated in three distinct instants of cultivation: at the end of exponential growth phase (indicated by “EXP”); when cells reached the maximum cell concentration (or beginning of stationary phase, indicated by “END”); and when cell concentration started to decrease (indicated by “DEATH”). The extent of the initial lag or adaptation phase (tLAG) was also determined in all experiments shown in Table 1, but no correlation was established between this variable and the run conditions, so it will not be discussed.
Fig. 1.
S2 cell growth, substrates uptake, and by-products synthesis for runs SF-1 and SF-3, carried out in shake flasks, in batch mode, in SF900 II® medium. (a) Cell growth; (b) Lactate production; (c) Glucose uptake; (d) Glutamine uptake; and (e) Glutamate uptake
Table 2.
Kinetic variables and parameters for S2 cell growth runs in SF900 II® medium, considering three distinct instants of cultivation: the end of exponential phase (EXP); maximum cell concentration (END); cell concentration decreasing (DEATH)
| End of exponential phase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Run | tLAG (h) | taEXP (h) | GLCEXP (g L−1) | GLNEXP (mg L−1) | LACEXP (g L−1) | μX,MAX (h−1) | YX/GLC (cells g−1) | YX/GLN (cells mg−1) | |
| SF-1 | 0.0 | 96.0 | 6.84 | 0.0 | 0.070 | 0.0283 | 3.69E + 09 | 5.76E + 06 | |
| SF-2 | 0.0 | 96.0 | 6.78 | 0.0 | 0.035 | 0.0298 | 4.37E + 09 | 5.30E + 06 | |
| SF-3 | 0.0 | 120 | 6.21 | – | 0.263 | 0.0311 | 3.29E + 09 | – | |
| SF-4 | 0.0 | 96.0 | 7.37 | – | 0.190 | 0.0318 | 4.63E + 09 | – | |
| Mean | 0.0 | 108 | 6.80 | 0.0 | 0.140 | 0.0303 | 3.99E + 09 | 5.53E + 06 | |
| Standard deviation | 0.0 | 17.0 | 0.474 | 0.0 | 0.106 | 0.00155 | 6.1E + 08 | 3.2E + 05 | |
| CV (%) | – | 15.7 | 6.98 | – | 75.8 | 5.1 | 15.4 | 5.86 | |
| Maximum cell growth | Beginning of death phase | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Run | taEND (h) | GLCEND (g L−1) | GLNEND (mg L−1) | LACEND (g L−1) | XV,END (cells mL−1) | PaX,END (cells mL−1 h−1) | tDEATH (h) | GLCDEATH (g L−1) | GLNDEATH (mg L−1) | LACDEATH (g L−1) |
| SF-1 | 144 | 4.70 | 0.0 | 0.313 | 1.45E + 07 | 9.27E + 04 | 408 | 0.0 | 0.0 | 0.0 |
| SF-2 | 144 | 4.11 | 0.0 | 0.194 | 1.61E + 07 | 1.04E + 05 | 336 | 0.0 | 0.0 | 0.0 |
| SF-3 | 120 | 6.21 | – | 0.263 | 1.42E + 07 | 1.18E + 05 | 264 | 1.53 | 0.0 | 1.51 |
| SF-4 | 120 | 5.97 | – | 0.710 | 1.20E + 07 | 1.00E + 05 | 216 | 4.10 | 0.0 | 1.58 |
| Mean | 120 | 5.25 | 0.0 | 0.370 | 1.42E + 07 | 1.09E + 05 | 240 | – | 0.0 | – |
| Standard deviation | 0.0 | 1.01 | 0.0 | 0.232 | 1.67E + 06 | 1.30E + 04 | 33.9 | – | 0.0 | – |
| CV (%) | 0.0 | 19.2 | – | 62.7 | 11.8 | 11.9 | 14.1 | – | – | – |
aMean and standard deviation consider only experiments carried out with initial cell concentration of 5.0 × 105 cells mL−1
Mean values, standard deviation, and coefficient of variance are also presented in Table 2, showing acceptable accuracy for most variables and parameters calculated, except for lactate measurement, what will be discussed later.
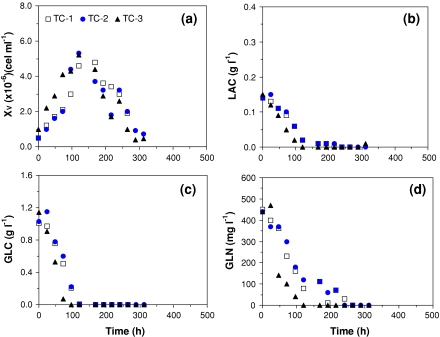
A similar set of results is shown in Fig. 2 and Table 3 for experiments carried out on serum-supplemented TC-100 medium.
Fig. 2.
S2 cell growth, substrates uptake, and by-product synthesis for runs TC-1 to TC-3, carried out in, shake flasks, in batch mode, with serum-supplemented TC-100 medium. (a) Cell growth; (b) Lactate production; (c) Glucose uptake; and (d) Glutamine uptake
Table 3.
Kinetic variables and parameters for S2 cell growth runs in serum-supplemented TC-100 medium, in batch mode, considering three distinct instants of cultivation: the end of exponential phase (EXP); maximum cell concentration (END); cell concentration decreasing (DEATH)
| End of exponential phase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Run | tLAG (h) | taEXP (h) | GLCEXP (g L−1) | GLNEXP (mg L−1) | LACEXP (g L−1) | μX,MAX (h−1) | YX/GLC (cells g−1) | YX/GLN (cells mg−1) | |
| TC-1 | 24.0 | 120 | 0.010 | 80.0 | 0.020 | 0.0167 | 3.26E + 09 | 9.41E + 06 | |
| TC-2 | 0.0 | 96.0 | 0.220 | 180 | 0.060 | 0.0205 | 30.87E + 09 | 1.07E + 07 | |
| TC-3 | 0.0 | 72.0 | 0.070 | 100 | 0.0 | 0.0188 | 2.99E + 09 | 8.67E + 06 | |
| TC-4 | 0.0 | 120 | 0.0 | – | 0.0 | 0.0192 | 3.19E + 09 | – | |
| TC-5 | 24.0 | 120 | 0.0 | – | 0.038 | 0.0244 | 4.75E + 09 | – | |
| Mean | 10 | 114 | 0.060 | 120 | 0.024 | 0.0199 | 3.61E + 09 | 9.60E + 06 | |
| Standard deviation | 13.1 | 12.0 | 0.094 | 52.9 | 0.026 | 0.0028 | 7.17E + 08 | 1.04E + 06 | |
| CV (%) | 137 | 10.5 | 157 | 44.1 | 109 | 14.2 | 19.9 | 10.8 | |
| Maximum cell growth | Beginning of death phase | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Run | taEND (h) | GLCEND (g L−1) | GLNEND (mg L−1) | LACEND (g L−1) | XV,END (cells mL−1) | PaX,END (cells mL−1 h−1) | tDEATH (h) | GLCDEATH (g L−1) | GLNDEATH (mg L−1) | LACDEATH (g L−1) |
| TC-1 | 168 | 0.0 | 20.0 | 0.010 | 5.30E + 06 | 2.86E + 04 | 168 | 0.0 | 20.0 | 0.010 |
| TC-2 | 120 | 0.010 | 120 | 0.020 | 5.30E + 06 | 4.42E + 04 | 120 | 0.01 | 120 | 0.020 |
| TC-3 | 120 | 0.0 | 0.0 | 0.050 | 5.20E + 06 | 4.33E + 04 | 120 | 0.0 | 0.0 | 0.050 |
| TC-4 | 144 | 0.0 | – | 0.0 | 4.70E + 06 | 2.92E + 04 | 144 | 0.0 | – | 0.0 |
| TC-5 | 168 | 0.0 | – | 0.008 | 6.40E + 06 | 3.51E + 04 | 168 | 0.0 | – | 0.008 |
| Mean | 150 | 0.002 | 46.7 | 0.018 | 5.38E + 06 | 3.43E + 04 | 144 | 0.002 | 46.7 | 0.018 |
| Standard deviation | 23.0 | 0.004 | 64.3 | 0.019 | 6.22E + 05 | 7.24E + 03 | 24.0 | 0.004 | 64.3 | 0.019 |
| CV (%) | 15.3 | 224 | 138 | 111 | 11.6 | 21.1 | 16.7 | 224 | 138 | 111 |
aMean and standard deviation consider only experiments carried out with initial cell concentration of 5.0 × 105 cells mL-1
In Fig. 1 and Table 2, maximum cell concentrations (XV,END) of 1.2 × 107 to 1.61 × 107 cells mL−1 are reported after 120–144 h of cultivation. The mean value for XV,END observed on SF900 II® medium (Table 2) is statistically higher than the values listed in Table 3 for serum-supplemented TC100 medium (t-test for α = 0.05). Besides this higher cell production, experiments carried out in SF900 II® medium also presents significant improvement in maximum specific growth rate (μX,MAX) in relation to serum-supplemented TC-100 medium (Tables 2 and 3). The individual calculations for μX,MAX also exhibit good accuracy with exponential linear regression showing coefficients of correlation always superior to 0.94. According to this data, maximum specific growth rate is 52% higher in SF900 II® medium—0.0303 h−1 in SF900 II® medium and 0.0199 h−1 in serum-supplemented TC-100 medium. These results corroborate the common sense idea that SF900 II® medium is one of the richest formulations for insect cell growth.
As previously shown, kinetic data of S2 cell line are not common in literature. Nevertheless, different authors present similar data for recombinant S2 cell lines. Swiech et al. (2007) and Galesi et al. (2007), both growing a recombinant S2 cell line transfected with rabies virus glycoprotein gene, achieved values of 0.027 and 0.037 h−1 for maximum specific growth rate of cells cultivated on a serum-free medium based on TC-100 and on SF900 II® media, respectively.
Glutamine (GLN) and glucose (GLC) are the main substrates consumed to provide energy and carbon source for cell growth (Doverskog et al 1997). In the experiments reported here, both nutrients are consumed to promote cell growth, up to complete depletion in some cases (Figs. 1c, d and 2c, d). The initial substrates concentrations (GLC and GLN), as well as the ratio between the substrates are different in SF900 II® and serum-supplemented TC-100 media. Therefore, cell response varied not only within the same formula but also for the different media studied.
GLN was totally consumed after 96 h of cultivation on SF900 II® medium (Fig. 1d), determining the end of the exponential growth phase. In Table 2, it is observed that GLN was also a limiting factor on run SF-2 (GLNEXP equal to zero). Although, data on GLN for runs SF-3 and SF-4 are not available, it is possible to infer that the same limiting condition applied also to these experiments.
Glutamate (GLU) is also consumed by S2 cells but only after GLN depletion (t ∼ 96 h) and only partially as shown in Fig. 1e. Apparently, after a period of constant concentration (∼260 h), GLU showed a slight increase, indicating a synthesis of these amino acid.
With a GLN to GLC relationship of 5:1 in SF900 II® medium, GLC is in excess at the end of the exponential phase and at the stationary phase (Fig. 1c; Table 2—GLCEXP and GLCEND). A different ratio between GLN and GLC for TC-100 medium formulation (1:2) determined the complete depletion of glucose earlier or at the same time of glutamine (Fig. 2c, d). This can also be confirmed by values attributed to GLC and GLN in Table 3, at the end of exponential phase (“EXP”) or at the end of cell growth (stationary phase; “END”).
Significant lactate synthesis (LAC) was observed only in cultures with SF900 II® medium (Figs. 1b and 2b). Lactate is a common by-product of GLC anaerobic metabolism reported for many different cell lines and the amount produced is normally dependent on the excess of GLC (Doverskog et al. 1997). In our experiments, although GLC was in excess from the beginning of cultures, LAC synthesis only occurred after complete depletion of GLN, i.e., after the end of exponential growth phase (Fig. 1b, d).
At least in runs SF-1 (Fig. 1b) and SF-2 (not shown), lactate uptake was observed at the end of experiment, when glucose concentration was already low (1–2 g L−1). Nevertheless, in run SF-3 (Fig. 1b) and SF-4 (not shown) LAC accumulated up to approximately 1.5 g L−1 and was not consumed by cells.
In contrast, no significant lactate production was observed in experiments carried out with serum-supplemented TC-100 medium. Nevertheless, LAC present at the initial phase of growth, probably derived from serum, was consumed during exponential phase (Mendonça et al. 1999). Since all GLC was also taken up during the exponential phase, no further by-product could be synthesised after that moment.
Finally, Figs. 1 and 2 also present a reduction in viable cells that can be attributed to different factors depending on culture media utilised and run performance. Glucose depletion is one of the hypotheses to explain cell death. This could be observed in run SF-1 (Fig. 1a, c) and SF-2 (not shown), with SF900 II® medium, as well in all experiments carried out with serum-supplemented TC-100 medium (Fig. 2a, c). Another possible explanation for the reduction of viable cells is an inhibitory effect caused by the high amount of LAC observed on runs SF-3 (Fig. 1a, c) and SF-4 (not shown).
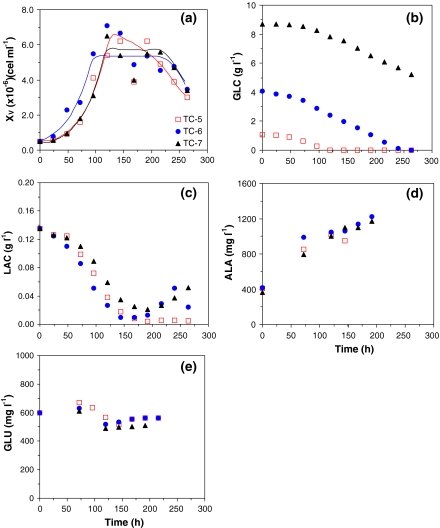
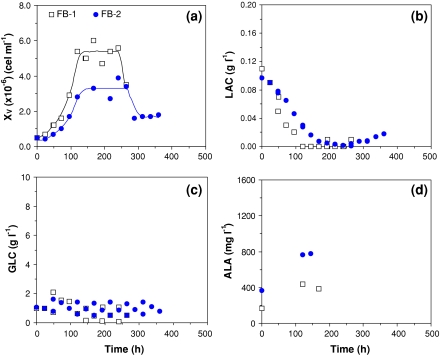
In order to clarify the importance of GLC on S2 cells viability, a set of experiments with higher amounts of initial glucose concentration (Fig. 3 and Table 4) or employing a feed strategy to ensure GLC concentration around 1 g L−1 (see Fig. 4 and Table 5) were carried out.
Fig. 3.
Influence of glucose concentration on S2 cell growth and death, substrates uptake, and by-product synthesis for runs TC-5 to TC-7 carried out in serum-supplemented TC-100 medium. (a) Cell growth; (b) Glucose uptake; (c) Lactate production; (d) Alanine production; and (e) Glutamate uptake
Table 4.
Kinetic variables and parameters for S2 cell growth runs in serum-supplemented TC-100 medium, in batch mode, with different initial GLC, considering three distinct instants of cultivation: the end of exponential phase (EXP); maximum cell concentration (END); cell concentration decreasing (DEATH)
| End of exponential phase | |||||||
|---|---|---|---|---|---|---|---|
| Run | tLAG (h) | tEXP (h) | GLCEXP (g L−1) | LACEXP (g L−1) | μX,MAX (h−1) | YX/GLC (cells g−1) | |
| TC-6 | 24.0 | 120 | 7.84 | 0.059 | 0.0257 | 5.35E + 09 | |
| TC-7 | 0.0 | 96.0 | 2.88 | 0.051 | 0.0251 | 4.14E + 09 | |
| Mean | 12.0 | 108 | 5.36 | 0.055 | 0.0254 | 4.74E + 09 | |
| Standard deviation | 17.0 | 17.0 | 3.51 | 5.66E–03 | 4.26E–04 | 8.59E + 08 | |
| CV (%) | 141 | 15.7 | 65.4 | 10.3 | 1.68 | 18.1 | |
| Maximum cell growth | Beginning of death phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | tEND (h) | GLCEND (g L−1) | LACEND (g L−1) | XV,END (cells mL−1) | PX,END (cells mL−1 h−1) | tDEATH (h) | GLCDEATH (g L−1) | LACDEATH (g L−1) |
| TC-6 | 144 | 7.53 | 0.035 | 5.50E + 06 | 3.40E + 04 | 240 | 0 | 0.05 |
| TC-7 | 96.0 | 2.88 | 0.051 | 5.40E + 06 | 5.21E + 04 | 216 | 6.10 | 0.03 |
| Mean | 120 | 5.21 | 0.043 | 5.45E + 06 | 4.31E + 04 | 228 | 3.05 | 0.04 |
| Standard deviation | 33.9 | 3.29 | 0.011 | 7.07E + 04 | 1.28E + 04 | 17.0 | 4.31 | 0.01 |
| CV (%) | 28.3 | 63.2 | 26.3 | 1.30 | 29.7 | 7.44 | 141 | 35.4 |
Fig. 4.
S2 cell growth and death, substrates uptake, and by-product synthesis for runs FB-1 and FB-2, in shake flasks, in fed batch mode, with serum-supplemented TC-100. (a) Cell growth; (b) Lactate production; (c) Glucose uptake; and (d) Alanine production
Table 5.
Kinetic variables and parameters for S2 cell growth runs in serum-supplemented TC-100 medium, in fed batch mode, considering three distinct instants of cultivation: the end of exponential phase (EXP); maximum cell concentration (END); cell concentration decreasing (DEATH)
| End of exponential phase | |||||||
|---|---|---|---|---|---|---|---|
| Run | tLAG (h) | tEXP (h) | GLCEXP (g L−1) | LACEXP (g L−1) | μX,MAX (h−1) | YX/GLC (cells g−1) | |
| FB-1 | 0.0 | 120 | 0.654 | 0.000 | 0.0196 | 2.46E + 09 | |
| FB-2 | 24.0 | 120 | 0.900 | 0.028 | 0.0192 | 2.72E + 09 | |
| Mean | 12.0 | 120 | 0.777 | 0.014 | 0.0194 | 2.59E + 09 | |
| Standard deviation | 17.0 | 0.0 | 0.174 | 0.020 | 0.0003 | 1.83E + 08 | |
| CV (%) | 141 | 0.0 | 22.4 | 141 | 1.31 | 7.07 | |
| Maximum cell growth | Beginning of death phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | tEND (h) | GLCEND (g L−1) | LACEND (g L−1) | XV,END (cells mL−1) | PX,END (cells mL−1 h−1) | tDEATH (h) | GLCDEATH (g L−1) | LACDEATH (g L−1) |
| FB-1 | 145 | 0.65 | 0.000 | 5.20E + 06 | 3.24E + 04 | 241 | 1.00 | 0.0 |
| FB-2 | 144 | 0.90 | 0.016 | 3.30E + 06 | 1.94E + 04 | 264 | 0.900 | 0.0 |
| Mean | 145 | 0.78 | 0.008 | 4.25E + 06 | 2.59E + 04 | 253 | 0.950 | 0.00 |
| Standard deviation | 0.85 | 0.18 | 0.011 | 1.34E + 06 | 9.14E + 03 | 16.3 | 0.071 | 0.00 |
| CV (%) | 0.59 | 22.8 | 141 | 31.6 | 35.3 | 6.4 | 7.4 | – |
The surplus of GLC at the beginning of runs TC-6 and TC-7 (Fig. 3b) resulted in long-lasting stationary growth phases, 70–100 h (Fig. 3a), when compared to the data obtained with supplemented TC-100 medium formulation (Fig. 2a). Data on GLN was not available for those experiments, but as the initial GLN concentration in these runs was the same as in other experiments with supplemented TC-100 medium (Table 1), a GLN limitation was expected at the end of exponential phase, with maximum cell concentration around 5.4 × 106 cells mL−1 (Table 3). Also confirming our previous observations, GLU was consumed only after the limitation on GLN (Fig. 3e).
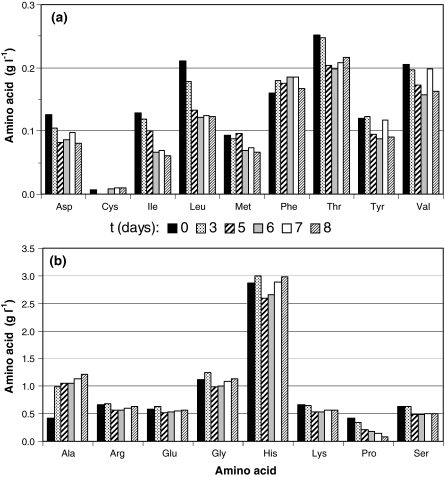
No other amino acid was completely consumed during these experiments (Fig. 5), barely constituting further limiting factors of the process. Alanine (ALA) was produced during the whole experiment, even after cell culture reached the stationary phase, i.e., after GLN depletion, usually considered as the main amino acid for alanine synthesis (Drews et al. 2000). It indicates that alanine can be the main destination of the exceeding piruvic acid generated from GLC uptake through the glycolytic pathway (Ikonomou et al. 2003).
Fig. 5.
Amino acids uptake for run TC-6, carried out in serum-supplemented TC-100 medium, supplemented with glucose (4 g L−1), operated in batch mode. Different columns indicate the sampling time (days)
In GLC-supplemented experiments, lactate was again produced and consumed (Fig. 3c, run TC-6 at ∼250 h), a pattern very similar to the one previously observed with SF900 II® medium (Fig. 1b). The fact that GLC was in excess in media was essential for the synthesis of LAC, nevertheless the amount of LAC produced was much lower than in cultures with SF900 II® medium, where similar initial GLC concentration was present.
A comparison between parameters obtained in experiments with and without GLC supplementation indicates no significant difference (t-test, α = 0.05) for maximum cell concentration (XEND), cell productivity (PX,END), and glucose-to-cell yield coefficient (YX/GLC). The only exception is the maximum specific cell growth rate (μX,MAX) that shows an increase of approximately 28%.
In runs operated in fed batch mode, GLC was maintained at an average of 1 g L−1 (Fig. 4c), and a stationary phase of 100–120 h was demonstrated (Fig. 4a). When compared to supplemented batch experiments (TC-5, TC-6, and TC-7), this fed batch runs showed lower lactate and alanine concentrations, probably as a consequence of lower glucose availability (Figs. 3b and 4c).
Discussion
The data presented above demonstrate that the D. melanogaster S2 cells used in this study could adjust its metabolism according to their environment, this variability being determined either by the specific medium formulation or through the culture condition of a given experiment.
The fact that S2 cells presented different values for maximum specific growth rate (μX,MAX) in SF900 II® and in serum-supplemented TC-100 media (Tables 2 and 3) is indicative of use of different metabolic pathways rather than a surplus of nutrients. A richer medium formulation, as that of SF900 II® medium, would lead to a long-lasting exponential phase, but Tables 2 and 3 showed no significant difference in the extent of exponential phase (tEXP) for the media studied.
The analysis of the exponential phase, particularly at the moment the specific growth rate started to decrease, indicated that the GLN depletion in SF900 II® medium (Fig. 1d), and the GLC total consumption, eventually associated with the GLN depletion, in the case of serum-supplemented TC-100 medium formulation (Fig. 2c) determined the growth limitation. Different limiting factors found in those media were a consequence of the balance between initial concentrations of GLN/GLC in the formulations: 1:5 in SF900 II® medium and 1:2.5 in serum-supplemented TC-100 medium formulation.
Although there is a correlation between the end of exponential growth phase and the depletion of GLN and/or GLC, the possibility of a double limiting condition must be considered. Swiech et al. (2007), studying a recombinant S2 in SF900 II® medium, indicated that proline was the main limiting substrate. Even though cells are not exactly the same in our work and in Swiech et al (2007), the metabolic machinery is likely to be similar, so a double limiting substrate is indeed possible. Oxygen is another possible limiting substrate, since volumetric oxygen transfer coefficient (KLa) in shake flasks is low particularly with the procedure adopted in our experiments that allowed gas exchange only during sampling, once or twice a day. As Swiech et al. (2007) discussed, S2 cells are remarkably well adapted to hypoxia, maintaining viability for long periods, but this characteristic does not avoid a growth limitation by oxygen. Such a limiting condition is much likely to happen in SF900 II® medium because cell concentration is higher, and consequently oxygen uptake should be also higher, leading to a lower profile of dissolved oxygen values.
Lactate production in many animal cell lines is a way of regenerate NADH when complete oxidation through the electron transport chain is not possible, as is the case when there is a limitation in oxygen supply or a an excess of GLC that generates a high glycolytic flux (Drews et al. 2000; Doverskog et al. 1997). Considering insect cell lines, the metabolic responses to such situations seems to be characteristically cell-dependent.
In non-limiting oxygen conditions, Sf9 cells are distinguished by the lack of accumulation of lactate even at high substrate concentrations (Drews et al. 2000), showing that these cells possess a fully functional TCA cycle (Bhatia et al. 1997). On the other hand, Trichoplusia ni and BTI-Tn-5B1-4 cells display a mammalian cell-like metabolism, characterized by the accumulation of alanine, lactate, and ammonium ions (Sugiura and Amann 1996; Rhiel et al. 1997). Finally, Galesi et al. (2007) and Swiech et al. (2007) showed that a recombinant S2 cell line did not produce expressive LAC (0.05–0.2 g/L) concentration even under oxygen limiting conditions.
This variability of responses is also verified within the experiments shown in this article. Different concentrations of LAC were observed in the media studied (Figs. 1b, 2b, and 3c), as well as within a specific formulation (Fig. 1b), under GLC excess conditions and possibly under oxygen limitation. It seems that a clear correlation between the depletion of GLN and the beginning of lactate production exists. Thus, the LAC appearance in culture media could be the result of S2 cell incapacity to synthesise ALA, after depletion of GLN, a conditions that that applies more to SF900 II®medium cultivation.
Glutamine is the major source of energy, carbon, and nitrogen for animal cells (Ikonomou et al. 2003; Mendonça et al. 1999). It plays two important functions: acts as an energy donor and plays a critical role in nitrogen metabolism, acting as a collection point for amino groups. According to Drews et al. (1995) the majority of other amino acids (hystidine, lysine, threonine, glycine, valine, leucine, isoleucine, phenylalanine, tyrosine, tryptophane) are incorporated in cellular protein, but are not significant for energy production purposes. The low consumption of those amino acids by S2 cells as shown in this study (Fig. 5), as well as in Swiech et al. (2008), is in accordance to the previous observation.
Concerning ALA production, Drews et al. (2000) demonstrated a lower production of this amino acid in glutamine-free medium, glutamate replacing glutamine as the major nitrogen-donor for ALA synthesis. The results shown in Fig. 3d indicated that ALA synthesis decreased after GLN depletion (possibly around 80 h), probably using other amino acids as ammonia donor. For S2 cell cultures in our study, it is not clear if GLU played this role, since it started to be consumed when GLN was limited, but did not have the same profile of ALA synthesis (Fig. 3d, e). Also, an increase in GLU is observed at the end of cultivation, while ALA is still being produced. Thus, another amino acid must be the responsible for ALA generation. As shown in Fig. 5, proline is the amino acid most consumed after GLN depletion and could play this role.
The apparently lower capacity of S2 cells to synthesise ALA after GLN depletion, leaded to a higher LAC accumulation in SF900 II® medium, up to deleterious concentrations (Fig. 1b). It occurred only in runs SF-3 and SF-4 (not shown), as a consequence of cultivation conditions. In comparison to experiments SF-1 and SF-2, runs SF-3 and SF-4 showed a high GLC at the end of exponential phase, when cells started to synthesize lactate. As presented before, cells produced lactate until GLC became limiting (1–2 g L−1) in cultivation, then LAC is consumed. As runs SF-3 and SF-4 had a relatively higher GLC availability, higher amounts of LAC were produced up to 1.5 g L−1. As a consequence, viable cells started to decrease and LAC synthesis was interrupted and no consumption of this by-product was observed in this condition.
Although different amounts of LAC were observed during our experiments, it is important to indicate that the S2 cell line did not alter its metabolism in GLC excess. The values for glucose-to-cells coefficient (YX/GLC) obtained for both media are similar (Tables 2–4). On the other hand, it was observed that the excess of GLN induced a less efficient metabolism, measured by the parameter YX/GLN.
Ammonia (NH3) was not determined in our experiments, but data on a derived recombinant S2 cell line indicate that the amount of NH3 synthesize, and its deleterious effects also depend on the culture medium employed (Swiech et al. 2008; Galesi et al. 2008). For SF900 II® (Swiech et al. 2008), it could be observed concentrations up to 340 mg L−1 at the end of exponential phase, without any indication of cell growth inhibition. Nevertheless, for the TC-100-based formulation (Galesi et al. 2008), a cell growth inhibition was attributed to the 50–60 mg L−1 of NH+4 accumulated in culture. As the initial GLN in our experiment was six times smaller than in Galesi et al. (2008), a much lower NH3 is expected, and possibly no growth inhibition could be attributed to this by-product.
Finally, this study demonstrated that GLN is an essential substrate for S2 cell growth, since its depletion limited the cell growth in all the experiments, while the GLC level, besides interrupting cell growth, is also responsible for maintenance of cell viability. Lactate synthesis is determined by GLN depletion and excess of GLC, but the amount release in culture depends on medium formulation and on experiment conditions. Its synthesis is lower in serum-supplemented TC-100 medium, possibly because in this case, alanine can be produced from other amino acids rather than GLN. Also, an excess of LAC accumulated in culture can lead to cell death.
Acknowledgments
This work was supported in part by grants from FAPESP, CNPq, and Fundação Butantan. A.M. Moraes and C. A. Pereira are the recipients of a CNPq research fellowship.
References
- Bachmann AS, Corpuz G, Hareld WP et al (2004) A simple method for the rapid purification of copia virus-like particles from Drosophila Schneider 2 cells. J Virol Methods 115:159–165 [DOI] [PubMed]
- Banks DJ, Hua G, Adang M (2003) Cloning of a Heliothis virescens 110 kda aminopeptidase N and expression in Drosophila S2 cells. Insect Biochem Mol Biol 33:499–508 [DOI] [PubMed]
- Bhatia R, Jesionowski G, Ferrance J et al (1997) Insect cell physiology. Cytotechnology 24:1–9 [DOI] [PMC free article] [PubMed]
- Deml L, Wolf H, Wagner R (1999) High level expression of hepatitis B virus surface antigen in stably transfected Drosophila Schneider-2 cells. J Virol Methods 79:191–203 [DOI] [PubMed]
- Doverskog M, Ljunggren J, Öhman L et al (1997) Physiology of cultured animal cells. J Biotechnol 59:103–115 [DOI] [PubMed]
- Drews M, Paalme T, Vilu R (1995) The growth and nutrient utilization of insect cell line Spodopterafrugiperda Sf9 in batch and continuous culture. J Biotechnol 40:187–198 [DOI]
- Drews M, Doverskog M, Öhman L et al (2000) Pathways of glutamine metabolism in Spodopterafrugiperda (Sf9) insect cells: evidence for the presence of the nitrogen assimilation system, and a metabolic switch by 1H/15N NMR. J Biotechnol 78:23–37 [DOI] [PubMed]
- Ferrance JP, Goel A, Ataai MM (1993) Utilization of glucose and amino acids in insect cell cultures: quantifying the metabolic flows within the primary pathways and medium development. Biotechnol Bioeng 42:697–707 [DOI] [PubMed]
- Freshney RI (2005) Culture of animal cells: a manual of basic technique, 5th edn. Wiley-Liss, New York, 642 p
- Galesi ALL, Pereira CA, Moraes AM (2007) Culture of transgenic Drosophila melanogaster Schneider S2 cells in serum free media based on TC100 basal media. Biotechnol J 2(11):1399–1407 [DOI] [PubMed]
- Galesi ALL, Aguiar MA, Astray RM et al (2008) Growth of recombinant Drosophila melanogaster Schneider 2 cells producing rabies virus glycoprotein in bioreactor employing serum-free medium. Cytotechnology. doi:10.1007/s10616-008-9139-y [DOI] [PMC free article] [PubMed]
- Gibson KR, Vanek PG, Kaloss WD et al (1993) Expression of dopamine-beta-hydroxylase in Drosophila Schneider 2 cells—Evidence for a mechanism of membrane-binding other than uncleaved signal peptide. J Biol Chem 268:9490–9495 [PubMed]
- Ikonomou L, Schneider YJ, Agathos SN (2003) Insect cell culture for industrial production of recombinant proteins. Appl Microbiol Biotechnol 62:1–20 [DOI] [PubMed]
- Jeon HK, Chang KH, Kim KI et al (2003) Functional expression of recombinant tumstatin in stably transformed Drosophilamelanogaster S2 cells. Biotechnol Lett 25:185–189 [DOI] [PubMed]
- Kirkpatrick RB, Ganguly S, Angelichio M et al (1995) Heavy chain dimers as well as complete antibodies are efficiently formed and secreted from Drosophila via a BiP-mediated pathway. J Biol Chem 270(34):19800–19805 [DOI] [PubMed]
- Lee JM, Park JH, Park JO et al (2000) Expression of recombinant erythropoietin in stably transformed Drosophila melanogaster S2 cells. In Vitro Cell Dev Biol Anim 36:348–350 [DOI] [PubMed]
- McCarroll L, King LA (1997) Stable insect cell cultures for recombinant protein production. Curr Opin Biotechnol 8:590–594 [DOI] [PubMed]
- Mendonça RZ, Palomares LA, Ramírez OT (1999) An insight into insect cell metabolism through selective nutrient manipulation. J Biotechnol 72:61–75 [DOI]
- Millar NS, Baylis HA, Reaper C et al (1995) Functional expression of a cloned Drosophila muscarinic acetylcholine receptor in a stable Drosophila cell line. J Exp Biol 198:1843–1850 [DOI] [PubMed]
- Öhman L, Ljunggren J, Häggström L (1995) Induction of a metabolic switch in insect cells by substrate-limited fed-batch. Appl Microbiol Biotechnol 43:1006–1013 [DOI] [PubMed]
- Park JH, Lee JM, Chung IS (1999) Production of recombinant endostatin from stably transformed Drosophila melanogaster S2 cells. Biotechnol Lett 21:729–733 [DOI]
- Perret BG, Wagner R, Lecat S et al (2003) Expression of EGFP-amino-tagged human mu opioid receptor in Drosophila Schneider 2 cells: a potential expression system for large-scale production of g-protein coupled receptors. Protein Expr Purif 31:123–132 [DOI] [PubMed]
- Rhiel M, Mitchell-Logean CM, Murhammer DW (1997) Comparison of Trichoplusia ni BTI-Tn-5B1-4 (High Five TM) and Spodoptera frugiperda Sf-9 insect cell line metabolism in suspension cultures. Biotechnol Bioeng 55:909–920 [DOI] [PubMed]
- Santos MG, Jorge SAC, Brillet K et al (2007) Improving heterologous protein expression in transfected Drosophila S2 cells as assessed by EGFP expression. Cytotechnology 54:15–24 [DOI] [PMC free article] [PubMed]
- Sondergaard L (1996) Drosophila cells can be grown to high cell densities in bioreactor. Biotechnol Tech 10:161–166 [DOI]
- Sugiura T, Amann E (1996) Properties of two insect cell lines useful for the baculovirus expression system in serum-free culture. Biotechnol Bioeng 51:494–499 [DOI] [PubMed]
- Swiech K, Silva CL, Arantes MK et al (2007) Characterization of growth and metabolism of Drosophila melanogaster cells transfected with the rabies virus glycoprotein gene. Biotechnol Appl Biochem 49:41–49 [DOI] [PubMed]
- Swiech K, Rossi N, Silva BG et al (2008) Bioreactor culture of recombinant Drosophila melanogaster S2 cells: characterization of metabolic features related to cell growth and production of the rabies virus glycoprotein. Cytotechnology. doi:10.1007/s10616-008-9130-7 [DOI] [PMC free article] [PubMed]
- Torfs H, Shariatmadari R, Guerrero F et al (2000) Characterization of a receptor for insect tachykinin-like peptide agonists by functional expression in a stable Drosophila Schneider 2 cell line. J Neurochem 74:2182–2189 [DOI] [PubMed]
- Tota MR, Xu L, Sirotina A et al (1995) Interaction of [fluorescein-Trp25] glucagon with the human glucagon receptor expressed in Drosophila Schneider-2 cells. J Biol Chem 270:26466–26472 [DOI] [PubMed]
- Valle MA, Kester MB, Burns AL et al (2001) Production and purification of human menin from Drosophila melanogaster S2 cells using stirred tank reactor. Cytotechnology 35:127–135 [DOI] [PMC free article] [PubMed]
- Vanden Broeck J, Vulsteke V, Huybrechts R et al (1995) Characterization of a cloned locust tyramine receptor cDNA by functional expression in permanently transformed Drosophila S2 cells. J Neurochem 64:2387–2395 [DOI] [PubMed]
- Yokomizo AY, Jorge SAC, Astray RM et al (2007) Rabies virus glycoprotein expression in Drosophila S2 cells. I. Functional recombinant protein in stable co-transfected cell line. Biotechnol J 2:1–8 [DOI] [PubMed]