Abstract
Polyglycylation, a posttranslational modification of tubulin, was discovered in the highly stable axonemal microtubules of Paramecium cilia where it involves the lateral linkage of up to 34 glycine units per tubulin subunit. The observation of this type of posttranslational modification mainly in axonemes raises the question as to its relationship with axonemal organization and with microtubule stability. This led us to investigate the glycylation status of cytoplasmic microtubules that correspond to the dynamic microtubules in Paramecium. Two anti-glycylated tubulin monoclonal antibodies (mAbs), TAP 952 and AXO 49, are shown here to exhibit different affinities toward mono- and polyglycylated synthetic tubulin peptides. Using immunoblotting and mass spectrometry, we show that cytoplasmic tubulin is glycylated. In contrast to the highly glycylated axonemal tubulin, which is recognized by the two mAbs, cytoplasmic tubulin reacts exclusively with TAP 952, and the α- and β- tubulin subunits are modified by only 1–5 and 2–9 glycine units, respectively. Our analyses suggest that most of the cytoplasmic tubulin contains side chain lengths of 1 or 2 glycine units distributed on several glycylation sites. The subcellular partition of distinct polyglycylated tubulin isoforms between cytoplasmic and axonemal compartments implies the existence of regulatory mechanisms for glycylation. By following axonemal tubulin immunoreactivity with anti-glycylated tubulin mAbs upon incubation with a Paramecium cellular extract, the presence of a deglycylation enzyme is revealed in the cytoplasm of this organism. These observations establish that polyglycylation is reversible and indicate that, in vivo, an equilibrium between glycylating and deglycylating enzymes might be responsible for the length of the oligoglycine side chains of tubulin.
INTRODUCTION
In eukaryotic cells, tubulin heterogeneity is generated at the genetic level and is considerably increased by several types of posttranslational modifications (PTMs)1 of primary gene products (for reviews, see MacRae, 1997; Ludueña, 1998). These tubulin PTMs are widely spread among species and they are found in diverse cell compartments. In ciliated protozoa and in flagellated and ciliated metazoan cells, immunoreactivity data indicated the existence of a particular PTM occurring in axonemal microtubules (Adoutte et al., 1985; 1991; Levilliers et al., 1995). The physico-chemical analysis of ciliary tubulin from the protist Paramecium led to the discovery of a new polymodification, polyglycylation, which consisted of an additional lateral chain of up to 34 glycine units on both axonemal tubulin subunits (Redeker et al., 1994). Since then, studies on polyglycylation, using either mass spectrometry (Rüdiger et al., 1995; Mary et al., 1996; Multigner et al., 1996; Weber et al., 1996) or two anti-glycylated tubulin monoclonal antibodies (mAbs), TAP 952 and AXO 49 (Bréet al., 1996), have involved principally axonemes of various cell types. Taken together, the data suggest that axonemal tubulin could be the preferred substrate for polyglycylation. This would contrast with the broad occurrence of another polymodification, polyglutamylation, in both cytoplasmic (Eddéet al., 1990; Alexander et al., 1991; Redeker et al., 1992; Rüdiger et al., 1992; Wolff et al., 1992; Mary et al., 1994) and axonemal tubulin (Bréet al., 1994; Fouquet et al., 1994; Mary et al., 1996; Schneider et al., 1997). Therefore, it may be asked whether polyglycylation is a selective marker of the most stable microtubules.
To determine whether the hyperstable microtubules represent the sole substrate for polyglycylation, we have investigated the glycylation status of cytoplasmic microtubules that correspond to the dynamic microtubules in Paramecium. The interest of this cellular model resides in the high glycylation level of its axonemal tubulin. Comparative immunoreactivity analyses of the tubulins from the two compartments show that they react differently with the two anti-glycylated tubulin mAbs, TAP 952 and AXO 49. Cytoplasmic tubulin was recognized solely by TAP 952, whereas axonemal tubulin reacted with both mAbs. Mass spectrometry analyses confirm that in Paramecium, cytoplasmic tubulin is polyglycylated, although at a much lower level than axonemal tubulin. To further understand the nature of the differences between the two polyglycylated tubulins, the molecular basis of the differential immunoreactivity of the two mAbs has been analyzed using glycylated synthetic tubulin peptides. Finally, the question of the mechanism accounting for the huge difference in the tubulin glycylation status between cytoplasmic and axonemal compartments has been addressed. Among the factors involved, the presence of a reverse enzyme activity has been investigated in the cytoplasm of Paramecium.
MATERIALS AND METHODS
Cells
Paramecium tetraurelia cells (strain d4–2) were grown at 27°C in phosphate buffered Wheat Grass Powder infusion supplemented with 0.4 μg β-sitosterol and bacterized with Klebsiella pneumoniae.
Paramecium Cytoplasmic Extract
The cytoplasmic extract was prepared by the method of Bréet al. (1994), which took advantage of the cold sensitivity of the intracytoplasmic microtubular network in Paramecium (Cohen and Beisson, 1988). Briefly, a cell pellet was resuspended in an equal volume of cold homogenization buffer (20 mM 2-morpholinoethanesulfonic acid, 2 mM EGTA, 1 mM MgCl2, 2 mM DTT, 0.34 M sucrose) supplemented with protease inhibitors (40 μg/ml leupeptin, 20 μg/ml pepstatin, 2 μg/ml o-phenantroline, 20 μg/ml aprotinin, 100 μg/ml ovomucoid trypsin inhibitor, 4 mM benzamidine, and 1 mM PMSF) and allowed to stand for 10 min at 4°C to depolymerize the intracytoplasmic microtubules before cell breakage with a Teflon-glass homogenizer. The crude extract was centrifuged at 6,000 × g for 5 min (SS-34 rotor, RC2-B centrifuge, Sorvall Instruments, Dupont, France), and then successively at 33,000 × g for 10 min (TL 100.3 rotor, TL-100 ultracentrifuge, Beckman, Gagny, France), and at 400,000 × g during 15 min (TLA-100.1 rotor, TL-100 ultracentrifuge). All steps were carried out at 4°C. The high-speed supernatant, referred to as cytoplasmic extract, was frozen and kept in liquid nitrogen until use.
Purification of Cytoplasmic and Axonemal Tubulins from Paramecium
Paramecium cytoplasmic tubulin was purified according to the method of Vallee and Collins (1986). The cytoplasmic extract was incubated for 30 min at room temperature with 20 μM taxol (kindly provided by Dr. D. Guénard, CNRS, Gif-sur-Yvette, France), followed by centrifugation through a sucrose cushion. Axonemal tubulin was purified from Paramecium cilia as previously described (Geuens et al., 1989). Cytoplasmic and axonemal tubulins were frozen and stored in liquid nitrogen until use.
Incubation of Paramecium Cytoplasmic Extract with Axonemal Tubulin
After thawing and centrifugation at 400,000 × g for 10 min, the Paramecium cytoplasmic extract, supplemented with protease inhibitors, was mixed with 3 μM axonemal tubulin. Incubation was carried out for up to 2 h at 33°C. At various time points, aliquots were taken and immediately boiled in sample buffer (Laemmli, 1970) and further processed for gel electrophoresis and immunoblotting.
Chemical Synthesis of Tubulin Peptides
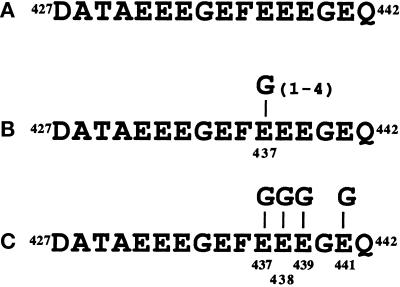
The glycylated peptides were synthesized by Neosystem (Strasbourg, France). Their structures are represented in Figure 4. They consist of the 16 residues −427 to 442 of the carboxy-terminal part of Paramecium β-tubulin (Dupuis, 1992) (Figure 4A), bearing either polyglycine chains of various lengths linked to the γ-carboxyl group of Glu437 residue (Figure 4B), or single glycine units added to each of the four glutamate residues, Glu437, Glu438, Glu439, and Glu441 (Figure 4C). Lyophilized synthetic peptides were dissolved either in 4 mM NaOH or in pure water and stored at −20°C.
Figure 4.
Structure of the synthetic peptides. (A) The peptide sequence corresponds to the carboxy-terminal residues 427–442 of Paramecium β-tubulin (β). (B) One to four glycine units are laterally linked to residue E437 of the above sequence (β-1 Gly, β-2 Gly, β-3 Gly, β-4 Gly). (C) One glycine unit is laterally linked to each of the residues, E437, E438, E439, and E441 of the sequence shown in panel A (β-4 × 1 Gly).
Preparation and Characterization of Paramecium Cytoplasmic Tubulin Carboxy-terminal Peptides
Paramecium tubulin was digested for 6 h at 36°C with endoproteinase Asp-N (1:400, wt/wt). Digestion products were separated by HPLC on an anion-exchange column (DEAE 5PW, Waters Associates, Waters, MA). They were further purified and desalted by reversed-phase HPLC on a C18 column (218TP52, Vydac, Hesperia, CA). Elution and detection conditions have been described previously (Redeker et al., 1994). For dot-blot analysis, the purified peptides were concentrated and recovered as previously reported (Bréet al., 1996). Reversed-phase purified peptides were sequenced by automated Edman degradation using a Procise pulsed-liquid protein sequencer (model 794, Perkin Elmer, Applied Biosystems Division, Norwalk, CT).
Mass Spectrometry
Mass spectra were recorded in positive and negative reflectron modes with a single-stage reflectron matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer (Voyager Elite, PerSeptive Biosystems, Framingham, CA) equipped with a delayed extraction device. α-Cyano-4-hydroxycinnamic acid (Sigma Chemical, St. Louis, MO) in the presence of pure nitrocellulose (0.45 μm pore size, from Bio-Rad [Richmond, CA] or from Millipore [Bedford, MA]) was the matrix used for all MALDI experiments. Thin-layer preparation of the samples and ionization conditions were performed as described by Vinh et al. (1997). External calibration was performed with a mixture of neurotensin and ACTH 18–39 and 7–38 clips (from Sigma), with monoisotopic m/z masses for [M+H]+ of 1672.92 Da, 2465.20 Da, and 3657.93 Da, respectively. Spectra were obtained with a resolving power M/ΔM = 2000 at 10% valley.
Antibodies
TAP 952 and AXO 49 mAbs, raised against Paramecium axonemal tubulin (Callen et al., 1994), are directed against polyglycylated tubulin (Bréet al., 1996). AXO 49 ascitic fluid was prepared by Dr. Jeanmaire-Wolf. 6-11B-1 mAb, directed against acetylated α-tubulin (Piperno and Fuller, 1985), was purchased from Sigma (Saint-Quentin Favallier, France). DM1A and DM1B anti-α- and anti-β-tubulin mAbs (Blose et al., 1984) were purchased from Amersham (Les Ulis, France). The peroxidase-labeled sheep anti-mouse IgG antibody was from Sanofi Diagnostics Pasteur (Marnes-la-Coquette, France).
Dot-Blot Analysis
Synthetic or Paramecium tubulin peptides were covalently bound to Immobilon-AV affinity membrane (Millipore, France), as previously described (Bréet al., 1996). The membrane was subsequently incubated for 2 h at room temperature with TAP 952 (1:50 to 1:300) or AXO 49 ascitic fluid (1:10,000 to 1:50,000) diluted in PBS containing 0.3% BSA and 0.1% Tween 20 (antibody buffer). After extensive washings with the same buffer, the dots were incubated with peroxidase-labeled secondary antibody (1:2,000). Detection was performed by enhanced chemiluminescence (ECL, Amersham). Exposure times were from 5 to 30 s.
Gel Electrophoresis and Immunoblotting
Proteins were separated by SDS-PAGE on 10% polyacrylamide mini-gels (Laemmli, 1970), containing 0.1% (wt/wt) SDS (99% pure; BDH, Poole, United Kingdom) at pH 8.3, according to Suprenant et al. (1985). Under these conditions, Paramecium α-tubulin migrates faster than β-tubulin. Proteins were stained with Coomassie blue or electro-transferred onto nitrocellulose by the method of Kyhse-Andersen (1984). The blots were stained with Ponceau red, washed in antibody buffer, and then incubated overnight with TAP 952 (1:100 to 1:500), AXO 49 ascitic fluid (1:10,000 to 1:50,000), 6-11B-1 (1:30,000), GT335 (1:10,000 to 1:30,000), DM1A (1:1,000 to 1:5,000), or DM1B (1:500), diluted with the latter buffer. After extensive washings, blots were incubated with peroxidase-labeled sheep anti-mouse IgG antibody and processed for ECL detection.
RESULTS
Cytoplasmic Tubulin from Paramecium Is Polyglycylated
The method of preparation of the cytoplasmic extract from Paramecium (cell homogenization at 4°C and at low ionic strength) was used to ensure selective depolymerization and recovery of the labile (intracytoplasmic) microtubules, whereas under these conditions the hyperstable and cold-resistant microtubules (such as those of the oral apparatus, contractile vacuoles, and cilia) are discarded.
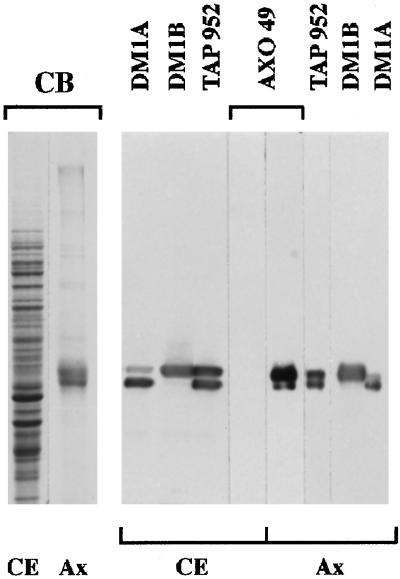
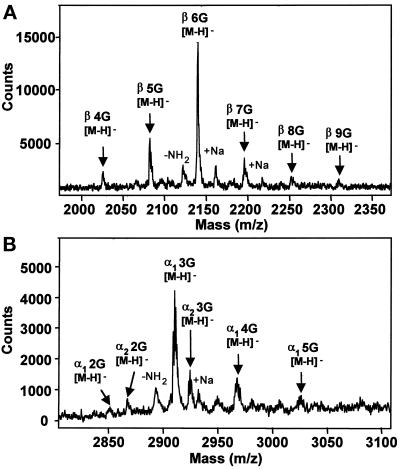
The α- and β-tubulin subunits in the cytoplasmic extract exhibited significant reactivity with the anti-glycylated tubulin mAb, TAP 952 (Figure 1), thereby indicating that cytoplasmic tubulin is glycylated. However, in contrast to Paramecium axonemal tubulin, which was labeled with both anti-glycylated tubulin mAbs, TAP 952 and AXO 49, cytoplasmic tubulin was not labeled with AXO 49. The huge difference in reactivity of the two types of tubulin with AXO 49 suggested that they might be differently glycylated. Consequently, we prepared cytoplasmic tubulin to specify its glycylation status. Tubulin recovered by centrifugation of the cytoplasmic extract after incubation with taxol was fairly pure, whereas the remaining supernatant was depleted of tubulin (Figure 2, lanes 1–3). Purified tubulin exhibited the same reactivity with TAP 952 and AXO 49 (Figure 2, lanes 5 and 7) as that of the initial extract (Figure 2, lanes 4 and 6), indicating that no change in glycylation level had occurred during purification. Edman degradation sequencing and mass spectrometry analyses of the purified carboxy-terminal peptides from cytoplasmic tubulin showed all of them to be glycylated, as previously found for axonemal tubulin (Redeker et al., 1994). The number of added glycine units ranged from 2 to 9 and from 1 to 5 for β- and α-tubulin, respectively (Figure 3, A and B), in contrast to axonemal β and α subunits, which are modified by the addition of 4–32 and 3–34 glycine units, respectively (Redeker et al., 1994). All the intermediate levels of glycylation were detected, as in axonemal tubulin. The hexaglycylated β and the triglycylated α peptides were the major forms found for cytoplasmic tubulin (Figure 3).
Figure 1.
Comparative distribution of glycylated tubulin isoforms in the soluble cytoplasmic pool (CE) and in axonemes (Ax) from Paramecium. The protein extracts were submitted to SDS-PAGE and stained with Coomassie blue (CB) or immunoblotted with TAP 952, AXO 49, DM1B, DM1A alone, or after DM1B. Protein amounts: 15 μg CE; 1.5 μg and 0.5 μg Ax for CB and immunoblotting, respectively. In contrast to axonemal tubulin, cytoplasmic tubulin is not reactive with AXO 49. However, at very long ECL exposure times, a faint staining of cytoplasmic tubulin with AXO 49 can be detected.
Figure 2.
Tubulin purification from the Paramecium cell extract, illustrated by Coomassie blue staining (CB) and immunoreactivity with TAP 952 and AXO 49. Cytoplasmic extract before (lanes 1, 4, and 6) and after (lane 2) taxol treatment; purified tubulin (lanes 3, 5, and 7). Protein amounts: lanes 1 and 2, 25 μg; lanes 4 and 6, 20 μg; lane 3, 2 μg; lanes 5 and 7, 0.5 μg.
Figure 3.
MALDI-TOF mass spectra of major reversed-phase HPLC fractions of Paramecium cytoplasmic β-tubulin (A) and α-tubulin (B) carboxy-terminal peptides. For β-tubulin (A), the major molecular ion of this series at m/z = 2138.9 corresponds to the theoretical monoisotopic mass of the hexaglycylated deprotonated β-tubulin carboxy-terminal peptide with the sequence 427DATAEEEGEFEEEGEQ442 ([M-H]-theoretical = 2138.8). The successive ions from this series are separated by mass increments of 57 atomic mass units (amu), the mass of one glycine unit. In this spectrum, β-tubulin carboxy-terminal peptides bear from four to nine additional glycine residues. For α-tubulin (B), the two major molecular ions of this series, at m/z = 2908.7 and 2922.6, correspond to the theoretical monoisotopic masses of the triglycylated deprotonated carboxy-terminal peptides of the α1-tubulin isotype with the sequence 424DLAALEKDYEEVGIETAEGEGEEGEG449 ([M-H]-theoretical = 2908.5), and of the α2-tubulin isotype with the sequence 424DLAALEKDYEEVGIETAEGEGEEGEA449 ([M-H]-theoretical = 2922.5), respectively. The α1-tubulin carboxy-terminal peptides gave more intense signals than the α2-tubulin ones. They carry from two to five additional glycine residues. Very minor reversed-phase fractions containing peptides corresponding to the lowest glycylation levels of β- and α-tubulin (bearing from two to three, and one glycine units, respectively) are not shown.
In conclusion, in Paramecium, cytoplasmic tubulin is polyglycylated, although at a much lower level than axonemal tubulin.
Evidence for the Presence of Short Oligoglycine Chains Distributed on Several Glycylation Sites in Cytoplasmic Tubulin
In the carboxy-terminal peptides from cytoplasmic β- and α-tubulin, respectively, the Glu437 and Glu445 residues were the first residues not to be detected by Edman degradation, as previously reported for axonemal tubulin (Redeker et al., 1994). This signifies that glycine units are linked at least to these glutamate residues. In either tubulin, cytoplasmic or axonemal, other glycylation sites at downstream glutamate residues cannot be excluded (Glu446, Glu448 in α-tubulin, and Glu438, Glu439, Glu441 in β-tubulin). In these studies, the overall number of glycine units, determined for each tubulin peptide by mass spectrometry, does not give any information about the length and the number of polyglycine chains.
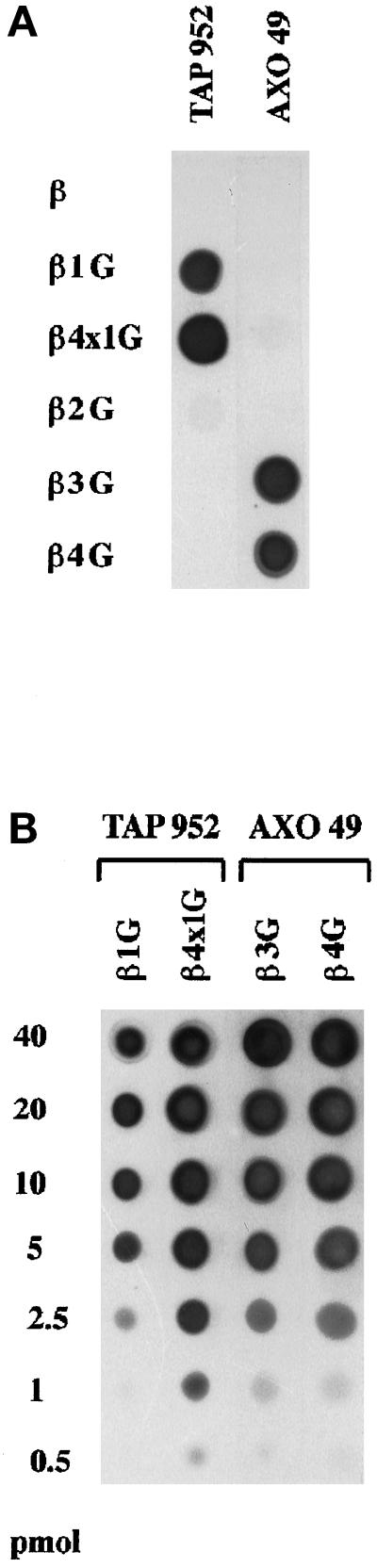
Previously, an immunological approach using the two mAbs, TAP 952 and AXO 49, revealed that these mAbs react differently with lowly and highly glycylated isoforms of Paramecium axonemal tubulin peptides (Bréet al., 1996). Therefore, we tried to determine whether the length of the polyglycine chains of cytoplasmic and axonemal tubulin could account for their differential reactivities with these mAbs. To approach this problem, synthetic peptides were designed to mimic presumptive structures of Paramecium tubulin peptides (see MATERIALS AND METHODS). They carried glycine units either bound as a single side chain of variable length to the previously identified glycylation site of β-tubulin, Glu437, or distributed as single units on each of the potential glycylation sites (Figure 4). These peptides were immunoprobed with TAP 952 and AXO 49 (Figure 5A). Neither of the antibodies recognized the unmodified β-tubulin carboxy-terminal sequence. TAP 952 strongly reacted with the β-1 Gly and β-4 × 1 Gly peptides. Its reactivity with β-4 × 1 Gly was higher than with β-1 Gly, the difference being magnified with antibody dilution (our unpublished results). In contrast, AXO 49 reacted strongly solely with the β-3 Gly and β-4 Gly peptides. It is worth noting that β-2 Gly was barely labeled by either of the two antibodies. The interactions between the mAbs and the synthetic peptides are concentration dependent, as expected, and peptide amounts as low as 1 pmol were detected by the two mAbs (Figure 5B).
Figure 5.
Immunoreactivities of TAP 952 (1:50) and AXO 49 (1:10,000) mAbs with β-tubulin synthetic peptides: β, β-1 Gly, β-4 × 1 Gly, β-2 Gly, β-3 Gly, β-4 Gly (β, β1G, β4 × 1G, β2G, β3G, β4G). Detection was carried out using ECL at the same exposure times for both mAbs. (A) Comparison of immunoreactivities of the two mAbs. The same quantity (160 pmol) of each peptide was dotted onto the membrane. (B) Sensitivities for the detection of the glycylated peptides. Picomole amounts are indicated on the left.
TAP 952 did not react with a peptide containing a monoglycylated glutamate (E) within a sequence unrelated to tubulin (AQGEEFGRSYEVHWKL). In addition, the synthetic peptide (DYEEVGIETAEGEGEEGEG), mimicking the carboxy-terminal end of the Paramecium α2-tubulin isotype, which possesses a glycine as last residue (Dupuis-Williams et al., 1996), was not reactive with TAP 952, thus showing that the lateral branching of the glycine unit to the tubulin sequence is required for recognition by this mAb.
In summary, the specificities of the two mAbs are different. TAP 952 exhibits a high affinity toward glycylated tubulin peptides bearing one glycine unit laterally linked either to one or to several specific glutamate residues, whereas AXO 49 specifically recognizes polyglycine chains of variable length from three residues upward.
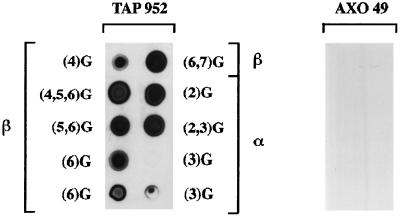
Since the length of the polyglycine side chain appeared to be responsible for the differential reactivity of the two mAbs, some of the purified cytoplasmic tubulin peptides were immunoprobed to examine the length of their polyglycine chains (Figure 6). The β- and α-tubulin peptides tested, bearing mostly from four to seven and from two to three extra glycine units, respectively, were all reactive with TAP 952, but not with AXO 49, as expected from the immunoreactivity of cytoplasmic tubulin (see Figure 2).
Figure 6.
Immunodot analysis of purified Paramecium cytoplasmic β- and α-tubulin peptides with TAP 952 and AXO 49. The nature of the peptide (β or α) tested with TAP 952 and the number of glycine units (G) per molecule (in parentheses) determined by mass spectrometry are indicated. Some fractions were heterogeneous and correspond to a mixture of isoforms comprising different levels of glycylation, as noted in parentheses. An estimated amount of 20 pmol of the peptides was bound to the membrane except for β(4)G, β(6)G, α(3)G for which 10 pmol were dotted. The two hexaglycylated β peptides, β(6)G, differ in the length of the peptide chain and result from complete (427DATAEEEGEFEEEGEQ442) or incomplete (417DLVSEYQQYQDATAEEEGEFEEEGEQ442) digestion with endoproteinase Asp-N. The two triglycylated α peptides α(3)G differ only by their salt content; the low peptide amount and a possible loss during concentration may account for the lack of reactivity of one of these α(3)G peptides. The same whole set of peptides was dotted on a second membrane and tested with AXO 49 following the same disposition; all peptides were unreactive with this antibody.
Therefore, given the respective specificities of the two mAbs, we can infer that most of the cytoplasmic tubulin contains at least one monoglycylated site per subunit and possibly additional sites bearing two glycine units (see DISCUSSION).
Evidence for the Existence of a Deglycylase Activity in a Cytoplasmic Extract of Paramecium
The presence of distinct glycylation levels of tubulin in Paramecium axonemal and cytoplasmic compartments raises the question as to the regulation of such PTM diversity. To account for the low glycylation level of cytoplasmic tubulin, one possible mechanism could involve a reverse deglycylation reaction.
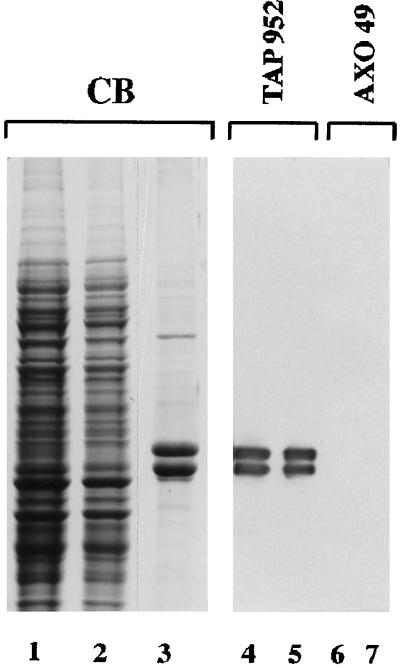
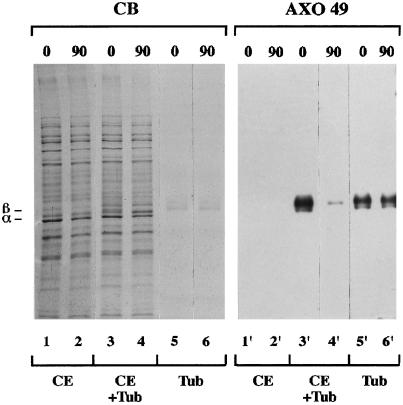
To search for the presence of a reverse enzymatic activity in the cytoplasmic compartment of Paramecium, the highly glycylated axonemal tubulin (Figure 7, lane 2) was incubated at 33°C for 1 h 30 min with the cellular extract (Figure 8, lane 3) and immunoprobed with AXO 49. Axonemal tubulin displayed a remarkable loss of immunoreactivity when it was incubated with the cytoplasmic extract (Figure 8, lanes 3, 4, 3′, and 4′), but not when it was incubated with the extraction buffer alone (Figure 8, lanes 5, 6, 5′, and 6′). No loss of reactivity was detected after incubation with the extract at 4°C (our unpublished results).
Figure 7.
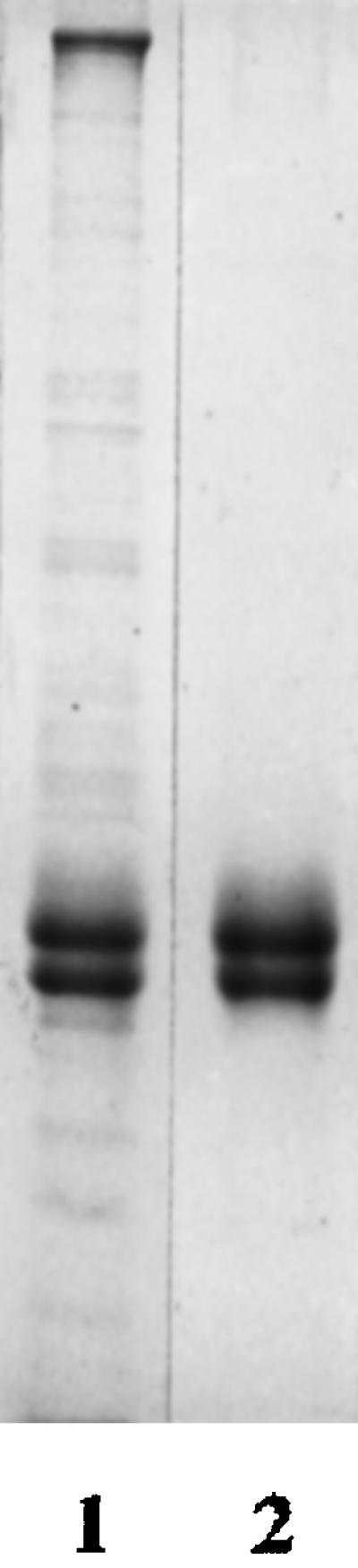
Coomassie blue staining of purified Paramecium axonemal tubulin (lane 2) from ciliary axonemes (lane 1). Protein amounts: lane 1, 2 μg; lane 2, 1 μg.
Figure 8.
Effect of Paramecium cytoplasmic extract on immunoreactivity of axonemal tubulin with AXO 49. The cytoplasmic extract either alone (CE, lanes 1, 2, 1′, and 2′), or in the presence of axonemal tubulin (CE + Tub, lanes 3, 4, 3′, and 4′), or tubulin alone (Tub, lanes 5, 6, 5′, and 6′) was incubated at 33°C. Incubation times (minutes) are noted above the immunoblot. Protein amounts: 4 μg CE and 0.2 μg Tub for Commassie blue staining (CB) and immunoblotting. The positions of α- and β-tubulin subunits are indicated on the left.
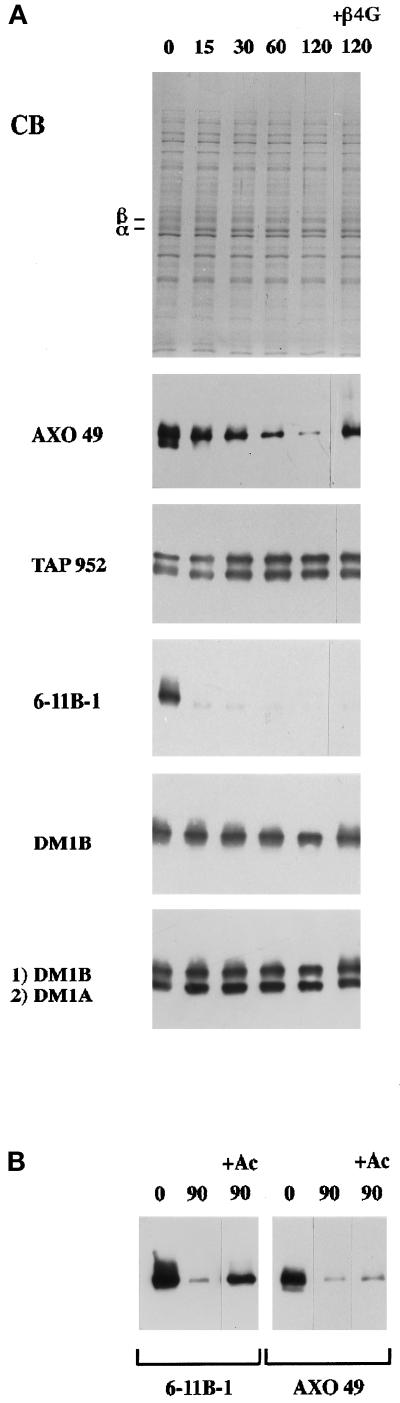
To follow the enzymatic reactions occurring in the course of incubation of axonemal tubulin with the cytoplasmic extract, samples were taken at various time points and immunoprobed with the anti-glycylated tubulin (AXO 49, TAP 952) or anti-acetylated tubulin (6-11B-1) antibodies, as well as with anti-tubulin sequence antibodies (DM1B, DM1A) (Figure 9A). AXO 49 reactivity progressively decreased to become almost undetectable after 2 h of incubation, whereas concomitantly TAP 952 reactivity increased. The opposite variations in reactivity observed with the two anti-glycylated tubulin mAbs suggest that a deglycylation reaction occurs progressively but not completely. 6-11B-1 reactivity dropped rapidly within the first 15 min. This reveals that, in addition to deglycylation, a deacetylation reaction takes place that proceeds quickly and reaches completion during the incubation. Noticeably, when axonemal tubulin was first mixed with the cytoplasmic extract, the bulk of the α-tubulin appeared as a doublet, and during the course of the incubation the upper band shifted toward the lower one (in Figure 8, compare lanes 3 and 4). This probably results from deacetylation of axonemal tubulin, which reaches completion during the same time frame (Figure 9A, CB and 6-11B-1, 15 min). In agreement with this inference, chemical acetylation of porcine brain tubulin has been shown to slow down its migration (Callen et al., 1994).
Figure 9.
Enzymatic reactions occurring in the course of incubation of axonemal tubulin with the Paramecium cytoplasmic extract. Times (minutes) are noted at the top of the figure. Protein amounts: 4 μg extract and 0.2 μg tubulin. (A) Kinetics of reactions and immunoreactivity with anti-tubulin PTM mAbs (AXO 49, TAP 952, or 6-11B-1), and anti-tubulin sequence mAbs (DM1B, or DM1B followed by DM1A) in the absence, or in the presence of 3 mM β-4 Gly peptide (+β4G). The migration levels of α- and β-tubulin bands are indicated on the Coomassie blue-stained gel (CB). (B) Immunoblotting analysis with 6-11B-1 and AXO 49 mAbs in the absence or in the presence of 20 mM sodium acetate (+Ac).
When the β-4 Gly peptide, a putative competitor for the deglycylation reaction, was added from the outset of incubation, the decrease in axonemal tubulin reactivity with AXO 49 was partially inhibited (Figure 9A, AXO 49). This peptide inhibited deglycylation specifically, since the other reverse reaction, deacetylation, was not affected (Figure 9A, 6–11B-1). Noticeably, the upward smear observed upon β-tubulin staining with DM1B, corresponding to highly modified axonemal tubulin isoforms (see Levilliers et al., 1995), disappeared after 2 h of incubation with the cytoplasmic extract, except in the presence of the deglycylation inhibitor (Figure 9A, DM1B). Conversely, sodium acetate was able to inhibit deacetylation specifically, without altering deglycylation (Figure 9B, 6–11B-1 and AXO 49). In this case, the smear of 6-11B-1 reactivity with α-tubulin was no longer observed (Figure 9B, 6–11B-1). This might be due to deglycylation of acetylated isoforms, leading to a downward shift in their migration. In addition, as is the case for tubulin deacetylation in Chlamydomonas (Piperno et al., 1987), butyric acid, an inhibitor of histone deacetylases (Allfrey et al., 1984), had no effect upon deacetylation of Paramecium axonemal tubulin under the present reaction conditions.
All these observations argue in favor of specific deglycylation and deacetylation reactions and provide evidence for the presence of enzymes reversing tubulin PTMs, namely a deglycylase and a deacetylase, in the cytoplasm of Paramecium.
DISCUSSION
Partition of Polyglycylated Tubulin Isoforms between the Dynamic Cytoplasmic and the Stable Axonemal Microtubules of Paramecium
We have shown that the two mAbs, TAP 952 and AXO 49, serve as complementary tools for detection of mono- and polyglycylated tubulin. Immunoblotting with these two mAbs and mass spectrometry analyses allowed us to establish that, in Paramecium, cytoplasmic tubulin is polyglycylated. This includes both cold-labile microtubules and the soluble pool of tubulin. Therefore, in Paramecium, the hyperstable microtubules do not represent the exclusive substrate for polyglycylation. This is the first example of polyglycylation of cytoplasmic tubulin. Other studies involving metazoan cells, both with and without axonemes, showed that cytoplasmic microtubules were not reactive with either of the two anti-glycylated tubulin mAbs, although cilia were reactive (Callen et al., 1994; Million and Tournier, unpublished results; Kann, Prigent, Levilliers, Bré, and Fouquet, in preparation). The difference in cytoplasmic microtubule reactivity between ciliated metazoa and protozoa could reflect either axonemal segregation of the glycylase in metazoan cells only, or the requirement for an axonemal-specific sequence for glycylase recognition in both metazoa and protozoa. The latter possibility is consistent with: 1) the existence of axonemal-specific tubulin isotypes in metazoa (see Raff, 1994; Hutchens et al., 1997); 2) the presence of a restricted pool of identical tubulin isotypes in the axonemes and the cytoplasm of ciliated and flagellated protists, and the assumption that, in consequence of the high constraints imposed by axoneme assembly, an axonemal signature would have been selected for within the set of protist tubulin isotypes (see Silflow, 1991; Gaertig et al., 1993; Raff et al., 1997). In fact, in Paramecium, the unique β-tubulin isotype (Dupuis-Williams, Neveu, and Klotz, in preparation) and the two α1 and α2 isotypes, which differ only in their last residue (Dupuis-Williams et al., 1996), are present in the cytoplasm (this report) as well as in the axonemes (Redeker et al., 1994), and all are glycylated. Accordingly, tubulin of axonemal type would then be the preferred substrate for polyglycylation.
The overall glycylation level of cytoplasmic tubulin is much lower than that of axonemal tubulin. This is unlikely to be due to an artefactual deglycylation occurring during the preparation of the cytoplasmic extract given that all the steps were carried out at 2–4°C, and at this temperature no deglycylase activity was detected in the extract.
The data on synthetic peptides show a higher reactivity of TAP 952 with the β-4 × 1 Gly peptide compared with β-1 Gly. This observation, together with a mutagenic analysis of the site(s) of α-tubulin polyglycylation in Tetrahymena thermophila in vivo (Hai, Gaertig, Xia, Levilliers, Bré, and Gorovsky, unpublished results), provides evidence that both mAbs, TAP 952 and AXO 49, are able to recognize glycine units bound to the identified glycylation sites and also to downstream glutamate residues. Thus, in the major hexaglycylated β peptide of Paramecium cytoplasmic tubulin, which is solely reactive with TAP 952, the glycine units would be expected to be distributed on all four potential glycylation sites: two monoglycylated sites recognized by TAP 952 alone, in addition to two biglycylated ones. In conclusion, the exclusive reactivity of TAP 952 with the major polyglycylated tubulin peptides suggests that most of the cytoplasmic tubulin displays a narrow panel of side chain lengths of 1 or 2 glycine units distributed on several modification sites. In axonemal tubulin, which carries up to 34 glycine units, the polyglycylated peptides reactive with both mAbs, TAP 952 and AXO 49 (Bréet al., 1996), would also possess several glycylation sites. Some sites would be monoglycylated, while others would bear polyglycine chains of variable length.
The multiplicity of sites seems to be a particular feature of polyglycylation, since this has never been reported for polyglutamylation, the other polymodification of tubulin. Thus polyglycylation, compared with other PTMs, has a higher potentiality to increase tubulin heterogeneity.
In summary, the present data have revealed that, at least in Paramecium, polyglycylation occurs not only in hyperstable microtubules, but also in dynamic ones. All cytoplasmic and axonemal tubulin peptides analyzed were glycylated. Therefore, in contrast to other PTMs, polyglycylation seems to affect the whole tubulin pool of this cell, although differentially. We show here a difference in tubulin glycylation level and side chain length between the axonemal and cytoplasmic compartments. Thus, this polymodification exhibits a peculiar capacity to differentiate distinct classes of microtubules. The data also suggest the presence of several glycylation sites on a single tubulin polypeptide, which fits well with most recent results obtained using a newly elaborated strategy of peptide fragmentation analyzed by mass spectrometry (Vinh et al., 1997). This fragmentation approach will be applied to a comparative structural study of axonemal and cytoplasmic tubulins from Paramecium, to visualize the variety of distributions of polyglycine chains added onto the molecules of the two tubulin pools. In cells where purified tubulin and/or polyglycylated isoform amounts are low, the combined use of the two specific antibodies will be suitable to examine the distribution of mono- and polyglycylated tubulin isoforms.
Polyglycylation, a Strategy for Microtubule Differentiation?
In Paramecium (Dupuis, 1992; Dupuis-Williams et al., 1996), as in other ciliates and in flagellates, the number of tubulin genes is reduced (Little and Seehaus, 1988; Silflow, 1991; Gaertig et al., 1993), in spite of the complexity of the microtubular cytoskeleton (Cohen et al., 1982; Cohen and Beisson, 1988; Fleury et al., 1995). In these cells, therefore, the combination of PTMs is likely to be a determinant for microtubule differentiation.
Compared with tubulins from the various species investigated so far, tubulin from Paramecium remains the most highly glycylated, both in terms of the extent and the level of glycylation. Indeed, the analysis of tubulin from sea urchin sperm (Mary et al., 1996; Multigner et al., 1996), bull sperm (Rüdiger et al., 1995), and Giardia lamblia cytoskeleton (Weber et al., 1996) show respective glycylation levels of up to 12, 13, and 23 glycine units per polypeptide chain, as well as substantial amounts of unglycylated carboxy-terminal tubulin peptides. Thus, the Paramecium cell represents a challenge to the question of the biological significance of tubulin PTMs, and especially polyglycylation.
At each generation, the Paramecium cell transmits and duplicates permanent microtubular networks and depolymerizes others before reassembling them in two copies. Consequently, the cell has to organize, spatially and temporally, various complex microtubular networks in a polarized manner from an almost homogeneous pool of tubulin isotypes. Our results reveal an asymmetrical partition of glycylated tubulin isoforms generated by a diversity of side chain lengths. “Short” chains in tubulin molecules appear to be associated with the soluble tubulin and dynamic microtubules, as well as with the hyperstable ones. In contrast, longer chains could represent a set of markers of the most stable microtubular structures. These results shed some light on immunofluorescence data concerning the morphogenesis of some hyperstable microtubular structures (such as the postoral fiber and the contractile vacuole rootlets) during cell division; in particular, the sequential appearance of TAP 952 and AXO 49 epitopes on newly assembled microtubules (Fleury et al., 1995) can be explained by a delay between the addition of the first glycine units and the lengthening of the polyglycine chains. Thus, in contrast to spermatozoa, in which glycylation takes place in axonemal microtubules only at the end of spermiogenesis (Bréet al., 1996; Iomini et al., 1998), in the Paramecium cell, it is the length of polyglycine chains that discriminates between young and mature stable microtubular assemblies. Therefore, regardless of the way of differentiating structures of distinct ages, either by the absence or presence of glycylation or by the side chain lengths, (poly)glycylation appears to be a marker of maturation during cell morphogenesis. In addition, polyglycylation has been postulated to be involved in spermatozoan motility (Bréet al., 1996).
The various glycylated isoforms within a cell could modulate tubulin association with diverse molecules aimed at distinct functions such as scaffolding of complex structures, microtubule stability, and axonemal motility.
In the ciliated protist, Tetrahymena, where gene replacement is feasible (Gaertig et al., 1994; Hai and Gorovsky, 1997), tubulin is also reactive with the two anti-glycylated tubulin antibodies. Using genetically engineered tubulin genes in this ciliate, an attempt to specify the in vivo role of polyglycylation in microtubule function is in progress.
The Length of the Oligoglycine Side Chain of Tubulin Is Regulated by an Equilibrium between Glycylating and Deglycylating Enzymes
To achieve tubulin polyglycylation, at least two classes of enzymes are expected to be required: one for the linkage of the first glycine of the nascent side chain to a glutamate residue of the tubulin polypeptide chain through a Glu γCOOH-Gly αNH2 isopeptide bond (Glu–Gly), and a second one for the linkage of the following glycine residues through Gly αCOOH-Gly αNH2 peptide bonds (Gly–Gly). If the glycylases operate with a strong selectivity toward each site of glycylation, more than one enzyme of the first class would be expected to catalyze polyglycylation of each tubulin subunit.
Within the Paramecium β-tubulin sequence (see Figure 4), it is striking that the sequence motif EEEGE is repeated almost in tandem; however, only the downstream 437EEEGE441 motif is glycylatable. Thus a larger motif than EEEGE must account for the strong selectivity of glycylases. Noticeably, the glycylatable sequence overlaps with the axonemal signature proposed by Raff et al. (1997).
Using the highly glycylated axonemal tubulin as substrate, and the TAP 952 and AXO 49 mAbs to trace the presence of mono- and polyglycylated sites in tubulin, the existence of a deglycylating enzymatic activity has been detected in the cytoplasm of Paramecium. These data suggest that, in the cytoplasm, the length of Paramecium tubulin oligoglycine chains is regulated by an equilibrium between glycylating and deglycylating enzymes. The presence of all the intermediate levels of glycylation for axonemal and cytoplasmic tubulin peptides detected by mass spectrometry, as well as the progressive deglycylation of axonemal tubulin upon incubation with the cytoplasmic extract, suggest the existence of sequential mechanisms of glycylation or deglycylation, involving a unit-by-unit addition or removal of glycine moieties. The TAP 952 reactivity increase during incubation of axonemal tubulin with the cytoplasmic extract implies a shortening of the polyglycine side chains, with at least one glycine residue per subunit remaining resistant to deglycylation. Therefore, the deglycylating enzymes may not have cleaved the amide link between the α-amino group of the first glycine residue and the γ-carboxylic group of the glutamate residue. This would account for the low average glycylation level of tubulin in the cytoplasmic compartment with respect to the axonemal compartment.
It is worth comparing the data on enzyme regulation of tubulin acetylation and polyglycylation in the two protozoa, Chlamydomonas and Paramecium. Unlike polyglycylated tubulin, which is found within both axonemal and cytoplasmic compartments of a single cell, acetylated tubulin is mainly localized in flagella or cilia (Greer et al., 1985; LeDizet and Piperno, 1986; Cohen and Beisson, 1988; Adoutte et al., 1991; Fleury et al., 1995). Rather than resulting from a strict segregation of the acetylase in the cilia, the low extent of acetylation in the cytoplasm reflects a balance between two opposing enzymes (L’Hernault and Rosenbaum, 1983; Greer et al., 1985; Maruta et al., 1986), as inferred for polyglycylation. Nevertheless, the lack of detection of deacetylase activity in flagella (Maruta et al., 1986) could indicate a segregation of the reverse reaction enzyme in the cytoplasm.
In the case of polyglutamylation in cultured mouse brain neurons, the presence of two opposing enzymatic activities has also been demonstrated (Audebert et al., 1993).
To assign modified tubulin isoforms to one cell compartment or to generate differentially polymodified tubulin isoforms in distinct compartments, a more general mechanism could therefore involve the distribution of a set of opposing enzymes throughout the cell, associated to distinct effectors (activators or inhibitors) of the enzymatic equilibrium (Maruta et al., 1986) in each compartment. Such a situation potentially offers to the cell the advantage of a rapid adaptation to diverse signals or stresses.
Paramecium, which exhibits the highest rate of tubulin glycylation described to date, should be a particularly good system for the purification of the enzymes involved in polyglycylation. In the future, inhibition of these enzymes or the knockout of the corresponding genes could provide a means to specify the function of polyglycylation.
Note added in proof. Our studies have provided evidence for a multiplicity of glycylation sites in Paramecium α- and β-tubulin. Very recently, the occurrence of several sites has also been reported for the other polymodification of tubulin, polyglutamylation (Schneider et al., 1998; Redeker, Rossier, and Frankfurter, unpublished data).
ACKNOWLEDGMENTS
We are grateful to Professor A. Adoutte for his interest in this work and for critical reading of the manuscript. We also would like to thank the colleagues who provided us with antibodies. We thank F. Iftode for help in Paramecium culture, L. Elu for photographic work and picture editing, and C. Couanon for manuscript editing.This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Université Paris-Sud, and the Association pour la Recherche contre le Cancer (ARC, France). It represents a part of the doctoral thesis of J. Vinh, who received a predoctoral fellowship from both the CNRS and Synthelabo Recherche (France), and grants from the Association pour le Developpement de la Formation par la Recherche Biomédicale (ADFRB, France).
Abbreviations used:
- MALDI-TOF
matrix-assisted laser desorption ionization-time of flight
- PTM
posttranslational modification
REFERENCES
- Adoutte A, Claisse M, Maunoury R, Beisson J. Tubulin evolution: ciliate-specific epitopes are conserved in the ciliary tubulin of metazoa. J Mol Evol. 1985;22:220–229. doi: 10.1007/BF02099751. [DOI] [PubMed] [Google Scholar]
- Adoutte A, Delgado P, Fleury A, Levilliers N, Lainé MC, Marty MC, Boisvieux-Ulrich E, Sandoz D. Microtubule diversity in ciliated cells: evidence for its generation by post-translational modification in the axonemes of Paramecium and quail oviduct cells. Biol Cell. 1991;71:227–245. doi: 10.1016/0248-4900(91)90069-y. [DOI] [PubMed] [Google Scholar]
- Alexander JE, Hunt DF, Lee MK, Shabanowitz J, Michel H, Berlin SC, Macdonald TL, Sundberg RJ, Rebhun LI, Frankfurter A. Characterization of posttranslational modifications in neuron-specific class III β-tubulin by mass spectrometry. Proc Natl Acad Sci USA. 1991;88:4685–4689. doi: 10.1073/pnas.88.11.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allfrey VG, Di Paola EA, Sterner R. Protein side-chain acetylations. Methods Enzymol. 1984;107:224–240. doi: 10.1016/0076-6879(84)07014-2. [DOI] [PubMed] [Google Scholar]
- Audebert S, Desbruyères E, Gruszczynski C, Koulakoff A, Gros F, Denoulet P, Eddé B. Reversible polyglutamylation of α- and β-tubulin and microtubule dynamics in mouse brain neurons. Mol Biol Cell. 1993;4:615–626. doi: 10.1091/mbc.4.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blose SH, Melzer DI, Feramisco JR. 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J Cell Biol. 1984;98:847–858. doi: 10.1083/jcb.98.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bré MH, de Néchaud B, Wolff A, Fleury A. Glutamylated tubulin probed in ciliates with the monoclonal antibody GT335. Cell Motil Cytoskeleton. 1994;27:337–349. doi: 10.1002/cm.970270406. [DOI] [PubMed] [Google Scholar]
- Bré MH, et al. Axonemal tubulin polyglycylation probed with two monoclonal antibodies: widespread evolutionary distribution, appearance during spermatozoan maturation and possible function in motility. J Cell Sci. 1996;109:727–738. doi: 10.1242/jcs.109.4.727. [DOI] [PubMed] [Google Scholar]
- Callen AM, et al. Isolation and characterization of libraries of monoclonal antibodies directed against various forms of tubulin in Paramecium. Biol Cell. 1994;81:95–119. doi: 10.1016/s0248-4900(94)80002-2. [DOI] [PubMed] [Google Scholar]
- Cohen J, Adoutte A, Grandchamp S, Houdebine LM, Beisson J. Immunocytochemical study of microtubular structures throughout the cell cycle of Paramecium. Biol Cell. 1982;44:35–44. [Google Scholar]
- Cohen J, Beisson J. The cytoskeleton. In: Görtz H-D, editor. Paramecium. Berlin: Springer-Verlag; 1988. pp. 363–392. [Google Scholar]
- Dupuis P. The beta-tubulin genes of Paramecium are interrupted by two 27 bp introns. EMBO J. 1992;11:3713–3719. doi: 10.1002/j.1460-2075.1992.tb05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis-Williams P, Klotz C, Mazarguil H, Beisson J. The tubulin gene family of Paramecium: characterization and expression of the αPT1 and αPT2 genes which code for α-tubulins with unusual C-terminal amino acids, GLY and ALA. Biol Cell. 1996;87:83–93. doi: 10.1016/s0248-4900(97)89840-1. [DOI] [PubMed] [Google Scholar]
- Eddé B, Rossier J, Le Caer JP, Desbruyères E, Gros F, Denoulet P. Posttranslational glutamylation of α-tubulin. Science. 1990;247:83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Fleury A, Callen AM, Bré MH, Iftode F, Jeanmaire-Wolf R, Levilliers N, Clérot JC. Where and when is microtubule diversity generated in Paramecium? Immunological properties of microtubular networks in the interphase and dividing cells. Protoplasma. 1995;189:37–60. [Google Scholar]
- Fouquet JP, Eddé B, Kann ML, Wolff A, Desbruyères E, Denoulet P. Differential distribution of glutamylated tubulin during spermatogenesis in mammalian testis. Cell Motil Cytoskeleton. 1994;27:49–58. doi: 10.1002/cm.970270106. [DOI] [PubMed] [Google Scholar]
- Gaertig J, Gu L, Hai B, Gorovsky MA. High frequency vector-mediated transformation and gene replacement in Tetrahymena. Nucleic Acids Res. 1994;22:5391–5398. doi: 10.1093/nar/22.24.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertig J, Thatcher TH, McGrath KE, Callahan RC, Gorovsky MA. Perspectives on tubulin isotype function and evolution based on the observation that Tetrahymena thermophila microtubules contain a single α- and β-tubulin. Cell Motil Cytoskeleton. 1993;25:243–253. doi: 10.1002/cm.970250305. [DOI] [PubMed] [Google Scholar]
- Geuens G, Hill AM, Levilliers N, Adoutte A, DeBrabander M. Microtubule dynamics investigated by microinjection of Paramecium axonemal tubulin: lack of nucleation but proximal assembly of microtubules at the kinetochore during prometaphase. J Cell Biol. 1989;108:939–953. doi: 10.1083/jcb.108.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer K, Maruta H, L’Hernault SW, Rosenbaum JL. α-Tubulin acetylase activity in isolated Chlamydomonas flagella. J Cell Biol. 1985;101:2081–2084. doi: 10.1083/jcb.101.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai B, Gorovsky MA. Germ-line knockout heterokaryons of an essential α-tubulin gene enable high-frequency gene replacement and a test of gene transfer from somatic to germ-line nuclei in Tetrahymena thermophila. Proc Natl Acad Sci USA. 1997;94:1310–1315. doi: 10.1073/pnas.94.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens JA, Hoyle HD, Turner FR, Raff EC. Structurally similar Drosophila α-tubulins are functionally distinct in vivo. Mol Biol Cell. 1997;8:481–500. doi: 10.1091/mbc.8.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iomini C, Bré MH, Levilliers N, Justine JL. Tubulin polyglycylation in Platyhelminthes: diversity among stable microtubule networks and very late occurrence during spermiogenesis. Cell Motil Cytoskeleton. 1998;39:318–330. doi: 10.1002/(SICI)1097-0169(1998)39:4<318::AID-CM6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984;10:203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeDizet M, Piperno G. Cytoplasmic microtubules containing acetylated α-tubulin in Chlamydomonas reinhardtii: spatial arrangement and properties. J Cell Biol. 1986;103:13–22. doi: 10.1083/jcb.103.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levilliers N, Fleury A, Hill AM. Monoclonal and polyclonal antibodies detect a new type of post-translational modification of axonemal tubulin. J Cell Sci. 1995;108:3013–3028. doi: 10.1242/jcs.108.9.3013. [DOI] [PubMed] [Google Scholar]
- L’Hernault SW, Rosenbaum JL. Chlamydomonas α-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol. 1983;97:258–263. doi: 10.1083/jcb.97.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M, Seehaus T. Comparative analysis of tubulin sequences. Comp Biochem Physiol. 1988;90B:655–670. doi: 10.1016/0305-0491(88)90320-3. [DOI] [PubMed] [Google Scholar]
- Ludueña RF. Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Tubulin post-translational modifications. Enzymes and their mechanisms of action. Eur J Biochem. 1997;244:265–278. doi: 10.1111/j.1432-1033.1997.00265.x. [DOI] [PubMed] [Google Scholar]
- Maruta H, Greer K, Rosenbaum JL. The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J Cell Biol. 1986;103:571–579. doi: 10.1083/jcb.103.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary J, Redeker V, Le Caer JP, Promé JC, Rossier J. Class I and IVa β-tubulin isotypes expressed in adult mouse brain are glutamylated. FEBS Lett. 1994;353:89–94. doi: 10.1016/0014-5793(94)01018-8. [DOI] [PubMed] [Google Scholar]
- Mary J, Redeker V, Le Caer JP, Rossier J, Schmitter JM. Posttranslational modifications in the C-terminal tail of axonemal tubulin from sea urchin sperm. J Biol Chem. 1996;271:9928–9933. doi: 10.1074/jbc.271.17.9928. [DOI] [PubMed] [Google Scholar]
- Multigner L, Pignot-Paintrand I, Saoudi Y, Job D, Plessmann U, Rüdiger M, Weber K. The A and B tubules of the outer doublets of sea urchin sperm axonemes are composed of different tubulin variants. Biochemistry. 1996;35:10862–10871. doi: 10.1021/bi961057u. [DOI] [PubMed] [Google Scholar]
- Piperno G, Fuller MT. Monoclonal antibodies specific for an acetylated form of α-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985;101:2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, LeDizet M, Chang X-j. Microtubules containing acetylated α-tubulin in mammalian cells in culture. J Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff EC. Microtubules. J.S. Hyams and C.W. Lloyd, New York: Wiley-Liss; 1994. The role of multiple tubulin isoforms in cellular microtubule function; pp. 85–109. [Google Scholar]
- Raff EC, Fackenthal JD, Hutchens JA, Hoyle HD, Turner FR. Microtubule architecture specified by a β-tubulin isoform. Science. 1997;275:70–73. doi: 10.1126/science.275.5296.70. [DOI] [PubMed] [Google Scholar]
- Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, Bré MH. Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science. 1994;266:1688–1691. doi: 10.1126/science.7992051. [DOI] [PubMed] [Google Scholar]
- Redeker V, Melki R, Promé D, Le Caer JP, Rossier J. Structure of tubulin C-terminal domain obtained by subtilisin treatment. The major α and β tubulin isotypes from pig brain are glutamylated. FEBS Lett. 1992;313:185–192. doi: 10.1016/0014-5793(92)81441-n. [DOI] [PubMed] [Google Scholar]
- Rüdiger M, Plessman U, Klöppel K-D, Wehland J, Weber K. Class II tubulin, the major brain β tubulin isotype is polyglutamylated on glutamic acid residue 435. FEBS Lett. 1992;308:101–105. doi: 10.1016/0014-5793(92)81061-p. [DOI] [PubMed] [Google Scholar]
- Rüdiger M, Plessmann U, Rüdiger AH, Weber K. β tubulin of bull sperm is polyglycylated. FEBS Lett. 1995;364:147–151. doi: 10.1016/0014-5793(95)00373-h. [DOI] [PubMed] [Google Scholar]
- Schneider A, Plessmann U, Felleisen R, Weber K. Posttranslational modifications of trichomonad tubulins: identification of multiple glutamylation sites. FEBS Lett. 1998;429:399–402. doi: 10.1016/s0014-5793(98)00644-9. [DOI] [PubMed] [Google Scholar]
- Schneider A, Plessmann U, Weber K. Subpellicular and flagellar microtubules of Trypanosoma brucei are extensively glutamylated. J Cell Sci. 1997;110:431–437. doi: 10.1242/jcs.110.4.431. [DOI] [PubMed] [Google Scholar]
- Silflow CD. Why do tubulin gene families lack diversity in flagellate/ciliate protists? Protoplasma. 1991;164:9–11. [Google Scholar]
- Suprenant KA, Hays E, LeCluyse E, Dentler WL. Multiple forms of tubulin in the cilia and cytoplasm of Tetrahymena thermophila. Proc Natl Acad Sci USA. 1985;82:6908–6912. doi: 10.1073/pnas.82.20.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Collins CA. Purification of microtubules and microtubule-associated proteins from sea urchin eggs and cultured mammalian cells using taxol, and use of exogenous taxol-stabilized brain microtubules for purifying microtubule-associated proteins. Methods Enzymol. 1986;134:116–127. doi: 10.1016/0076-6879(86)34080-1. [DOI] [PubMed] [Google Scholar]
- Vinh J, Loyaux D, Redeker V, Rossier J. Sequencing branched peptides with CID/PSD MALDI-TOF in the low-picomole range: application to the structural study of the posttranslational polyglycylation of tubulin. Anal Chem. 1997;69:3979–3985. doi: 10.1021/ac970449j. [DOI] [PubMed] [Google Scholar]
- Weber K, Schneider A, Müller N, Plessmann U. Polyglycylation of tubulin in the diplomonad Giardia lamblia, one of the oldest eukaryotes. FEBS Lett. 1996;393:27–30. doi: 10.1016/0014-5793(96)00848-4. [DOI] [PubMed] [Google Scholar]
- Wolff A, de Néchaud B, Chillet D, Mazarguil H, Desbruyères E, Audebert S, Eddé B, Gros F, Denoulet P. Distribution of glutamylated α and β-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur J Cell Biol. 1992;59:425–432. [PubMed] [Google Scholar]