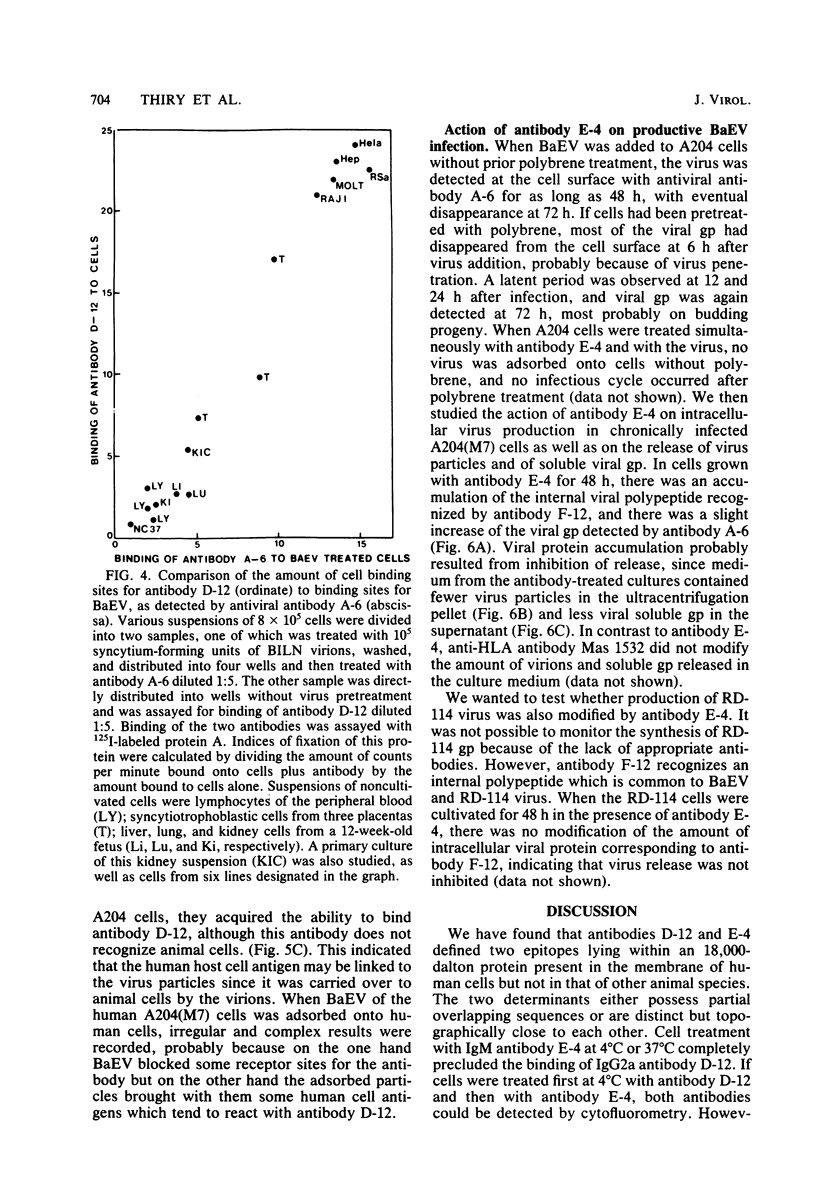
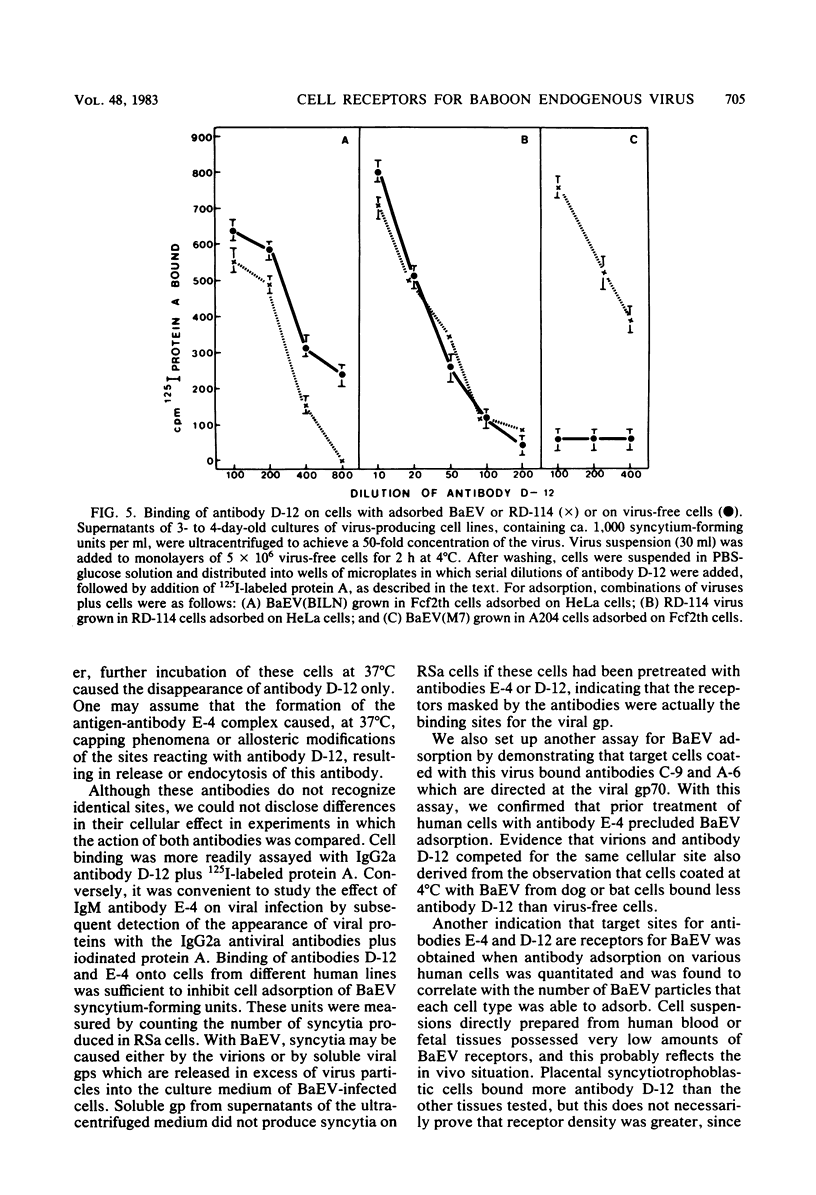
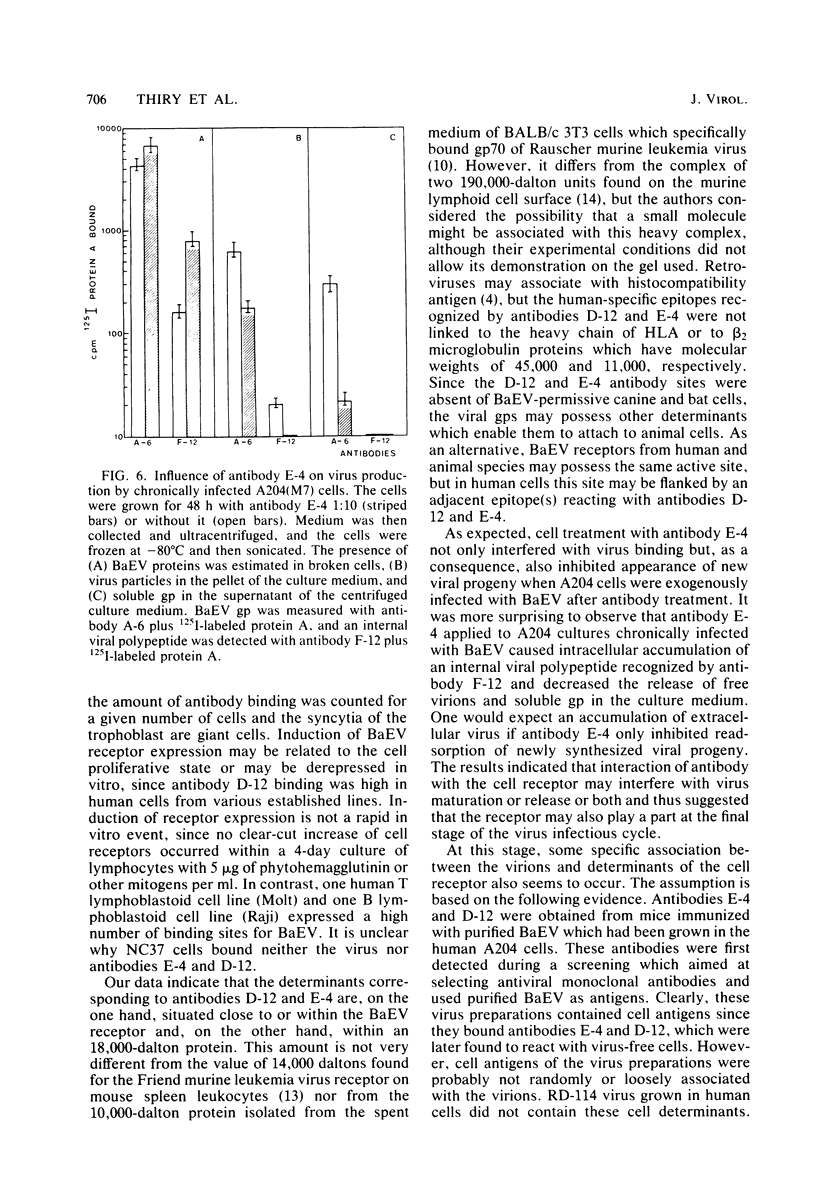
Abstract
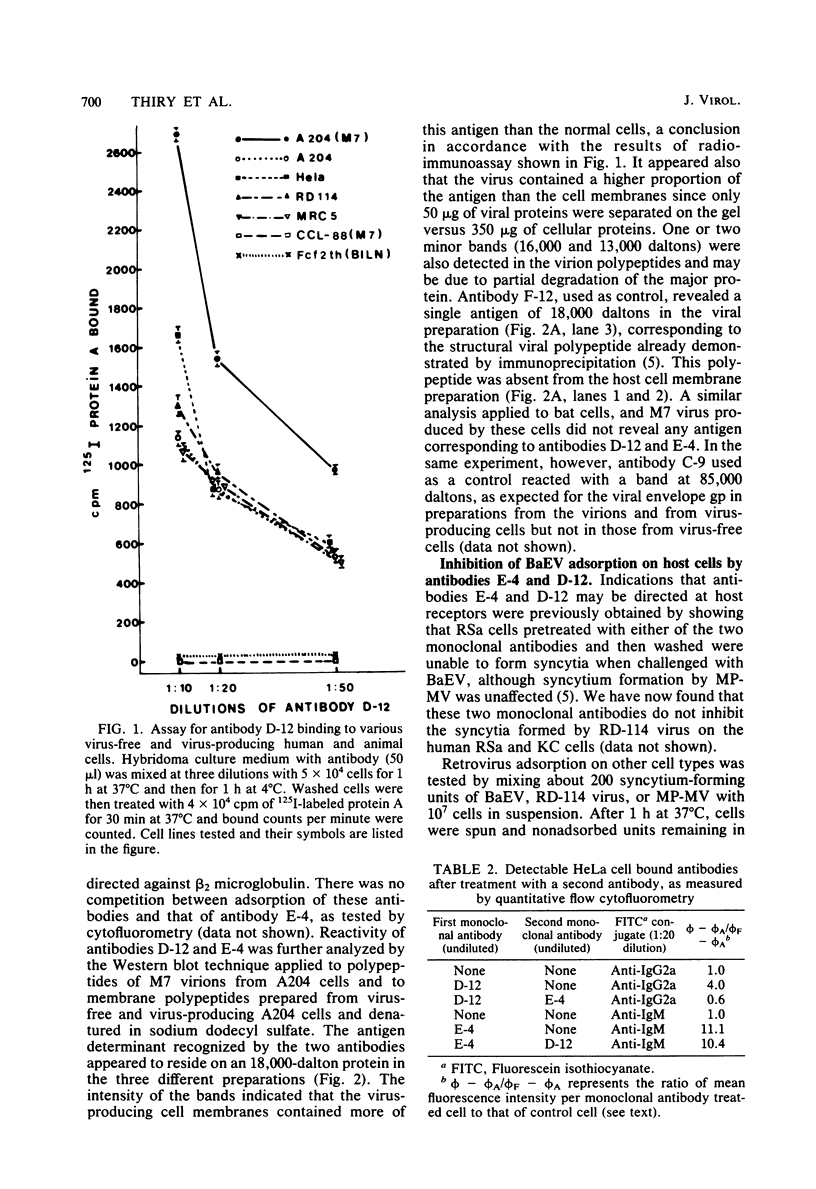
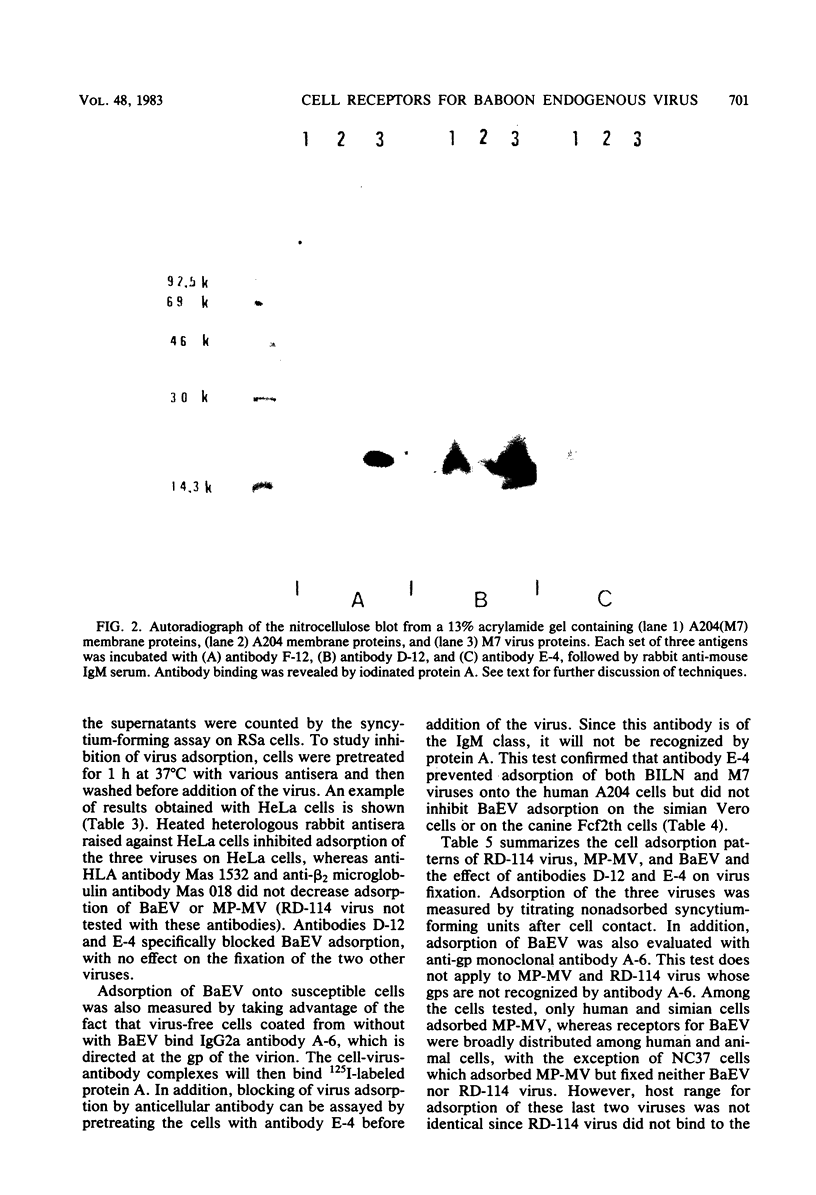
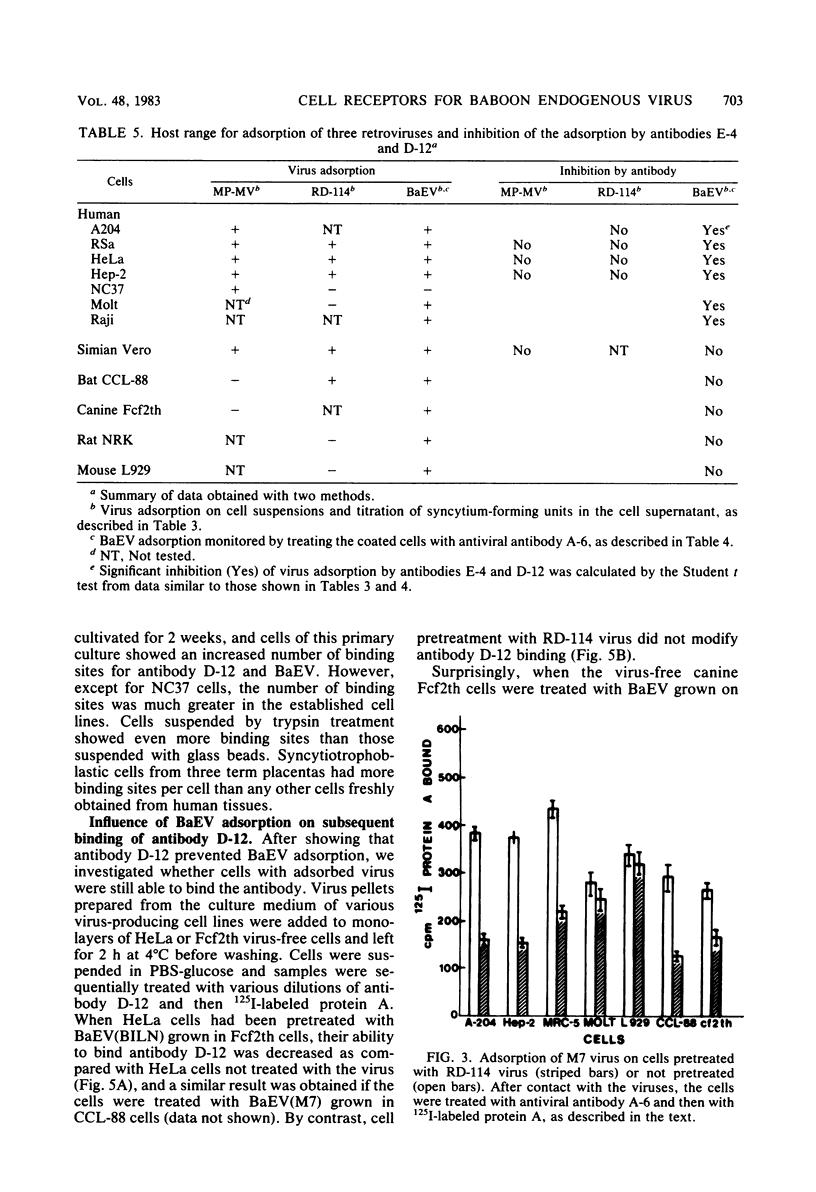
Hybridomas from mice immunized with baboon endogenous virus (BaEV) from A204(M7) cells produced several antiviral monoclonal antibodies and, in addition, antibodies D-12 and E-4, which appeared to be virus specific because they reacted with BaEV but not with Mason-Pfizer virus or RD-114 virus. However, they also bound to human virus-free cells, and they did not recognize BaEV from bat or canine host cells. Cell membrane targets for these antibodies comigrated with an 18,000-dalton protein, which may contain specific determinants of BaEV receptors since antibody masking of these cell sites prevented BaEV but not Mason-Pfizer virus or RD-114 virus adsorption. However, RD-114 virus interfered with BaEV adsorption. Thus, the two viral receptors must be adjacent, but the antibody D-12 and E-4 targets are not within the active site of RD-114 virus receptor. Conversely, cell coating with BaEV from bat or canine hosts inhibited antibody D-12 binding. Noncultivated human lymphocytes and cells from fetal organs bound much less antibody D-12 than did cells from established cell lines, with a correlation between amounts of antibody D-12 acceptor sites and BaEV receptors. Thus, in vivo, BaEV infection of human cells may be inefficient. In vitro, antibody D-12 treatment of chronically infected A204(M7) cells caused intracellular accumulation of viral proteins and decreased virus release, with no such effect on RD-114 virus-producing cells. Canine cells bound antibody D-12 only if coated with BaEV from A204(M7) cells, indicating that the human determinant coadsorbed with the virions to animal cells. Possibly, determinants of cell receptors participate in BaEV maturation and become associated with the virions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azocar J., Essex M., Yunis E. J. Cell culture density modulates the incorporation of HLA antigens by enveloped viruses. Hum Immunol. 1983 Jun;7(2):59–65. doi: 10.1016/s0198-8859(83)80006-8. [DOI] [PubMed] [Google Scholar]
- Brown S., Oie H. K., Gazdar A. F., Minna J. D., Francke U. Requirement of human chromosomes 19, 6 and possibly 3 for infection of hamster x human hybrid cells with baboon M7 type C virus. Cell. 1979 Sep;18(1):135–143. doi: 10.1016/0092-8674(79)90362-3. [DOI] [PubMed] [Google Scholar]
- Bubbers J. E., Chen S., Lilly F. Nonrandom inclusion of H-2K and H-2D antigens in Friend virus particles from mice of various strains. J Exp Med. 1978 Feb 1;147(2):340–351. doi: 10.1084/jem.147.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogniaux J., Olislager R., Sprecher-Goldberger S., Thiry L. Monoclonal antibodies against baboon endogenous virus and against host cell antigens. J Virol. 1982 Aug;43(2):664–672. doi: 10.1128/jvi.43.2.664-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Krueger J. G., Hanafusa H., Goldberg A. R. Temperature-sensitive membrane association of pp60src in tsNY68-infected cells correlates with increased tyrosine phosphorylation of membrane-associated proteins. Virology. 1983 Apr 15;126(1):73–86. doi: 10.1016/0042-6822(83)90462-2. [DOI] [PubMed] [Google Scholar]
- Hellman A., Peebles P. T., Strickland J. E., Fowler A. K., Kalter S. S., Oroszlan K. S., Gilden R. V. Baboon virus isolate M-7 with properties similar to feline virus RD-114. J Virol. 1974 Jul;14(1):133–138. doi: 10.1128/jvi.14.1.133-138.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen S., Stokke T., Prydz H. HeLa cell plasma membranes. I. 5'-Nucleotidase and ouabain-sensitive ATPase as markers for plasma membranes. J Cell Biol. 1974 Nov;63(2 Pt 1):357–363. doi: 10.1083/jcb.63.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landen B., Fox C. F. Isolation of BPgp70, a fibroblast receptor for the envelope antigen of Rauscher murine leukemia virus. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4988–4992. doi: 10.1073/pnas.77.8.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons R. S., Nash W. G., O'Brien S. J., Benveniste R. E., Sherr C. J. A gene (Bevi) on human chromosome 6 is an integration site for baboon type C DNA provirus in human cells. Cell. 1978 Aug;14(4):995–1005. doi: 10.1016/0092-8674(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Lemons R. S., O'Brien S. J., Sherr C. J. A new genetic locus, Bevi, on human chromosome 6 which controls the replication of baboon type C virus in human cells. Cell. 1977 Sep;12(1):251–262. doi: 10.1016/0092-8674(77)90203-3. [DOI] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J. Monoclonal antibodies derived during graft-versus-host reaction. II. Antibodies detect unique determinants common to many MCF viruses. Virology. 1983 Apr 15;126(1):96–105. doi: 10.1016/0042-6822(83)90464-6. [DOI] [PubMed] [Google Scholar]
- Schaffar-Deshayes L., Choppin J., Lévy J. P. Lymphoid cell surface receptor for Moloney leukemia virus envelope glycoprotein gp71. II. Isolation of the receptor. J Immunol. 1981 Jun;126(6):2352–2354. [PubMed] [Google Scholar]
- Schnitzer T. J., Weiss R. A., Juricek D. K., Ruddle F. H. Use of vesicular stomatitis virus pseudotypes to map viral receptor genes: Assignment of RD114 virus receptor gene to human chromosome 19. J Virol. 1980 Aug;35(2):575–580. doi: 10.1128/jvi.35.2.575-580.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J., Weiss R. A., Zavada J. Pseudotypes of vesicular stomatitis virus with the envelope properties of mammalian and primate retroviruses. J Virol. 1977 Sep;23(3):449–454. doi: 10.1128/jvi.23.3.449-454.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiry L., Sprecher-Goldberger S., Cogniaux-Leclerc J., Cappel R., Bossens M. Comparison of different tests to measure immune responses to primate retroviruses. J Immunol Methods. 1979;25(3):197–209. doi: 10.1016/0022-1759(79)90108-x. [DOI] [PubMed] [Google Scholar]
- Thiry L., Sprecher-Goldberger S., Hard R. C., Bossens M., Neuray F. Expression of retrovirus-related antigen in pregnancy. I. Antigens cross-reacting with simian retroviruses in human foetal tissues and cord blood lymphocytes. J Reprod Immunol. 1981 Mar;2(6):309–322. doi: 10.1016/0165-0378(81)90001-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]